Abstract
Introduction
Cancer care providers have faced many challenges in delivering safe care for patients during the COVID-19 pandemic. This cross-sectional survey-based study investigated the impact of the pandemic on clinical practices of Portuguese medical oncologists caring for patients with breast cancer.
Methods
An anonymous online survey comprising 42 questions gathered information regarding COVID-19 testing, treatment in (neo)adjuvant and metastatic settings, and other aspects of breast cancer management. Practices before and during the pandemic were compared, and potential differences in outcomes according to respondents’ regions, case volumes, and practice type were explored.
Results
Of 129 respondents, 108 worked in the public health system, giving a representative national picture of the impact of the COVID-19 pandemic on breast cancer management. Seventy-one percent of respondents reported a reduction in visits for new cases of breast cancer, and there was a shift towards increased use of telemedicine. Clinical decision-making was largely unaffected in the most aggressive indications (i.e., triple-negative, HER2-positive, visceral crisis). The use of neoadjuvant therapy increased when access to surgery was difficult, whereas dose-dense regimens decreased, and cyclin-dependent kinase 4/6 inhibitor treatment decreased for less aggressive disease and increased for more aggressive disease. The use of oral formulations and metronomic chemotherapy regimens increased, and clinical trial participation decreased. Some differences by respondents’ region and case volume were noted.
Conclusion
Medical oncologists in Portugal implemented many changes during the COVID-19 pandemic, most of which were logical and reasonable responses to the current healthcare emergency; however, the true impact on patient outcomes remains unknown.
Plain Language Summary
This study was an online survey of Portuguese medical oncologists to determine how they managed patients with breast cancer during the COVID-19 pandemic. Forty-two questions covered topics such as how COVID testing was done, the types of cancer treatments used, and how this compared to before the pandemic. It also examined whether the geographic region, the number of patients each doctor was responsible for (caseload), and the type of medical institution influenced how patients with breast cancer were managed. One hundred and twenty-nine oncologists completed the survey, of whom 108 worked in the public health system, making this survey representative of breast cancer management during the COVID-19 pandemic across Portugal. Most (71%) said there were fewer visits for new cases of breast cancer during lockdown. The use of telemedicine increased, as did the use of pre-surgery hormone therapy or chemotherapy when access to surgery was difficult, and the use of anticancer medications taken orally or metronomically (low doses given frequently over a long time period). Chemotherapy given very frequently (dose-dense) was used less often, and fewer patients participated in clinical trials. Treatment decisions for patients with aggressive breast cancer types (e.g., triple-negative breast cancer) were largely unchanged, except for greater use of cyclin-dependent kinase 4/6 inhibitors—drugs targeting the cell cycle and cell division control. Geographic region and caseload influenced treatment decisions. All of these changes in breast cancer treatment during the COVID-19 pandemic were logical and reasonable for the circumstances, but their long-term impact is not yet known.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
We wanted to investigate how clinical practices for breast cancer had changed in Portugal in response to the COVID-19 pandemic. |
We performed this via an anonymous peer-reviewed online survey that was answered by 129 medical oncologists in Portugal. |
What was learned from the study? |
Compared with pre-pandemic clinical practice, respondents reported a reduction in visits for new breast cancer diagnoses; increased use of telemedicine, oral formulations and metronomic regimens; and reduced use of dose-dense regimens. |
Decision-making in the most aggressive forms of breast cancer, such as triple-negative disease, was largely unchanged by the pandemic. |
It is not known whether changes in practice have affected breast cancer outcomes in Portugal. |
Introduction
Rapidly escalating death rates due to coronavirus disease 2019 (COVID-19) [1], caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), led the World Health Organization to declare COVID-19 a pandemic on March 11, 2020 [2, 3].
The COVID-19 pandemic has had a negative impact on cancer care. Given that older people and patients with underlying comorbidities have an increased risk of complications and severe consequences of COVID-19 [3,4,5], patients with cancer are a particularly high-risk population [6,7,8,9,10]. Cancer patients have an increased risk of developing infections [8], and the risk of SARS-CoV-2 infection is further increased by frequent hospital visits required for regular treatment [9]. Thus, delivering safe care for patients with cancer, or suspected cancer, during the COVID-19 pandemic is challenging. Moreover, the reported negative effects of the pandemic on hospital practices and resources, such as reduced screening programs, delayed diagnosis, and delayed or suboptimal treatment, could all impact the overall survival of patients with cancer [11,12,13,14,15,16,17,18,19,20].
