Abstract
In this work, we report on a clinically significant response of meningeal carcinomatosis to repotrectinib in a woman with a heavily pretreated ROS1-rearranged non-small cell lung cancer (NSCLC) that harbored the concomitant solvent front G2032R mutation. Meningeal carcinomatosis has a higher incidence in oncogene addicted NSCLC due to increased life expectancy, yet no report has ever documented the activity of repotrectinib in this context. In line with its activity, we documented the presence of the drug at potentially active concentrations in the cerebrospinal fluid. Nevertheless, the short-lived response reported by our patient highlights the importance for novel ROS1-tyrosine kinase inhibitors (TKIs) to be specifically developed to be able to penetrate the blood–brain barrier.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Due to increased life expectancy, the prevention of disease spread to the brain and/or treatment of active central nervous system (CNS) metastases is a relevant issue in ROS1-positive non-small cell lung cancer (NSCLC). |
Particularly, meningeal carcinomatosis is a devastating complication for whom very limited treatment options are available. |
Here, we report the case of a patient with ROS1-positive, G2032R-mutated NSCLC with meningeal carcinomatosis who derived a clinically significant benefit from the novel ROS1-TKI repotrectinib. In addition, we documented that this drug is present in the cerebrospinal fluid (CSF) at a potentially active concentration, with a CSF/plasma ratio of approximately 1%. |
Our case provides for the first-time evidence that repotrectinib may be active against meningeal carcinomatosis from ROS1-positive NSCLC. |
Introduction
ROS proto-oncogene 1 (ROS1)-rearranged non-small cell lung cancer (NSCLC) represents a molecularly defined disease subgroup that can be effectively targeted with ROS1-tyrosine kinase inhibitors (TKIs). More in detail, the ROS1/anaplastic lymphoma kinase (ALK)-TKI crizotinib and the ROS1/ALK/pan-tropomyosin receptor kinase (TRK)-TKI entrectinib have both demonstrated to be exceptionally active for ROS1-positive advanced NSCLC, with objective response rate (ORR) being 72% for crizotinib and 68% for entrectinib. The median overall survival was 51.4 months with crizotinib and 47.8 months with entrectinib [1, 2]. Notably, entrectinib efficacy was also demonstrated in patients with intracranial disease. In this setting, the ORR was 80%, with a median intracranial duration of response (DoR) of 12.9 months and a median intracranial progression free survival (PFS) of 8.8 months.
Nevertheless, the management of central nervous system (CNS) metastases from ROS1-positive disease remains a relevant issue, since 25–35% of ROS1-rearranged advanced NSCLCs present with brain metastases at first diagnosis, while more than 40% of patients will develop CNS metastases after treatment with ≥ 1 ROS1-TKI(s) [3]. The highly CNS-penetrant ROS1/ALK-TKI lorlatinib could be a valid option in ROS1-TKI(s)-pretreated patients with CNS-only progression [4]. However, lorlatinib lacks efficacy against the ROS1 solvent front mutation (SFM) G2032R, which occurs in approximately one third of ROS1-TKI(s)-pretreated patients, thus potentially limiting its use in the refractory setting [5,6,7]. By contrast, the next-generation ROS1/ALK/pan-TRK-TKI repotrectinib has recently demonstrated clinical activity against the SFM G2032R [3, 8]. Nevertheless, repotrectinib’s efficacy in patients with meningeal carcinomatosis has not been reported so far. We describe the case of a heavily pretreated ROS1-positive patient with meningeal carcinomatosis and a ROS1 G2032R SFM who derived a clinically significant, though transitory, benefit from repotrectinib, and put in context the ability of this drug to cross the blood–brain barrier (BBB).
