Abstract
Introduction
Despite the growing evidence for the anticancer effect of metformin or its combination with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), the efficacies and side effects of such strategies in non-small cell lung cancer (NSCLC) patients with or without type 2 diabetes mellitus (T2DM) are not well understood. This meta-analysis was performed to determine the efficacy and side effects of metformin combined with EGFR-TKIs (MET-EGFR-TKIs) for the treatment of NSCLC with or without T2DM.
Methods
PubMed and Cochrane Library databases were used to retrieve relevant studies through August 2020 using the keywords “metformin”, “EGFR-TKIs” (“gefitinib” or “erlotinib” or “afatinib” or “icotinib” or “dacomitinib”) and “lung cancer”. The patients in the experimental group received MET-EGFR-TKIs, while those in the control group received only EGFR-TKIs. The outcome analysis reported overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR). Random-effect models and fixed-effect models were used to estimate the combined hazard ratio (HR) and odds ratio (OR) depending on the data heterogeneity. Three studies (including 1996 patients) were included in the current meta-analysis.
Results
There were significant differences in PFS (HR 0.84; 95% confidence interval (CI) 0.75–0.95; P = 0.004) and OS (HR 0.77; 95% CI, 0.50–1.04; P < 0.001) between the MET-EGFR-TKI and EGFR-TKI groups. Although the ORR (OR 1.38; 95% CI 0.66–2.88; P = 0.105) and DCR (OR 2.61, 95% CI 0.68–9.95, P = 0.160) were improved, there was no statistical significance. OS subgroup analysis showed that the combination was more effective in NSCLC with T2DM than in NSCLC without T2DM (HR 0.84; 95% CI 0.74–0.95; P < 0.005).
Conclusions
MET-EGFR-TKIs provided benefits for PFS and OS, and OS subgroup analysis showed that patients with NSCLC with T2DM received greater benefit than NSCLC patients without T2DM. However, further large-scale, well-designed randomized controlled trials (RCTs) are warranted to confirm the findings in the present investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lung cancer is divided into small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), and NSCLC accounts for approximately 80–85% of cases, with the most predominant types being adenocarcinomas and squamous cell carcinomas. |
Despite the growing evidence for the anticancer effect of metformin or its combination with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), the efficacies and side effects of such strategies in non-small cell lung cancer (NSCLC) patients with or without type 2 diabetes mellitus (T2DM) are not well understood. |
Metformin has been increasingly valued for its antitumor effects. |
Retrospective studies have shown that metformin can reduce the incidence of cancer and improve the survival rate in patients with type 2 diabetes. |
Further large-scale, well-designed randomized controlled trials (RCTs) are warranted to confirm the findings in the present investigation. |
Introduction
The World Health Organization (WHO) states that lung cancer is one of the most common cancers worldwide and has a high mortality rate [1, 2]. Based on histology, lung cancer is divided into small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), and NSCLC accounts for approximately 80–85% of cases, with the most predominant types being adenocarcinomas and squamous cell carcinomas [3]. Various treatments are administered according to the individual pathological type and stage of each tumor.
Platinum-based chemotherapy is the first-line treatment for advanced NSCLC, but the prognosis from such therapy is poor due to drug resistance [4]. The emergence of targeted therapy has changed the treatment model for NSCLC, including ALK inhibitors, ALK/ROS1/NRTK inhibitors, EFGR inhibitors, HER1/HER2/HER4 inhibitors, KRAS inhibitors, and EGFR-TKIs. For example, the emergence of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) has improved patient progression-free survival (PFS) and overall survival (OS) [5, 6]. Despite initially high response rates, patients will inevitably gradually become resistant to EGFR-TKI treatment [7], so there is a crucial need to develop treatment strategies with good curative effects and low drug resistance.
