Abstract
Introduction
We evaluated real-world outcomes in patients with advanced ovarian cancer (AOC) based on their cumulative risk profile and maintenance therapy (MT) status following first-line (1L) treatment.
Methods
This retrospective observational study of a nationwide electronic health record-derived de-identified database included adult patients diagnosed with stage III/IV OC from January 1, 2011 to February 28, 2021, who received 1L therapy and had ≥ 12 weeks of follow-up after the index date (end of 1L therapy). Patients were grouped according to whether they received MT or active surveillance (AS) following 1L treatment and by the cumulative number of risk factors (RF) present (stage IV disease; no surgery/treated with neoadjuvant therapy and interval debulking surgery; had postoperative visible residual disease; and had BRCA wild-type disease/unknown BRCA status). Time to next treatment (TTNT) and overall survival (OS) were assessed with a cloning and inverse probability of censoring (IPC)-weighted Kaplan–Meier method.
Results
Among 1920 patients, 22.2% received MT and 77.8% received AS. Median IPC-weighted TTNT and OS were 13.3 months (95% CI 11.7–15.8) and 39.1 months (95% CI 32.5–48.6) in the MT cohort, respectively, and 8.6 months (95% CI 8.0–9.5) and 38.4 months (95% CI 36.4–41.0) in the AS cohort, respectively. Almost all patients had ≥ 1 RF (MT 95.3%; AS 96.7%). Median IPC-weighted TTNT was shorter among patients with more RF in both cohorts (MT: 1 RF, 19.3 months, 95% CI 13.5–37.8; 2 RF, 17.2 months, 95% CI 12.8–20.2; 3 RF, 11.0 months, 95% CI 8.2–13.8; 4 RF, 7.0 months, 95% CI 6.2–8.8; AS: 1 RF, 17.7 months, 95% CI 13.5–22.3; 2 RF, 10.2 months, 95% CI 9.1–11.5; 3 RF, 6.5 months, 95% CI 5.8–7.4; 4 RF, 4.1 months, 95% CI 3.5–4.5).
Conclusion
Regardless of RF number, MT was associated with longer TTNT in real-world patients with AOC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients with advanced ovarian cancer are at a high risk for disease progression or death and are typically managed with either maintenance therapy or active surveillance following first-line treatment. |
Understanding real-world clinical outcomes in patients with advanced ovarian cancer by maintenance therapy status and by the number of high-risk factors for disease progression or death can help inform treatment decisions. |
What was learned from the study? |
Most patients had at least one high-risk factor for disease progression or death, and the distribution of patients by the total number of high-risk factors present was similar in patients who received maintenance therapy and those treated with active surveillance. |
Maintenance therapy was associated with improved outcomes regardless of the number of high-risk factors present. |
Introduction
Despite the slowly declining incidence over the past 20 years [1], ovarian cancer continues to be the leading cause of gynecological cancer death among women worldwide [2], with approximately 20,000 new diagnoses and more than 13,000 deaths attributed to the disease each year in the USA alone [1]. Up to 90% of ovarian cancer cases are epithelial in origin, 70% of which present as high-grade serous tumors [3]. Moreover, approximately 70% of women with advanced stage ovarian cancer at diagnosis experience recurrence, and each successive line of treatment raises the likelihood of incurable ovarian cancer due to platinum-based chemotherapy resistance [4,5,6,7]. Patients with advanced disease at diagnosis are most commonly treated with a combination of surgery plus platinum-based chemotherapy with or without anti-angiogenic therapy [8,9,10]. To prolong progression-free survival (PFS) following first-line therapy, maintenance therapy with poly(ADP-ribose) polymerase (PARP) inhibitors is now an option for patients with primary advanced epithelial ovarian cancer following a complete or partial response to platinum-based chemotherapy [11,12,13].
Although the PFS benefit of first-line maintenance therapy has been well-established in clinical trials [12], further evidence is needed to fully characterize the outcomes associated with first-line maintenance therapy in clinical practice. In addition, it remains unknown how a patient’s risk profile affects the efficacy of maintenance therapy. A greater understanding of maintenance therapy and whether patients with multiple high-risk factors experience different outcomes in a real-world setting could help inform clinical decision-making. In this retrospective real-world study, we describe the clinical characteristics and outcomes (i.e., disease progression and mortality) of patients with ovarian cancer, based on their risk category, who received first-line maintenance therapy or were under active surveillance following the completion of first-line treatment.