Several organizations, including the European Society for Medical Oncology (ESMO), have released recommendations for managing patients with cancer during the COVID-19 pandemic [12, 21,22,23]. ESMO recommended a tiered approach to patient management (i.e., patients categorized according to high, medium, or low priority), in order to help mitigate the negative effects of the COVID-19 pandemic on the diagnosis and treatment of patients with breast cancer [24].
The aim of the current study was to investigate the impact of the COVID-19 pandemic on the clinical practice of Portuguese oncologists toward breast cancer care.
Methods
Study Design
This was a cross-sectional survey-based study. Medical oncologists, including those in their last year of training and qualified specialists, were invited to complete an anonymous peer-reviewed online survey. The study was endorsed by the Portuguese Society of Senology and Portuguese Medical Association, and complied with the rules of the European Union’s General Data Protection Regulations. Since this was a survey-based study with a targeted audience of anonymized medical oncologists, local ethics committee approval was not necessary.
Survey
The online survey, created using Google Forms software, contained 42 questions regarding the sociodemographic characteristics of respondents (questions 1–10), COVID-19 testing of patients (questions 11–14), (neo)adjuvant treatment (questions 15–22 and 26), treatment of metastatic disease (questions 27–32), and other aspects of breast cancer management (questions 23–25 and 33–42). Completion time was approximately 10 min.
The survey was sent by email to members of the Portuguese Society of Senology and the Portuguese Medical Association on December 3, 2020. It was also available online for 3 months (from December 2020 to February 2021) via the websites of these associations, and links to the survey on these websites were shared on social media (Facebook, LinkedIn, and WhatsApp).
Endpoints and Outcome Measures
The study examined the impact of the COVID-19 pandemic on (neo)adjuvant treatment (i.e., type and frequency) and metastatic treatment (i.e., type, frequency, and formulation) in patients with triple-negative, human epidermal growth factor receptor 2 (HER2)-positive or hormone receptor-positive disease. Additionally, the survey evaluated eventual changes in some aspects of breast cancer management since the start of the pandemic, including prescription of ovarian suppression, bisphosphonates, granulocyte colony-stimulating factor (G-CSF), and corticosteroids; the use of genomic profiling to determine treatment; referrals to clinical trials and palliative care; and the use of telemedicine.
Statistical Analysis
With regard to sample size, it was estimated that, among an overall population of 293 medical oncologists working in the public health system [25] (in the case of a two-category grouping of answers), 100 respondents would allow a margin of error in the estimate of approximately 8.0%, with a 95% confidence level.
The answers obtained for each specific topic were compared by applying a McNemar test to detect differences between practices in the period before the COVID-19 pandemic and those reported during the pandemic. Changes in practices were examined between respondents, as well as comparing practices before versus during the pandemic. Additionally, descriptive analyses were conducted to explore whether changes in practices differed according to respondents’ country regions, case volumes, and type of practice. Chi-squared tests were two-sided and a P-value of < 0.05 was considered statistically significant. Statistical analyses were conducted with SPSS for MacOS, version 28.0, and Excel v.16.52.
Results
Respondents
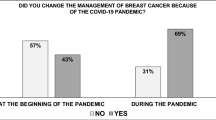
A total of 129 medical oncologists involved in breast cancer care completed the survey. Of these, 108 worked in the public health system, thus meeting the desired sample size permitting adequate detection of statistical differences. The majority of respondents were aged < 40 years (57.4%) and female (65.1%); almost half (48.1%) were from the Lisbon and Tagus Valley Region (Table 1). Most respondents worked exclusively in public practice (65.1%), in large institutions (i.e., > 200 new breast cancer cases per year; 48.1%), with a multidisciplinary team dedicated exclusively to breast cancer (77.5%) and/or multidisciplinary tumor board in breast cancer care (98.4%) (Table 1). The majority of respondents (71.3%) reported a decrease in new breast cancer cases between March and May 2020 (i.e., the first lockdown period due to the COVID-19 pandemic in Portugal).