Metformin is both a hypoglycemic agent and an anticancer agent [8,9,10]. Metformin has been increasingly valued for its antitumor effects. Retrospective studies have shown that metformin can reduce the incidence of cancer and improve the survival rate in patients with type 2 diabetes (T2DM) [11, 12]. Additionally, in advanced NSCLC, there is some evidence that metformin synergizes with standard therapy [13, 14]. More importantly, metformin has been shown to enhance the sensitization of NSCLC to EGFR-TKIs based on in vitro studies and clinical trials [15,16,17]. Metformin combined with EGFR-TKIs (MET-EGFR-TKIs) has been utilized in previous trials for NSCLC patients, however, the results have been inconclusive. Therefore, we conducted a systematic review to determine the efficacy of MET-EGFR-TKIs in patients with NSCLC and T2DM to provide a safe and effective reference for clinicians.
Methods
The present investigation utilized meta-analysis based on Quality of Reporting of Meta-analyses (QUORUM) guidelines [18] and recommendations of the Cochrane Collaboration [19].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Sources and Searches
The electronic databases PubMed and Cochrane Library were searched by two of the authors independently, from their establishment to August 2020. The Cochrane Library includes the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews (CDSR), the Database of Reviews of Effects (DARE), and Health Technology Assessments (HTA). The search was limited to studies in humans but not to studies in a specific language. The keywords “metformin” and “EGFR-TKIs” (“gefitinib” or “erlotinib” or “afatinib” or “icotinib” or “dacomitinib”) and “lung cancer” (“lung cancer” and “T2DM”) were used. Related to the limited number of available studies, filters were not applied for screening (such as the Clinical Trials filter in PubMed), and studies were selected item by item. We checked the novelty of this analysis by entering keywords through the “system review” and “meta-analysis” filters. In addition to reviewing the research listed in the references, a comprehensive search was performed; if the full text was unavailable or the research information was incomplete, the author was contacted [16]. An example of a conducted search on PubMed is as follows: the keywords “metformin” and “EGFR-TKIs” and “lung cancer” were typed into the search bar.
Selection Criteria
-
1.
Population: adult patients with histologically or cytologically confirmed NSCLC or NSCLC with T2DM. The population was not limited by sex, race, nationality, clinical tumor stage, histology, smoking or drinking history, or EGFR status.
-
2.
Intervention: MET-EGFR-TKIs.
-
3.
Comparison intervention: EGFR-TKIs with or without placebo.
-
4.
Outcome: PFS as the primary prognostic indicator; OS, ORR, and DCR as secondary prognostic indicators.
-
5.
Study design: randomized controlled trials (RCTs) or non-RCTs.
Data Extraction
Data extraction was carried out by two evaluators, Wang and Wei, independently, and the extracted information included the following: basic author information (first author and year of publication); characteristics of the study subjects (sex, age, ethnicity, tumor origin, number of tumors, tumor histology, clinical stage, and EGFR status); intervention measures (MET-EGFR-TKIs in the experimental group and EGFR-TKIs with or without placebo in the control group); outcome indicators (PFS and OS); and study design (RCT or non-RCT). We entered the raw data and the information obtained into Table 1. The hazard ratios (HRs) between the combination treatment group and the monotherapy group were also recorded, and any differences arising from this process were resolved by consensus.
Statistical Analysis
The purpose of this study was to investigate the PFS and OS of patients with NSCLC with or without T2DM (diagnosed according to fasting blood sugar and hemoglobin A1c levels and confirmed using tests such as oral glucose tolerance test) treated with MET-EGFR-TKIs or EGFR-TKI monotherapy. The effects on PFS and OS are summarized as HRs and 95% confidence intervals (CIs). HR < 1 indicated that the combination therapy extended OS or PFS more than did the monotherapy. The effects on ORR and DCR were measured as odds ratios (ORs) and 95% CIs, and OR > 1 suggested that the combination therapy provided a higher ORR or DCR than monotherapy. Some studies directly reported HR data related to our findings. In other studies, the HR could not be obtained, and only Kaplan–Meier curves were provided. According to the research of Tierney et al. [20], we extracted relevant data from the Kaplan–Meier curve and estimated the HRs and the 95% CIs of time-event variables, including PFS and OS. For the evaluation of heterogeneity, we used the Q chi-square test and I2 statistics [21]; significant heterogeneity was suspected with I2 > 50% or Q test P < 0.1 [22]. We used a fixed-effects model to process the data, and when significant heterogeneity was found among the studies, a random-effects model was used. If I2 was ≤ 50% or P was > 0.10, similar studies were suspected to be similar; in contrast, if I2 was > 50% or P ≤ 0.10, heterogeneity was suspected, and sensitivity analysis and subgroup analysis were carried out. Publication bias was assessed via Begg’s and Egger’s tests [23, 24]. All statistical analyses were performed with Stata 15.0 (Stata Corporation, College Station, TX, USA). All analyses were based on previously published studies; thus, no ethical approval or patient consent was required. Two-sided P < 0.05 was considered statistically significant, unless otherwise specified.