Methods
Data Source
The Flatiron Health database is a longitudinal electronic health record (EHR)-derived database comprised of de-identified patient-level data from approximately 280 cancer clinics (ca. 800 sites of care) across the USA [14, 15]. The database includes both structured (e.g., laboratory values, prescribed drugs) and unstructured patient-level data curated via technology-enabled chart abstraction from physicians’ notes and other unstructured documents. The study was conducted using the Flatiron Health ovarian cancer database, which included women with a diagnosis of ovarian, fallopian tube, or peritoneal cancer (collectively referred to as ovarian cancer) as defined by International Classification of Diseases (ICD) codes 183x and 158x (ICD 9) and C56x, C57.0x, C48x (ICD-10) and at least two documented clinical visits between January 1, 2011 and February 28, 2021, hereafter referred to as the study period. Although this study used the Flatiron Health database, Flatiron Health was not involved in the study, its design, analysis, or interpretation, or in the drafting of this manuscript.
Study Design, Patient Population, and Cohort Assignment
This retrospective observational cohort study included patients aged 18 years or older diagnosed with stage III or IV ovarian cancer during the study period, who received first-line therapy. The index date was defined as the end date of non-maintenance first-line treatment. Lines of therapy were oncologist-defined and rules-based according to the Flatiron Health database. Patients were required to have at least 12 weeks of follow-up after the index date to enable evaluation of the type of management used after first-line treatment (i.e., maintenance or active surveillance). Patients with incomplete or missing data regarding surgery type [i.e., primary debulking surgery (PDS), interval debulking surgery (IDS), or no surgery] and postoperative residual disease status (i.e., non-visible residual disease or visible residual disease) were excluded because this information was needed to determine each patient’s risk profile. Additionally, patients who had gaps in their medical history after diagnosis were excluded because later treatments may have been mistakenly listed as earlier lines of therapy resulting in treatment line misclassification. For example, patients who received PARP inhibitor monotherapy first-line maintenance therapy may have been misclassified as receiving first-line non-maintenance PARP inhibitor treatment because of missing data. Bevacizumab, PARP inhibitors (olaparib, rucaparib, niraparib), paclitaxel, and gemcitabine were all eligible to be considered maintenance therapies if given in the maintenance setting. Combination therapy with paclitaxel plus gemcitabine, bevacizumab plus a PARP inhibitor, and bevacizumab plus paclitaxel and/or gemcitabine were also eligible to be considered maintenance therapies if given in the maintenance setting. Patients were placed into either the maintenance therapy cohort or active surveillance cohorts based on whether or not they had received maintenance treatment within 120 days of the end of first-line therapy (index date). Patients were considered eligible for first-line PARP inhibitor maintenance therapy if they had received first-line platinum-based treatment containing cisplatin, carboplatin, or oxaliplatin and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. All patients were followed from their index date to the first occurrence of last clinical activity, or the end of the study period.
Four high-risk factors that have been reported in previous studies to increase the risk for disease progression and mortality among patients with ovarian cancer were used to define risk categories [16,17,18]. Patients were grouped according to the cumulative number of risk factors present that would place patients at moderate or high risk for disease progression. High-risk factors were (1) stage IV disease at initial diagnosis, (2) did not undergo surgery, or were treated with neoadjuvant therapy and IDS, (3) had visible residual disease following surgery, and (4) had BRCA wild-type (BRCAwt) disease or unknown BRCA status (Fig. S1 in the supplementary material). Patients who did not undergo debulking surgery were assumed to have visible residual disease. Patients were considered at moderate risk for disease progression if they had none of the high-risk factors and instead had all of the following: stage III disease, nonvisible residual disease following initial surgery, received PDS, and had BRCA mutated (BRCAm) disease.