Case Report
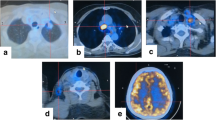
A never smoker, 42-year-old woman was diagnosed with advanced NSCLC (extra-thoracic lymph-nodes and bone) in November 2014, for which she received two cycles of cisplatin/pemetrexed. In February 2015, ROS1 rearrangement was detected by FISH, and the patient started crizotinib 250 mg orally (p.o.) twice daily (BID), with the achievement of a long-lasting partial response. After approximately 5 years of crizotinib, the disease progressed in lung and bone, so that in June 2020 she initiated lorlatinib 100 mg orally once a day (QD), with stable disease as best response. At that time, a rebiopsy of the lung lesion showed G2032R, and lorlatinib was initiated based on the availability of a compassionate use program regardless of the mechanisms of resistance. Upon further progression of bone metastases, the patient was rechallenged with carboplatin/pemetrexed chemotherapy (4 cycles of induction + 2 cycles of maintenance pemetrexed, which was stopped because of patient’s withdrawal) while continuing lorlatinib beyond progression, with partial disease response as best response. In March 2023, the patient was hospitalized because of severe frontal lobe headache, projectile vomiting and associated nausea. A magnetic resonance imaging (MRI) of the brain (Fig. 1A, B) was compatible with the occurrence of meningeal carcinomatosis, which was cytologically confirmed in the cerebrospinal fluid (CSF) by lumbar puncture. The patient was put on symptomatic therapy with steroids (dexamethasone p.o. 4 mg BID) with low effects on symptoms improvement. At that time, CSF liquid biopsy confirmed the presence of ROS1 rearrangement (CD74-ROS1) through next-generation sequencing (NGS), with the concomitant presence of the SFM G2032R mutation (allelic frequency = 29.18%). Interestingly, NGS testing detected the same resistance mutation on plasma (allelic frequency = 1.29%). On this basis the patient started anticancer treatment with repotrectinib p.o. 160 mg QD × 2 weeks followed by 160 mg BID within a compassionate use program. Notably, on day 5 of treatment, the patient’s clinical condition progressively improved, and she was discharged with complete regression of neurologic symptoms on day 14 since the start of repotrectinib. Collaterally, steroids were slowly tapered until permanent discontinuation on day 25 of repotrectinib. A brain MRI performed on day 28 showed stable disease (Fig. 1C, D), while a positron emission tomography (PET)-computed tomography (CT) scan was compatible with partial extra-cranial metabolic response (Fig. 2A, B). Overall, repotrectinib treatment was well tolerated with no relevant side effects. However, on day 75 of repotrectinib the patient was readmitted to the hospital because of the reemergence of headache, nausea/vomiting, and the new onset of severe bilateral hypoacusia. At that time, a brain MRI showed progression of meningeal carcinomatosis (Fig. 1E, E), while the extra-cranial disease was still responding according to the absence of clinical symptoms. On that occasion, a lumbar puncture was repeated, which confirmed cytological involvement of CSF, with positivity for ROS1 rearrangement (CD74-ROS1) and G2032R mutation (allelic frequency = 23.7%) by NGS. We also collected plasma and CSF samples to measure repotrectinib’s concentrations, which were found to be 1317 ng/mL and 14.9 ng/mL, respectively, for a CSF-to-serum ratio of 0.0113 (Table 1). At that time (October 2023), the patient was still on repotrectinib from which she was experiencing both persistent extracranial response and intracranial disease stabilization (as confirmed by the most recent brain MRI and PET-CT) with no worsening of neurological symptoms and partial autonomy in daily living activities.
MRI postcontrast T1-weighted fat saturated (fat-sat) sequences. Sagittal scans show (A) pachimeningeal enhancement of the tent, the posterior region of falx cerebri along with its tentorial insertion, and the pineal region at baseline prior to repotrectinib at (C) the time of stable disease on day 28 and (E) at the time of progressive disease on day 75 of repotrectinib. (B) Axial scan reveals enhancement of the ependyma that covers the cerebral tonsils at baseline prior to repotrectinib at (D) the time of stable disease on day 28 and at (F) the time of progressive disease on day 75 of repotrectinib
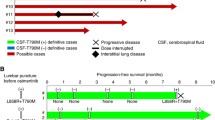
Disease course is represented in Fig. 3, which also focuses on molecular monitoring throughout the years.
Methods and Ethics statement
For cytology examination, CSF was treated by thin-prep cytology technology and stained by Papanicolau stain. Plasma and CSF NGS were carried out through the Oncomine pan-cancer cell-free assay (Thermo Fisher Scientific) available for the Ion Torrent platform. Measurement of plasmatic and CSF concentrations of repotrecinib was performed by reverse phase liquid chromatography with tandem mass spectrometric detection. A validated method was performed using ibrutinib as internal standard [9]. Linear range of detection was 2000–7.8 ng/mL.
The patient signed written informed consent to authorize this case report. No further consents or approvals are required by local institutions.