Results
Search Results
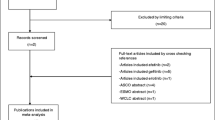
A total of 43 studies were retrieved by searching the databases, among which 19 duplicates were excluded. By reading the titles and abstracts, a total of 16 articles were excluded because they contained animal experiments, were phase I trials, were research reports or were irrelevant studies. After reading the full text, five studies were excluded, including one study that used a treatment other than metformin in the control group [11] and a dose-determining study [25]; ultimately, three studies were included. This meta-analysis included three studies [12, 16, 17] comprising 1996 participants, 554 of whom were in the MET-EGFR-TKI group and 1442 of whom were in the EGFR-TKI monotherapy group. The main research characteristics are described in Fig. 1.
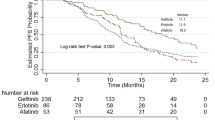
The three studies we selected were published in 2019 and were conducted in China, Mexico, and Taiwan. The tumors in all three studies were adenocarcinomas. In one study [16], squamous cell carcinoma was included, while another study [12] included not only adenocarcinomas but also some NSCLCs with unclear tissue histology. Two studies [16, 17] provided definite tumor stages and clear types of EGFR mutation, while in another study [12], stage was ambiguous, and no specific EGFR mutation type was noted. In that study, response to EGFR-TKIs was used as a surrogate for EGFR mutation, while EGFR mutations were only found in 40.2% of participants. All studies used MET-EGFR-TKIs in the experimental group, and the monotherapy group used EGFR-TKIs. One study [17] used EGFR-TKIs (gefitinib/afatinib/erlotinib) in combination with metformin as the experimental group, one study12 used EGFR-TKIs (gefitinib/erlotinib/both) combined with prescribed metformin as the experimental group, and patients who used metformin without prescription were included in the single EGFR-TKI group. Another study [16] used only gefitinib and metformin in the experimental group. Two studies [16, 17] reported PFS, OS, ORR and DCR, and one study [12] only reported PFS and OS. Two [16, 17] of the three studies were phase 2 RCTs, and the other study [12] was not an RCT and did not mention a study period. We also analyzed the population data: one study [16] was from the Instituto Nacional de Cancerología (INCAN) in Mexico, one study was from nine hospitals in China, one study [17] was from patients in the INCAN database, and one study [12] was from the NHIRD. All three studies were published in English.
Main Outcome Analysis
Pooled Analysis of PFS
The results of the present investigation demonstrated that PFS data were available for all included studies, enabling analysis of a total of 1996 patients. There was little heterogeneity among all eligible studies (I2 = 49.9%, P = 0.136). Therefore, the HRs and 95% CIs for PFS were determined with a fixed-effects model. Compared with that of EGFR-TKIs, the PFS of MET-EGFR-TKIs was longer (HR 0.84; 95% CI 0.75–0.95; P = 0.004), and the difference was statistically significant (Fig. 2A).
Fixed-effects model analysis of PFS and DCR and random-effects model analysis of OS and ORR. A Forest plot of the effect of treatment on PFS. B Forest plot of the effect of treatment on OS. C Forest plot of the effect of treatment on the ORR. D Forest plot of the effect of treatment on the DCR. CI confidence interval, HR hazard ratio, OS overall survival, PFS progression-free survival, OR odds ratio, ORR overall response rate, DCR disease control rate
Pooled Analysis of OS
All included studies contained OS data, enabling analysis of a total of 1996 patients. A fixed-effect model was used for the 1996 patients. The fixed-effect model implied that the studies were heterogeneous, so a random-effect model was selected (I2 = 66.9%, P = 0.058). Compared with that EGFR-TKIs, the OS of MET-EGFR-TKIs was extended (HR 0.77; 95% CI 0.50–1.04; P < 0.001), and the difference was statistically significant (Fig. 2B).