Study Outcomes
Outcomes of interest included time to next treatment (TTNT) and overall survival (OS) assessed by treatment cohort (maintenance therapy or active surveillance) and by cumulative risk category. TTNT was used as a surrogate for PFS and defined as the time from the index date to the earliest occurrence of second-line therapy initiation or death; patients without an event indicating disease progression (i.e., initiation of second-line therapy or death) were censored at last activity. OS was defined as the time from index date to the date of death. Patients who did not have an event (i.e., death) were censored at the last activity or at the end of the study period. Patient demographic and clinical characteristics were defined on the basis of information from the time of diagnosis to the index date. The following index variables were described: age, weight, race, ethnicity, practice type, patient’s region of residence, ECOG performance status, disease stage at diagnosis, tumor location, histology at diagnosis, BRCA mutation status, homologous recombination deficiency (HRD) status, therapy type (i.e., PDS with adjuvant therapy, IDS with neoadjuvant therapy, or no surgery), residual disease status following initial surgical treatment, hemoglobin level, neutrophil level, and platelet level. Full details on how variables were captured and/or derived from the database can be found in the supplemental methods.
Statistical Methods
Because treatments are not assigned at random in real-world clinical practice, rigorous methods were defined a priori and used to minimize biases and maximize the comparability of baseline characteristics across the two cohorts. To address any potential immortal time bias introduced by assessing treatments that occurred after the index date (i.e., maintenance therapy or active surveillance), a trial emulation cloning methodology was applied [19]. Additional details on the cloning methodology can be found in the supplemental methods. Potential selection bias associated with cohort assignment was addressed using inverse probability of censoring (IPC) weighting (see supplemental methods for additional details). For each outcome of interest (TTNT, OS), survival curves for each cohort and risk group were estimated using an IPC-weighted non-parametric Kaplan–Meier estimator. For index characteristics, medians and interquartile ranges were reported for continuous variables and frequencies and percentages were reported for categorical variables prior to cloning the patient population.
Lastly, we conducted a series of sensitivity analyses. To assess the completeness of data, we required patients to have at least one confirmed clinical activity between 30 and 90 days post-index date. We also applied a grace period of 90 days to assign patients to first-line maintenance therapy or active surveillance groups and restricted the analysis to patients whose index date was on or after January 1, 2017, to align with PARP inhibitor commercial availability. All analyses were performed using SAS® 9.4 (SAS Institute Inc., Cary, NC).
Study Ethics
This study complied with all applicable laws regarding patient privacy. Institutional review board approval of the study protocol was obtained before study conduct and included a waiver of informed consent.
Results
Participants
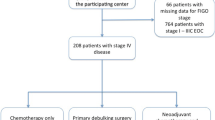
In total, 1920 patients with advanced ovarian cancer diagnosed between January 1, 2011 and February 28, 2021 and who met all eligibility criteria were included in the analysis (Fig. 1). Of these, 426 patients (22.2%) received maintenance therapy within 120 days of completing first-line treatment and were placed into the maintenance therapy cohort; 1494 patients (77.8%) did not receive maintenance therapy and were placed in the active surveillance cohort. Among the patients who received first-line maintenance therapy, 43.7% received bevacizumab-containing regimens, 35.0% received a PARP inhibitor, 5.4% received bevacizumab plus PARP inhibitor combination therapy, and 16.0% of patients received other agents. Focusing on PARP inhibitors, 13.1% (56/426) in the maintenance therapy cohort received niraparib monotherapy, 18.1% (77/426) received olaparib monotherapy, and 1.6% (7/426) received rucaparib monotherapy.
Patient attrition chart for the selection of patients with advanced ovarian cancer who received first-line platinum-based chemotherapy prior to cloning and IPC weighting. aPatients who underwent debulking surgery and were missing either the surgical date or postoperative residual disease status were excluded. bPatients with incomplete medical history within 90 days of diagnosis or debulking surgery (no visit or non-cancelled medication order within 90 days of diagnosis or debulking surgery) were excluded. cPatients who received maintenance treatment within 120 days of the end of first-line therapy (index date). dPatients who did not receive maintenance treatment within 120 days of the end of first-line therapy (index date). 1L, first-line; 2L, second-line; IPC, inverse probability of censoring; PARP, poly(ADP-ribose) polymerase
Overall, the majority of patients originated from a community practice (1685/1920, 87.8%). Demographic characteristics at index were generally similar across the maintenance therapy and active surveillance cohorts prior to cloning and IPC weighting (Table 1). However, the proportion of patients who had stage IV disease at diagnosis (40.4% and 31.7%), BRCAm disease (19.5% and 11.2%), ECOG performance status 0–1 (76.1% and 66.4%), and originated from a community practice (93.2% and 86.2%) was larger in the maintenance therapy cohort than in the active surveillance cohort.