Discussion
Meningeal carcinomatosis has a higher incidence in patients with oncogene-addicted NSCLCs such as ROS1-positive disease, mainly due to the availability of effective therapies associated with unprecedented overall survival [1, 2]. Accordingly, our patient developed meningeal carcinomatosis following a long disease history, during which she received two ROS1-TKIs in sequence, namely crizotinib and lorlatinib. At the time of diagnosis of meningeal carcinomatosis, the SFM G2032R was detected in both plasma and CSF, which prompted us to employ repotrectinib, a drug with known activity against this resistant variant of ROS1-positive lung cancer [8]. Whole brain radiotherapy (WBRT) was not considered based on the fact that we expected a CNS response given the preliminary activity reported with repotrectinib in the brain [3, 8]. Another reason for avoiding WBRT was its uncertain activity in leptomeningeal disease, especially in oncogene-addicted NSCLC, and our intention to spare the toxic effect of brain radiation and preserve quality of life [10]. Of note, after roughly 1 week of treatment, symptoms of increased intracranial pressure rapidly improved, until complete resolution at approximately week 2 of therapy. In addition, steroids were safely tapered and completely discontinued within 1 month of treatment. Importantly, steroids could not be the reason for symptom improvement because: (1) steroids were administered at the time of admission to the hospital 2 weeks before repotrectinib, and no improvement was noted thereafter; (2) neurologic manifestations of disease did not reappear for a relatively long period of time despite steroid discontinuation ever since. Unfortunately, despite a remarkable partial metabolic extracranial response, neurologic symptoms re-emerged after approximately 3 months, with pathological and radiological confirmation of progression of meningeal carcinomatosis.
To test the ability of repotrectinib to cross the BBB, we measured its concentrations in plasma and CSF. Of note, the CSF/plasma albumin quotient of our patient (= 33.8) was compatible with marked disruption of the BBB, which led us to hypothesize that the drug could effectively penetrate into the CSF (Table 1). We found a plasma and CSF concentrations of 1317.0 ng/mL and 14.9 ng/mL, respectively, for a CSF/plasma ratio of 0.0113, which suggests that approximately 1% of the drug is able to penetrate through the BBB into the CSF (Table 1). Therefore, the presence of repotrectinib in the CSF could justify the exceptional symptomatic response observed in our case. Importantly, the intracranial activity of repotrectinib against brain metastases from ROS1-positive NSCLC has been recently reported. In the phase 1/2 TRIDENT-1 trial, intracranial response of CNS metastases was 33% (8 out of 24 patients with measurable and not measurable lesions) in patients pretreated with one ROS1-TKI [3]. In this sense, repotrectinib appears to have some degree of anti-tumor activity against ROS1-positive CNS metastases in ROS1-TKI refractory patients. Our report extends the potential CNS activity of repotrectinib also to meningeal carcinomatosis, which represents a devastating complication from NSCLC, generally associated with an overall survival of less than 1 year [11].
Nevertheless, we must acknowledge that other factors beyond mere drug penetration through the BBB may contribute to the antitumor effectiveness of a drug against CNS metastases, which may explain the short duration of CNS-response observed in our patient. For instance, repotrectinib is found to be highly bound (95.4%) to plasma proteins, thus possibly limiting the effectiveness of target inhibition by the low fraction of unbound repotrectinib [repotrectinib investigator’s brochure, data on file]. Furthermore, repotrectinib has been shown to act as substrate for the efflux transport P-glycoprotein, which may further limit repotrectinib’s activity in the CNS by keeping the drug out of the brain [12].
Interestingly, the present report suggests that the CSF penetration of repotrectinib is intermediate between crizotinib (median = 0.2%) and lorlatinib (median for unbound drug = 67.9%) (Table 2) [13,14,15,16]. In line with this, lorlatinib has demonstrated an exceptional antitumor activity in ROS1-positive NSCLCs with CNS-only disease progression who were refractory to the ROS1-TKI crizotinib [4]. Consistently, a recently published case report has described a “Lazarus” response to lorlatinib in a patient with meningeal carcinomatosis without additional mutation(s) potentially implicated in resistance across the ROS1 kinase domain (e.g., G2032R) [17]. By contrast, in our case, in which meningeal carcinomatosis was detected after crizotinib and lorlatinib along with documentation of the SFM G2032R mutation, repotrectinib appeared to be the optimal treatment choice.
In conclusion, we report for the first time a clinically significant, though short-lived, response of meningeal carcinomatosis to repotrectinib in a heavily ROS1-TKI-pretreated patient with a G2032R mutation, which is in line with its 1% CSF/plasma penetration that was found in this specific case. Evidently, the penetration of repotrectinib may depend on several factors such as type of CNS disease (parenchymal and/or meningeal involvement) and type/number of prior local treatments received (stereotactic and/or WBRT) that may influence BBB permeability and that cannot be captured by a single case report. Given the common involvement of CNS as metastatic site following treatment with a ROS1-TKI, novel active agents should be specifically developed to both address ROS1-dependent resistance mechanisms and actively penetrate the BBB.
Data Availability
Clinical and molecular data are available upon request.
Change history
03 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s40487-023-00258-z
References
Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30:1121–6.
Drilon A, Chiu CH, Fan Y, et al. Long-term efficacy and safety of entrectinib in ROS1 fusion-positive NSCLC. JTO Clin Res Rep. 2022;3: 100332.