COVID-19 Testing
Overall, 99.2% of respondents reported COVID-19 testing of patients before each new treatment (e.g., chemotherapy, radiation therapy, or surgery), and 90.7% reported testing of patients before each cycle of outpatient-based treatment. During radiation therapy, 123 respondents (95.3%) reported COVID-19 testing, either weekly (65.1%), every 2 weeks (29.3%), every 3 weeks (4%), or according to other schedules (1.6%).
Impact of the COVID-19 Pandemic on (neo)Adjuvant Treatment of Breast Cancer
Survey responses regarding the use of (neo)adjuvant treatment during the COVID-19 pandemic are shown in Table 2; a number of trends were observed. All respondents maintained the use of neoadjuvant therapy for triple-negative or HER2-positive tumors ≥ 2 cm and/or lymph node-positive disease (Table 2). Comparing practices before and during the pandemic, 5.4% of respondents reported an increase in the use of neoadjuvant versus adjuvant therapy for operable locally advanced breast cancer (luminal B-like/HER2-negative) (P = 0.343; Figure S1a in the Supplementary Material), and 41.9% of respondents reported an increase in the use of neoadjuvant hormone therapy or chemotherapy for luminal A-like/HER2-negative disease because surgery became more difficult to access (Table 2). Also, regarding adjuvant short-term chemotherapy regimens, a slightly greater proportion of respondents changed their preference to anthracycline-free regimens versus an anthracycline-containing regimen (Table 2), although this difference did not reach statistical significance (P = 0.435). However, data differed according to respondents’ case volume, with a higher proportion of the respondents with larger case volumes maintaining or changing to anthracycline-free regimens (P = 0.002; Figure S2a in the Supplementary Material).
The use of weekly rather than 3-weekly docetaxel in the sequential chemotherapy regimen decreased by 21.7% and use of weekly versus 3-weekly paclitaxel in combination with anti-HER2 therapy decreased by 16.3% during the pandemic compared with before (P = 0.003; Figures S1b and S1c in the Supplementary Material). Moreover, 10.8% of respondents stated that they reduced the use of dose-dense anthracycline-based (neo)adjuvant chemotherapy (P = 0.144 vs. before the pandemic; Figure S1d in the Supplementary Material). There were statistically significant regional differences (P < 0.001) in medical oncologists’ preferences regarding the use of taxane-based regimens associated with anti-HER2 blockade (Figure S3a in the Supplementary Material). There were also statistically significant differences in short-term chemotherapy preferences, according to case-volume (P = 0.040; Figure S2b in the Supplementary Material). The use of neoadjuvant carboplatin and adjuvant capecitabine for triple-negative breast cancer remained mostly unchanged (72.1% and 82.2%, respectively), with only 12.4% and 4.7% of respondents, respectively, claiming that they used it less frequently.
Impact of the COVID-19 Pandemic on Treatment of Metastatic Breast Cancer
Survey responses regarding treatment of metastatic disease during the COVID-19 pandemic are shown in Table 3. The COVID-19 pandemic did not affect the clinical decision regarding breast cancer treatment for the most aggressive scenarios.
For triple-negative breast cancer without visceral crisis, first-line preferences for either single-agent therapy or polychemotherapy/chemoimmunotherapy remained largely unchanged during the pandemic compared with before (P = 0.886; Figure S4a in the Supplementary Material).
Similarly, for triple-negative breast cancer with visceral crisis, first-line preferences remained largely unchanged during the pandemic, with only 2.3% of respondents decreasing the use of combination regimens (P = 0.923; Figure S4b in the Supplementary Material); however, statistically significant regional differences in medical oncologists' preferences were observed (P = 0.014; Figure S3b in the Supplementary Material). When a chemotherapy agent was available in both oral and intravenous formulations, 37.2% of respondents preferred the oral formulation, with changes in practices noted during the pandemic compared with before (P = 0.119; Figure S4c in the Supplementary Material). Also, the use of metronomic chemotherapy significantly increased during the pandemic were compared with before (P < 0.001).