In terms of timing, 70.7% of maintenance therapy patients (301/426) and 41.6% of active surveillance patients (622/1494) had an index date on or after January 1, 2017. Within the maintenance therapy cohort, 59.1% of patients (110/186) who received bevacizumab-containing regimens, 100% of patients who received PARP inhibitor monotherapy or bevacizumab plus PARP inhibitor combination therapy (n = 172), and 27.9% of patients (19/68) who received other agents had an index date on or after January 1, 2017. In terms of risk classification, almost all patients in the maintenance and active surveillance cohorts had at least one high-risk factor (95.3% and 96.7%, respectively). The distribution of patients by cumulative number of high-risk factors was similar across cohorts, with the highest proportion of patients having two high-risk factors (maintenance therapy, 32.4%; active surveillance, 34.2%; Fig. 2). The post–IPC-weighted key baseline characteristics were generally similar between the maintenance treatment and active surveillance cohorts (see supplemental methods and Figs. S2 and S3 for standardized mean differences).
Percentage of patients by cumulative number of high-risk factors prior to cloning. The percentage of patients by cumulative number of high-risk factors present in the a maintenance therapy cohort and b active surveillance cohort. Patients who did not undergo debulking surgery were assumed to have visible residual disease
TTNT and OS
By the end of follow-up, 59.6% (254/426) and 82.3% (1230/1494) of patients in the maintenance therapy and active surveillance cohorts, respectively, progressed to second-line therapy or died. The IPC-weighted median TTNT for patients in the maintenance therapy cohort was 13.3 months (95% CI 11.7–15.8) and 8.6 months (95% CI 8.0–9.5) in patients in the active surveillance cohort (Fig. 3a). Overall, 32.9% (140/426) of the maintenance cohort and 53.9% (805/1494) of the active surveillance cohort died during the study period. The IPC-weighted median OS in patients in the maintenance therapy cohort was 39.1 months (95% CI 32.5–48.6) and 38.4 months (95% CI 36.4–41.0) in patients in the active surveillance cohort (Fig. 3b). Sensitivity analyses results were consistent with the main analysis results.
TTNT and OS by Cumulative Risk
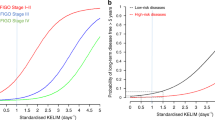
To assess whether there was an association with having multiple high-risk factors, TTNT and OS were evaluated by the cumulative number of high-risk factors in each cohort. Patients with more risk factors had shorter IPC-weighted median TTNT in both the maintenance and active surveillance cohorts (Fig. 4a, b). For patients with moderate risk (no high-risk factors), the IPC-weighted median TTNT was not reached (95% CI 12.7–not reached) in patients who received maintenance therapy and 26.4 months (95% CI 16.4–49.2) in patients who received active surveillance. In patients with one risk factor, the IPC-weighted median TTNT in the maintenance group was 19.3 months (95% CI 13.5–37.8) and 17.7 months (95% CI 13.5–22.3) in the active surveillance group. In patients with two and three high-risk factors, respectively, the IPC-weighted median TTNT was 17.2 months (95% CI 12.8–20.2) and 11.0 months (95% CI 8.2–13.8) in maintenance therapy patients and 10.2 months (95% CI 9.1–11.5) and 6.5 months (95% CI 5.8–7.4) in active surveillance patients. Patients with all four high-risk factors had the shortest IPC-weighted median TTNT in both cohorts (maintenance therapy, 7.0 months, 95% CI 6.2–8.8; active surveillance, 4.1 months, 95% CI 3.5–4.5). In addition, patients with more risk factors had shorter IPC-weighted median OS in both cohorts (Fig. 4c, d).