Pooled Analysis of ORR
ORRs for tumors were obtained from all eligible studies, enabling analysis of 341 participants. Heterogeneity between studies was detected with the fixed-effect model, so a random-effect model was used to collect ORR data (I2 = 62.0%, P = 0.105). The ORR of the combined treatment group was 68.1% (113/166), while that of the monotherapy group was 61.7% (108/175). Further analysis showed that MET-EGFR-TKIs improved the ORR in patients compared with EGFR-TKI monotherapy (OR 1.38; 95% CI 0.66–2.88; P = 0. 105), but the difference was not statistically significant (Fig. 2C).
Pooled Analysis of DCR
A total of 341 patients were eligible for the study. According to the forest map, there was no heterogeneity in the two studies when the OR of DCR was pooled using the fixed-effect model (I2 = 0.0%, P = 0.356). The DCR in the combination treatment group was 98.2% (163/166), and that in the EGFR-TKI group was 95.4% (167/175). The forest map further showed that combination therapy improved the DCR (OR 2.61, 95% CI 0.68–9.95, P = 0.160) compared with monotherapy, but the difference was not statistically significant (Fig. 2D).
Sensitivity Analysis and Subgroup Analysis
There was no heterogeneity in the HR of PFS or the OR of the DCR, and there were few included studies, so no sensitivity analysis was conducted. Since there was no heterogeneity between the studies in terms of the PFS and DCR indexes and only two studies were included in the ORR analysis, only OS was evaluated in terms of age, region, tumor stage, type of EGFR-TKI in the experimental group and type of study. As shown in the forest map, stratified analysis revealed an increase in OS after combination treatment (HR 0.85; 95% CI 0.77–0.95; P < 0.001), and no heterogeneity was found in the intragroup analysis (I2 = 40.3%, P = 0.187). In the analysis of each group, it was found that the OS value from combination treatment increased in groups with the following characteristics: age ≥ 65 years, region other than Asia, unclear tumor stage and non-RCT design. However, the experimental group was categorized by EGFR-TKI type, and no significant differences were found in the subgroup analyses. As such, age, study type, tumor stage, and study region are also points for further exploration (Fig. 3).
Additionally, since the experimental group receiving MET-EGFR-TKIs included patients with NSCLC with or without T2DM, further subgroup analysis for OS was performed for the experimental group. As seen in the forest map, there was no heterogeneity in the intragroup analyses (I2 = 40.3%, P = 0.187). Compared with patients with NSCLC, patients with NSCLC and T2DM were more likely to see a benefit from MET-EGFR-TKIs (HR 0.84; 95% CI 0.74–0.95; P < 0.005), which may be related to them having diabetes (Fig. 4).
Publication Bias
There was no evidence of publication bias for PFS (Begg’s test: P = 1.000; Egger’s test: P = 0.43) or OS (Begg’s test: P = 1.000; Egger’s test: P = 0.999) (Figs. S1 and S2, Tables S1 and S2). Since there were only two studies included in the ORR and DCR analyses, no publication bias analysis was conducted.
Discussion
Targeted therapy for NSCLC, especially the application of MET-EGFR-TKIs, has attracted increasing attention. The purpose of this meta-analysis was to determine the efficacy of MET-EGFR-TKIs in the treatment of patients with NSCLC with or without T2DM. In this study, the combination therapy reduced the risk of disease progression by 16% and the risk of OS by 20% compared to EGFR-TKI monotherapy.