For hormone receptor-positive breast cancer, 10.9% of respondents reduced their use of cyclin inhibitors in combination with hormone therapy to hormone therapy alone in patients with less aggressive features (e.g., those of older age or with bone-only disease) (P = 0.393 vs. before the pandemic; Figure S4d in the Supplementary Material), whereas 6.2% of respondents increased their use of cyclin inhibitors in combination with hormone therapy in patients with more aggressive features (e.g., visceral disease) (P = 0.519 vs. before the pandemic; Figure S4e in the Supplementary Material). The majority of respondents monitored adverse effects in patients receiving cyclin inhibitors during the pandemic either every 2 weeks during the first two cycles and then monthly thereafter (recommended) or monthly; only two respondents (1.6%) used another monitoring schedule (e.g., every 2 months, or monthly until dose stabilization and then every two cycles).
Impact of the COVID-19 Pandemic on Other Aspects of Breast Cancer Management
Responses regarding complementary care during the COVID-19 pandemic are shown in Table 4. Regarding ovarian suppression, about one half of the respondents chose chemical ovarian suppression over surgical methods, and about 50% preferred quarterly to monthly regimens. There was an overall increase in the use of oral bisphosphonates of 15.4% compared with before the pandemic (P = 0.015). Also, in the metastatic setting, while nearly 60% of respondents maintained schedule of bisphosphonate administration, 41.1% of respondents changed their preference from monthly to quarterly administration during the pandemic (P = 0.25). Overall, the use of G-CSF increased in more than half of the respondents and 9.3% described reducing the use of corticosteroids (Table 4). There was an increased awareness of the importance of seasonal influenza and pneumococcal vaccination in cancer patients (66.9%).
The use of first- or second-generation platforms/signatures (i.e., MammaPrint, Oncotype Dx, EndoPredict, Prosigna, or Breast Cancer Index) and molecular profiling tests (i.e., next-generation sequencing) to guide therapy decision-making increased only slightly (Table 4). About half of the respondents reported lower referral of patients to clinical trials, and there was a small increase in early referral to palliative care. There were statistically significant regional differences in medical oncologists’ attitudes regarding palliative care referral, with respondents from the Lisbon and Tagus Valley region being less likely to refer patients to palliative care than those from Northern Portugal (P = 0.004).
Almost 80% of respondents reported a reduction in the use of face-to-face appointments and increased use of telemedicine (Table 4). Respondents who worked in public centers were significantly more likely to use telemedicine almost exclusively than those who worked exclusively in private centers (69/84 [82%] vs. 14/21 [66%]) and to promote the use of telemedicine over face-to-face visits (P = 0.027; Figure S5 in the Supplementary Material), although it is important to note the difference in numbers of respondents in public versus private centers when making this comparison.
Discussion
Our study showed that 71.3% of respondents reported a reduction in visits for new cases of breast cancer during the COVID-19 pandemic (i.e., March–May 2020), and a preference for the use of telemedicine rather than face-to-face visits by 79.1% of medical oncologists. Also, most patients (> 90%) were tested for COVID-19 before each cycle of therapy and during radiotherapy. This is to be expected given that, at the time this survey was conducted, Portugal had reported more than half a million SARS-CoV-2 infections and almost 9000 COVID-19-related deaths, and the country was facing a third wave of infections with their national health system on the brink of collapse [26]. However, during this time, it is reassuring to note that the COVID-19 pandemic did not appear to affect clinical decision-making with regard to the most aggressive breast cancer indications, such as triple-negative and HER2-positive disease, and in patients with visceral crisis.