IPC-weighted Kaplan–Meier survival curves for TTNT and OS by risk category (N = 1920). Kaplan–Meier estimated TTNT and OS by cohort and cumulative number of high-risk factors using IPC-weighted cloning method. IPC-weighted TTNT in a patients who received maintenance therapy and b patients who were treated with active surveillance. IPC-weighted OS in c patients who received maintenance therapy and d patients who were treated with active surveillance. Patients who did not undergo debulking surgery were assumed to have visible residual disease. IPC, inverse probability of censoring; OS, overall survival; TTNT, time to next treatment
Patients Eligible for First-Line PARP Inhibitor Maintenance Therapy
Because first-line PARP inhibitor maintenance therapy is recommended for patients with advanced ovarian cancer who had a complete or partial response to chemotherapy, outcomes were also evaluated in the 1251 patients who were considered eligible for first-line PARP inhibitor maintenance therapy. Within this subpopulation, 323 (25.8%) patients received first-line maintenance therapy and 928 (74.2%) of patients were treated with active surveillance prior to cloning and IPC weights. Demographic and clinical characteristics in this subpopulation were generally similar to the overall population (Table S1 in the supplementary material). Similar to the overall population, the IPC-weighted median durations of TTNT and OS were longer in the maintenance therapy cohort than in the active surveillance cohort in patients who were considered eligible for first-line PARP inhibitor maintenance therapy (Table S2 in the supplementary material). The trends for shorter IPC-weighted median TTNT and OS among patients with more high-risk factors were also observed in PARP inhibitor eligible patients in both cohorts (Table S2).
Discussion
In this retrospective analysis of real-world patients with stage III or IV ovarian cancer at diagnosis who received first-line therapy, almost all patients (ca. 96%) had at least one high-risk factor for disease progression or death. The individual high-risk factors for disease progression or death of stage IV disease at diagnosis, BRCAwt or unknown BRCA status, IDS, and visible residual disease, are well known, and have been studied using both clinical trial and real-world data [2, 16,17,18, 20, 21]. Patients with advanced ovarian cancer are generally considered at high risk for disease progression or death, with a 5-year survival rate of patients with distant disease at diagnosis of approximately 30% [2]. Interestingly, the percentage of patients with high-risk factors was similar across the maintenance therapy and active surveillance cohorts. Because most patients (77.8%) in this study did not receive maintenance therapy, this suggests that in clinical practice a high percentage of patients who were at risk for recurrence did not receive maintenance therapy.
Additionally, the study results also showed that patients who received maintenance therapy had both longer IPC-weighted median TTNT and OS than those who received active surveillance. This result is consistent with those of the PRIMA/ENGOT-OV26 [22], OVARIO [23], SOLO-1 [24], and PAOLA-1/ENGOT-OV25 [25] clinical trials that demonstrated the benefit of PARP inhibitor monotherapy or PARP inhibitor plus bevacizumab first-line maintenance therapy in patients with advanced ovarian cancer. The observed difference in outcomes between patients receiving maintenance therapy and active surveillance was more pronounced in the TTNT than OS results.
Furthermore, the study found that across both cohorts, TTNT and OS outcomes were shorter among patients with more high-risk factors (stage IV disease at initial diagnosis; no surgery or treated with neoadjuvant therapy and IDS; had visible residual disease following surgery; and BRCAwt disease or unknown BRCA status). Importantly, the IPC-weighted median TTNT in patients in the maintenance therapy cohort was consistently longer than in patients in the active surveillance cohort regardless of the cumulative number of risk factors present. Even among patients with all four high-risk factors who had the shortest outcomes, the IPC-weighted median TTNT was longer among patients treated with maintenance therapy than in patients who received active surveillance. These data are generally consistent with findings from clinical trials showing that patients benefited from first-line maintenance therapy regardless of whether they were classified as being at a “low” or “high” risk for progression [26].