A review of the literature identified two studies [12, 17], and the combination treatment prolonged the survival of lung cancer patients; one study [16] showed that monotherapy with EGFR-TKIs showed greater benefit and a lower occurrence of adverse events than the combination therapy. However, according to the results of the meta-analysis, the combination treatment group had better PFS than the monotherapy group (HR 0.84; 95% CI 0.75–0.95; P = 0.004); therefore, in general, the combination therapy was superior to monotherapy in terms of prolonging the PFS of NSCLC patients. The treatment results regarding disease progression were also surprising, showing that OS (HR 0.77; 95% CI 0.50–1.04; P < 0.001) was significantly longer in patients receiving combination therapy than in patients receiving EGFR-TKIs. Previous studies have shown that the median OS in patients receiving MET-EGFR-TKIs is 31.7 months (95% CI 20.5–42.8 months), compared to 17.5 months (95% CI 11.4–23.7 months) in patients receiving EGFR-TKIs17. However, in the Li et al. [16] study, a slight difference between the combination therapy and the monotherapy groups was observed. Subgroup analysis showed that OS increased after combination treatment (HR 0.85; 95% CI 0.77–0.95; P < 0.001), and intragroup analysis found no heterogeneity (I2 = 40.3%, P = 0.187). In the analysis of each group, it was found that age, region, tissue stage, study type, and combination treatment were related to OS. Therefore, further studies are needed to determine the efficacies of combination treatment and monotherapy in terms of OS for NSCLC patients with and without T2DM. Additionally, a subgroup analysis found that MET-EGFR-TKIs was more likely to produce an OS benefit in NSCLC with T2DM than in NSCLC without T2DM (HR 0.84; 95% CI 0.74–0.95; P = 0.005), which may be related to the fact that the patients had diabetes. Due to the small number of studies that were included, larger studies and further clinical trials will be conducted to confirm our findings.
This meta-analysis also identified experimental evidence for therapeutic response.
Though the ORR (OR 1.38; 95% CI 0.66–2.88; P = 0.105) and DCR (OR 2.61, 95% CI 0.68–9.95, P = 0.160) were improved, there was no statistical significance. Only two experiments reported relevant data that could be used in the analyses. The main reason is that there are too few studies on MET-EGFR-TKIs available. Due to the insufficient relevant information reported in the studies included in this meta-analysis, subgroup analysis of ORR and DCR was not conducted. This deficit will no doubt need to be carefully studied in the future, but there were some direct and reliable conclusions from this research that may benefit MET-EGFR-TKIs treatment of NSCLC.
This meta-analysis has several advantages. First, direct evidence, which is more reliable than indirect evidence, of the effectiveness of MET-EGFR-TKIs was summarized. Second, this meta-analysis generated a large and comprehensive dataset, enabling the generation of accurate estimates and the performance of group analyses based on important factors. Importantly, there was no evidence of publication bias. Third, interstudy statistical heterogeneity was not significant in most of the major meta-analyses.
Limitations
This meta-analysis also had many shortcomings, mainly regarding the following aspects. First, not all the studies in this meta-analysis were RCTs, and not all RCTs were double-blinded. Second, EGFR-TKIs include gefitinib, erlotinib, afatinib, icotinib, and dacomitinib. In one study [16], the only EGFR-TKIs used in the intervention group was gefitinib, while the other two studies [12, 17] included at least two EGFR-TKIs, which might also affect the validity of the data. Fourth, there was a large discrepancy between drug withdrawal times and follow-up times between the studies. One study [17] had a higher discontinuation rate (23.3%) and a shorter follow-up time than the other studies. The median follow-up time of the other two studies was over 19 months. Fifth, one of the three studies had unclear information regarding pathological stage, smoking status, and genetic factors. Sixth, this study had two target populations: NSCLC patients with T2DM and NSCLC patients without T2DM. Last but not least, the number of studies on MET-EGFR-TKIs and monotherapy was very limited, which made it impossible for us to draw clear conclusions about the relative efficacy of metformin in certain situations.
MET-EGFR-TKIs in the treatment of NSCLC has only been discovered in recent years, and a large number of studies are currently underway. At this time, a phase II open-label study [24] is being conducted using metformin in combination with erlotinib in approximately 60 patients to determine the maximum tolerated dose of both agents and the time until disease progression. Our results suggest that MET-EGFR-TKIs may be a new treatment regimen for NSCLC that is worthy of further study. Overall, the meta-analysis showed that MET-EGFR-TKIs was superior to monotherapy.
Conclusions
MET-EGFR-TKIs provided PFS and OS benefits to patients, and OS subgroup analysis showed that NSCLC combined with T2DM gained more benefits than NSCLC patients without T2DM. However, there is still a need for further large-scale, well-designed RCTs to confirm our findings.