Lin JJ, Drilon A, Cho BC, et al. Intracranial and systemic efficacy of repotrectinib in advanced ROS1 fusion-positive (ROS1+) non-small cell lung cancer (NSCLC) and central nervous system metastases (CNS mets) in the phase 1/2 TRIDENT-1. J Clin Oncol. 2023;41(suppl 16):9017.
Schneider JL, Muzikansky A, Lin JJ, et al. A phase 2 study of lorlatinib in patients with ROS1-rearranged lung cancer with brain-only progression on crizotinib. JTO Clin Res Rep. 2022;3: 100347.
Shaw AT, Solomon BJ, Chiari R, et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019;20:1691–701.
Lin JJ, Choudhury NJ, Yoda S, et al. Spectrum of mechanisms of resistance to crizotinib and lorlatinib in ROS1 fusion–positive lung cancer. Clin Cancer Res. 2021;27:2899–909.
Landi L, Tiseo M, Heukamp LC, et al. Secondary ROS1 mutations and lorlatinib sensitivity in crizotinib-refractory ROS1 positive NSCLC: results of the prospective PFROST trial. Ann Oncol. 2019;30(suppl 5):v609–10.
Drilon A, Ou S-HI, Cho BC, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent-front Mutations. Cancer Discov. 2018;8:1227–36.
Li W, Perpinioti N, Schinkel AH, et al. Bioanalytical assay for the new-generation ROS1/TRK/ALK inhibitor repotrectinib in mouse plasma and tissue homogenate using liquid chromatography-tandem mass spectrometry. J Chromatog B Analyt Technol Biomed Life Sci. 2020;1144: 122098.
Zhen J, Wen L, Lai M, et al. Whole brain radiotherapy (WBRT) for leptomeningeal metastasis from NSCLC in the era of targeted therapy: a retrospective study. Radiat Oncol. 2020;15:185.
Li Q, Lin Z, Hong Y, et al. Brain parenchymal and leptomeningeal metastasis in non-small cell lung cancer. Sci Rep. 2022;12:22372.
Li W, Sparidans RW, Lebre MC, et al. ABCB1 and ABCG2 control brain accumulation and intestinal disposition of the novel ROS1/TRK/ALK inhibitor repotrectinib, while OATP1A/1B, ABCG2, and CYP3A limit its oral availability [Internet]. Pharmaceutics. 2021;13:1761.
Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–5.
Metro G, Lunardi G, Floridi P, et al. CSF concentration of crizotinib in two ALK-positive non-small-cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J Thorac Oncol. 2015;10:e26–7.
Okimoto T, Tsubata Y, Hotta T, et al. A low crizotinib concentration in the cerebrospinal fluid causes ineffective treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer with carcinomatous meningitis. Intern Med. 2019;58:703–5.
Sun S, Pithavala YK, Martini J, et al. Evaluation of lorlatinib cerebrospinal fluid concentrations in relation to target concentrations for anaplastic lymphoma kinase (ALK) inhibition. J Clin Pharmacol. 2022;62:1170–6.
Facchinetti F, Levy A, Ammari S, et al. Meningeal “Lazarus response” to lorlatinib in a ROS1-positive NSCLC patient progressing to entrectinib. Cancer Manag Res. 2021;13:2805–10.
Acknowledgements
The authors thank the participant of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Giulio Metro and Eleonora Gariazzo conceived of the presented idea, wrote the original draft, and designed the figures. Eleonora Gariazzo, Fausto Roila, and Stefania Gori were responsible for reviewing and editing the manuscript. Sara Baglivo performed the liquid biopsies and Gianluigi Lunardi and Antonio Conti carried out the measurements of repotrectinib concentrations both in plasma and CSF. Guido Bellezza analyzed CSF and confirmed the diagnosis of leptomeningeal disease. Barbara Palumbo and Pietro Chiarini interpreted MRI and PET-CT assessments. Luca Marcomigni, Silvia Costabile, and Francesca Colamartini contributed to the preparation of biologic samples. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All named authors confirm they have nothing to disclose.
Ethical Approval
The patient signed written informed consent to authorize this case report. No further consents or approvals are required by local institutions.
Additional information
The original online version of this article was revised: The authors erroneously mentioned the drug repotrectinib in the introduction of the paper (attached screenshot). This should be replaced with the drug entrectinib.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Metro, G., Gariazzo, E., Costabile, S. et al. Repotrectinib’s Clinical Benefit and Its Brain Penetration in a Patient with Meningeal Carcinomatosis from G2032R-Mutated ROS-1 Positive Non-Small Cell Lung Cancer. Oncol Ther 12, 163–171 (2024). https://doi.org/10.1007/s40487-023-00251-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-023-00251-6