However, IPC-weighted median OS was similar between the maintenance and active surveillance cohorts. In part, this could have been because some of the patients in the active surveillance cohort may have gone on to receive maintenance therapy after subsequent lines of treatment. Additionally, the duration of follow-up for the study population was relatively short (median 16.2 months for the maintenance therapy cohort and 24.0 months for the active surveillance cohort) to evaluate OS. IPC-weighted median OS in both cohorts was shorter with multiple risk factors, with the shortest OS seen among patients with a total of four high-risk factors. Dissimilarities in the IPC-weighted median OS in the maintenance therapy and active surveillance cohorts were inconsistent across risk groups. In this study, most patients received active surveillance (ca. 80%) instead of maintenance therapy of any kind. This analysis included patients with ovarian cancer diagnosed between January 1, 2011 and February 28, 2021. During this time, the treatment landscape for first-line maintenance therapy evolved significantly, with the US Food and Drug Administration (FDA) approval of PARP inhibitors alone and in combination with bevacizumab [11]. The bulk of these treatment options were not widely available until the later portion of the study period, which could have contributed to the low percentage of patients who received maintenance therapy. In addition, approvals for different first-line maintenance therapies vary on the basis of BRCA and HRD status. Among patients who received first-line maintenance therapy, bevacizumab and PARP inhibitors were most commonly used, with only a small percentage (ca. 5%) of patients receiving bevacizumab plus PARP inhibitor combination therapy. In a European survey-based study conducted between September 2013 and March 2016, less than half (ca. 45%) of patients with primary epithelial ovarian cancer were aware that maintenance therapy was an option; in these patients, most (ca. 70%) were made aware of the option by doctors [27]. In terms of patient preference, extended OS and PFS were strong motivating factors for choosing maintenance therapy [27, 28]. In a discrete choice experiment, patients with ovarian cancer expected a PFS and OS benefit with maintenance therapy to offset treatment-related side effects; of note, the magnitude of the expected PFS benefit increased in relation to the expected severity of the queried adverse event [28]. Analysis of two online community events for patients with ovarian cancer promoted to US audiences reported patient interest in side effects, expectations of benefit, information on the relationship between genetic mutations and PARP inhibitors, and a desire for more information regarding clinical trial results [29]. Although it is expected that the level of awareness about maintenance therapy has increased over time since the initial approvals, results from these previous studies and our findings suggest that additional work may be needed to increase patient awareness of maintenance therapy options and their potential benefits and risks.
This study provides new data showing how the risk of disease progression and mortality varies by the overall risk profile of patients, which should be considered when making treatment decisions. Previous studies have only evaluated the impact of individual risk factors on patient outcomes. To our knowledge, no other studies have assessed outcomes in patients with multiple risk factors by the type of disease management received after first-line treatment (i.e., maintenance therapy and active surveillance). Clinical trials are often conducted with highly selected patient populations with similar medical histories, thereby providing results that are limited in their generalizability to broader patient populations [30]. Data from EHRs are increasingly being used to augment results from clinical trials by providing valuable real-world information [30, 31]. This study used real-world data that aggregates medical information at the point of care, allowing for data capture from a much larger, more diverse patient population.
Some methodological limitations must be taken into consideration when interpreting the findings from this retrospective analysis. The Flatiron Health population may not be representative of the overall population of patients with ovarian cancer in the USA. In particular, because the database is focused primarily on patients cared for in US community-based practice, it may not be fully representative of patients treated in academic center practices. Therefore, the study results may not be generalizable to all ovarian cancer populations. In addition, as an EHR database, the Flatiron Health database is subject to data entry errors and missing data. Further, as the database does not uniformly capture health visits and treatments outside of the Flatiron Health Network, incomplete medical histories may have occurred, contributing to missing data for some of the study variables. There were several factors that may have also impacted the TTNT and OS estimates. Some patients may have initiated second-line treatment during the grace period, and accordingly would not have been eligible for maintenance therapy for the remainder of the grace period. However, this additional person-time contributed to both cohorts as both clones were not censored during the grace period.