References
Hashim D, Boffetta P, La Vecchia C, Rota M, Bertuccio P, Malvezzi M, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol. 2016;27:926–33.
Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin N Am. 2019;103:463–73.
Skřičková J, Kadlec B, Venclíček O, Merta Z. Lung cancer. Cas Lek Cesk. 2018;157:226–36.
Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIS) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019;137:113–22.
Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105:595–605.
Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36:2244–50.
Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17:38.
Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:2184–95.
Meireles CG, Pereira SA, Valadares LP, Rêgo DF, Simeoni LA, Guerra ENS, et al. Effects of metformin on endometrial cancer: systematic review and meta-analysis. Gynecol Oncol. 2017;147:167–80.
Zhong S, Wu Y, Yan X, Tang J, Zhao J. Metformin use and survival of lung cancer patients: meta-analysis findings. Indian J Cancer. 2017;54:63–7.
Chen H, Yao W, Chu Q, Han R, Wang Y, Sun J, et al. Synergistic effects of metformin in combination with EGFR-TKI in the treatment of patients with advanced non-small cell lung cancer and type 2 diabetes. Cancer Lett. 2015;369:97–102.
Hung MS, Chuang MC, Chen YC, Lee CP, Yang TM, Chen PC, et al. Metformin prolongs survival in type 2 diabetes lung cancer patients with EGFR-TKIS. Integr Cancer Ther. 2019;18:1534735419869491.
Wink KC, Belderbos JS, Dieleman EM, Rossi M, Rasch CR, Damhuis RA, et al. Improved progression free survival for patients with diabetes and locally advanced non-small cell lung cancer (NSCLC) using metformin during concurrent chemoradiotherapy. Radiother Oncol. 2016;118:453–9.
Tan BX, Yao WX, Ge J, Peng XC, Du XB, Zhang R, et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer. 2011;117:5103–11.
Morgillo F, Sasso FC, Della Corte CM, Vitagliano D, D’Aiuto E, Troiani T, et al. Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin Cancer Res. 2013;19:3508–19.
Li L, Jiang L, Wang Y, Zhao Y, Zhang XJ, Wu G, et al. Combination of metformin and gefitinib as first-line therapy for nondiabetic advanced NSCLC patients with EGFR mutations: a randomized, double-blind phase ii trial. Clin Cancer Res. 2019;25:6967–75.
Arrieta O, Barrón F, Padilla MS, Avilés-Salas A, Ramírez-Tirado LA, Arguelles Jiménez MJ, et al. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5: e192553.
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Br J Surg. 2000;87:1448–54.
Scholten RJ, Clarke M, Hetherington J. The Cochrane Collaboration. Eur J Clin Nutr. 2005;59:S147–96.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6:341–50.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Morgillo F, Fasano M, Della Corte CM, Sasso FC, Papaccio F, Viscardi G, et al. Results of the safety run-in part of the metal (metformin in advanced lung cancer) study: a multicentre, open-label phase I-II study of metformin with erlotinib in second-line therapy of patients with stage IV non-small-cell lung cancer. ESMO Open. 2017;2: e000132.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Medical writing, editorial, and other assistance
The author would like to thank Professor Wang Wen for his suggestions, Wei Feilong for his help in language improvement, and all the patients who participated in this study. We thank the authors of some original studies who provided additional data to us for this meta-analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
Qiman Zhang, Jin Zheng, Wen Wang, Elyse M. Cornett, Alan David Kaye, Ivan Urits, Omar Viswanath and Fei-Long Wei contributed to the design and implementation of the research, and to the analysis of the results and to the writing of the manuscript.
Disclosures
Qiman Zhang, Jin Zheng, Wen Wang, Elyse M. Cornett, Alan David Kaye, Ivan Urits, Omar Viswanath, and Fei-Long Wei confirm that they have no conflicts of interest to disclose.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, Q., Zheng, J., Wang, W. et al. The Anticancer Effect of Metformin Combined with Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-small Cell Lung Cancer Patients with or Without Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Oncol Ther 10, 363–375 (2022). https://doi.org/10.1007/s40487-022-00209-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-022-00209-0