Within the USA, first-line PARP inhibitor maintenance therapy is indicated for use in patients with a complete or partial response to first-line platinum-based treatment [32, 33]. Because response to first-line treatment was not available in the database, it is likely that not all patients in the analysis were eligible for maintenance therapy. The cloning approach with IPC weighting ensured that the baseline characteristics between the two cohorts (i.e., maintenance therapy and active surveillance) were similar by accounting for differences in the factors that may have affected treatment assignment and resulted in informative censoring during the grace period. For risk factor assessment, patients who had no documented information on surgery were assumed to have visible residual disease, resulting in these patients having a minimum of two high-risk factors (IDS/no surgery and visible residual disease), which could have led to the misclassification of the risk profile for some patients. However, there was a similar proportion of patients who did not undergo surgery in both cohorts (18.1% maintenance, 15.4% active surveillance), likely resulting in minimal bias. Also, data were not adjusted for future therapies that could have affected the outcomes of interest, particularly OS. Finally, results stratified by cumulative risk factors should be interpreted with caution because of the small sample size prior to the cloned analysis, particularly in the moderate risk and PARP inhibitor maintenance therapy eligible group. In the future, risk level assignment in this patient population should be validated in the context of clinical studies. In addition, as the first-line maintenance therapy landscape has shifted in recent years, it will be important to evaluate maintenance therapy treatment patterns and outcomes over time as maintenance therapies have become more widely available.
Conclusions
The findings from this study contribute to real-world evidence on longer IPC-weighted clinical outcomes in patients with advanced ovarian cancer who received first-line maintenance therapy than in patients monitored with active surveillance, especially among those grouped as high risk. In addition, the results provide important information about the clinical outcomes of patients with advanced ovarian cancer with multiple risk factors in the real world.
References
American Cancer Society. Key statistics for ovarian cancer. 2021. https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. Accessed 4 Dec 2022.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Tew WP, Lacchetti C, Ellis A, et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:3468–93.
Matsuo K, Bond VK, Eno ML, Im DD, Rosenshein NB. Low drug resistance to both platinum and taxane chemotherapy on an in vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian and peritoneal cancer. Int J Cancer. 2009;125:2721–7.
Tapia G, Diaz-Padilla I. Molecular mechanisms of platinum resistance in ovarian cancer. In: Diaz-Padilla I, editor. Ovarian cancer: a clinical and translational update. InTech; 2013. https://doi.org/10.5772/55562.
Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23:2605–12.
Giornelli GH. Management of relapsed ovarian cancer: a review. Springerplus. 2016;5:1197.
Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30:672–705.
Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24-32.
Musella A, Vertechy L, Romito A, et al. Bevacizumab in ovarian cancer: state of the art and unanswered questions. Chemotherapy. 2017;62:111–20.
Colombo N, Ledermann JA, ESMO Guidelines Committee. Updated treatment recommendations for newly diagnosed epithelial ovarian carcinoma from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32:1300–3.
Mirza MR, Coleman RL, Gonzalez-Martin A, et al. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol. 2020;31:1148–59.
Musella A, Marchetti C, Gasparri ML, et al. PARP inhibition: a promising therapeutic target in ovarian cancer. Cell Mol Biol (Noisy-le-grand). 2015;61:44–61.
Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv [preprint]. 2001.09765.
Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv [preprint]. 2020;2020.03.16.20037143.
Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2011. https://doi.org/10.1002/14651858.CD007565.pub2.
Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
Westin SN, Louie-Gao M, Gupta D, Thaker PH. Risk factors for progression or death after first-line platinum-based chemotherapy in real-world patients in the USA with ovarian cancer from 2011 to 2018. Future Oncol. 2021;17:4263–74.
Maringe C, Benitez Majano S, Exarchakou A, et al. Reflection on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data. Int J Epidemiol. 2020;49:1719–29.
PDQ Adult Treatment Editorial Board. PDQ Ovarian Epithelial, Fallopian Tube, and Primary Peritoneal Cancer Treatment (PDQ®): Health Professional Version. In: PDQ Cancer Information Summaries. National Cancer Institute; February 9, 2023.
Di Donato V, Kontopantelis E, Aletti G, et al. Trends in mortality after primary cytoreductive surgery for ovarian cancer: a systematic review and metaregression of randomized clinical trials and observational studies. Ann Surg Oncol. 2017;24:1688–97.
González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402.
Hardesty MM, Krivak TC, Wright GS, et al. OVARIO phase II trial of combination niraparib plus bevacizumab maintenance therapy in advanced ovarian cancer following first-line platinum-based chemotherapy with bevacizumab. Gynecol Oncol. 2022;166:219–29.
Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505.
Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–28.
Chambers LM, O’Malley DM, Coleman RL, Herzog TJ. Is there a “low-risk” patient population in advanced epithelial ovarian cancer?: a critical analysis. Am J Obstet Gynecol. 2022;227:728–34.
Rohr I, Alavi S, Richter R, et al. Expectations and preferences of patients with primary and relapsed ovarian cancer to maintenance therapy: a NOGGO/ENGOT-ov22 and GCIG survey (Expression IV). Int J Gynecol Cancer. 2020;30:509–14.
Havrilesky LJ, Lim S, Ehrisman JA, et al. Patient preferences for maintenance PARP inhibitor therapy in ovarian cancer treatment. Gynecol Oncol. 2020;156:561–7.
Monuszko KA, Fish LJ, Sparacio D, et al. Understanding the needs and perspectives of ovarian cancer patients when considering PARP inhibitor maintenance therapy: findings from two online community events. Gynecol Oncol Rep. 2022;43: 101050.
Rudrapatna VA, Butte AJ. Opportunities and challenges in using real-world data for health care. J Clin Invest. 2020;130:565–74.
Nordo AH, Levaux HP, Becnel LB, et al. Use of EHRs data for clinical research: historical progress and current applications. Learn Health Syst. 2019;3: e10076.
Olaparib. Package insert. AstraZeneca; 2022.
Niraparib. Package insert. GSK; 2022.
Acknowledgements
Funding
This study (OneCDP 213807) and its publication, including the journal’s Rapid Service fee, was supported by GSK.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial assistance, funded by GSK (Waltham, Massachusetts) and coordinated by Hasan Jamal, MSc, of GSK, were provided by Gauri Saal, MA Economics, Medical Division, London, UK, Betsy C. Taylor, PhD, CMPP, and Jennifer Robertson, PhD, of Ashfield MedComms, an Inizio company (Middletown, Connecticut).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conception and design: All authors. Provision of study materials or patients: Jessica Perhanidis, Divya Gupta, Linda Kalilani, Amanda Golembesky. Collection and assembly of data: Jessica Perhanidis, Linda Kalilani. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Prior Presentation
Portions of these data were previously presented at the European Society of Gynaecological Oncology (ESGO) 22nd European Congress of Gynaecological Oncology, October 23–25, 2021, Prague, Czech Republic.
Disclosures
Dana Chase reports speakers’ bureau fees and/or advisory roles from, AstraZeneca, Clovis, Genentech/Roche, and GSK and consulting fees from GSK, AstraZeneca, Clovis, and Genentech/Roche. Jessica Perhanidis is a current employee of GSK and reports financial interest (stock) in Boston Scientific and GSK. Linda Kalilani and Amanda Golembesky are current employees of GSK. Divya Gupta was an employee of GSK at the time the analysis was conducted, and is currently affiliated with Mersana Therapeutics, Inc, Cambridge, MA, USA. Antonio González-Martín reports honoraria as advisor from Alkermes, Amgen, AstraZeneca, Clovis Oncology, Genmab, GSK, HederaDx, ImmunoGen, Merck Sharp & Dohme, MacroGenics, Novartis, Oncoinvent, Pfizer/Merck, PharmaMar, Roche, Sotio, Sutro; honoraria as speaker for AstraZeneca, Clovis, GSK, PharmaMar, Roche; institutional funding (GEICO) for clinical research from Roche and GSK; and non-remunerated activities as chair in GEICO and ENGOT (for the period 2018–2020).
Compliance with Ethics Guidelines
This study complied with all applicable laws regarding patient privacy. Institutional review board approval of the study protocol was obtained prior to study conduct and included a waiver of informed consent.
Data Availability
The data that support the findings of this study have been originated by Flatiron Health, Inc. These de-identified data may be made available upon request and are subject to a license agreement with Flatiron Health; interested researchers should contact <DataAccess@flatiron.com> to determine licensing terms.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chase, D., Perhanidis, J., Gupta, D. et al. Real-World Outcomes Following First-Line Treatment in Patients with Advanced Ovarian Cancer with Multiple Risk Factors for Disease Progression who Received Maintenance Therapy or Active Surveillance. Oncol Ther 11, 245–261 (2023). https://doi.org/10.1007/s40487-023-00227-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-023-00227-6