In the current study, the use of neoadjuvant chemotherapy was maintained by all medical oncologists for triple-negative or HER2-positive tumors ≥ 2 cm and/or lymph node-positive disease, increased use of neoadjuvant treatment was reported by 5.4% and 41.9%, when access to surgery was difficult, in patients with luminal B-like and luminal A-like breast cancer, respectively. In the (neo)adjuvant setting, the use of weekly paclitaxel, carboplatin, and dose-dense anthracycline regimens decreased, but the use of adjuvant capecitabine in patients with triple-negative residual disease after neoadjuvant treatment was maintained. Omitting anthracyclines can result in a substantial reduction in potential serious toxicities while still achieving optimal treatment outcomes [27]. Results from ongoing de-escalation trials may provide answers regarding how to maximize chemotherapy benefits while minimizing undesired impacts of adverse events on patients’ quality of life [27]. Also, considering the similar efficacy of the two taxanes [28], an increased preference for docetaxel every 3 weeks appears reasonable during the COVID-19 pandemic in order to reduce the number of hospital visits. However, the increased risk of hematologic toxicity associated with the use of docetaxel every 3 weeks, including the higher rates of febrile neutropenia and infections [28], needs to be taken into account. The anticipated increase in hematologic toxicity may explain the observed increase in G-CSF prescription as a primary prophylactic measure, and potentially the lower rates of corticosteroid prescription as supportive treatment, given the immunosuppressive effects of these agents [29]. Interestingly, only about 4% of the respondents used dose-dense paclitaxel, despite studies demonstrating superiority of this regimen versus a 3-weekly regimen [30]. Studies suggest that routine G-CSF during dose-dense paclitaxel can be omitted without compromising safety [31].
Results of the current study also show that, in the metastatic setting, first-line therapies were maintained for triple-negative disease, the use of oral formulations of chemotherapy agents and metronomic chemotherapy regimens increased, and the use of cyclin-dependent kinase (CDK) 4/6 inhibitors in combination with hormone therapy decreased for less aggressive disease and increased for disease with more aggressive features.
For patients with less aggressive and more aggressive metastatic disease, more than 50% and 90% of responding medical oncologists, respectively, acknowledged maintaining the use of CDK4/6 inhibitors in combination with first-line hormone therapy. In addition, while almost 11% of respondents reported a reduced use of CDK4/6 inhibitors in patients with less aggressive disease, the same was not true for those with more aggressive disease, with almost 97% of respondents prescribing these agents. In patients with less aggressive disease, the risk of hematologic toxicity and the need for close monitoring may be possible explanations for the observed decrease in CDK4/6 inhibitor use. Despite high rates of grade 3–4 neutropenia reported with cyclin inhibitors [32], the incidence of febrile neutropenia is low; thus, the COVID-19 pandemic had less impact on the use of these agents for the treatment of patients with more aggressive disease scenarios (e.g., visceral crisis). Almost 30% of responding medical oncologists indicated that they used monthly monitoring for adverse events during the pandemic in patients receiving cyclin inhibitors instead of every 2 weeks during the first two cycles, then monthly as recommended in the manufacturers’ labelling information for these agents [33,34,35]. Instead of reducing important monitoring for adverse events, alternative strategies may include the use of external laboratories for blood testing, telephone follow-up conducted by nurses, or home delivery of drugs in order to limit the number of hospital visits. The use of oral rather than IV formulations, where available, and metronomic chemotherapy regimens, where appropriate, help in this regard.
Regarding complementary therapy, the use of oral and quarterly schedules of bisphosphonates was reportedly increased during the COVID-19 pandemic in the current study, early referral to palliative care increased, and participation in clinical trials was reduced. A speculative reason for increased early referral to palliative care may be that more late-stage breast cancer diagnoses were made, related to the delays in breast cancer screening and lockdown periods. Another hypothetical explanation could be that a reduction in the usage of later lines of chemotherapy with less benefit and higher risk of cumulative toxicity may have resulted in early referral.
It is important to compare the “Portuguese reality” during the worst COVID-19 wave with that of other countries under similar circumstances. To this end, results of the current study are in line with those reported in studies in other countries [36, 37]. For example, a similar study utilizing a 29-question survey with responses from 165 medical oncologists accessed via the Italian Association of Medical Oncology (AIOM) and Italian Breast Cancer Study Group (GIM) mailing lists, showed reduced use of (neo)adjuvant weekly paclitaxel or a dose-dense schedule for anthracycline-based chemotherapy during the COVID-19 outbreak, and reduced use of first-line weekly paclitaxel for HER2-positive disease or CDK4/6 inhibitors for luminal tumors with less aggressive characteristics in the metastatic setting [36, 37]. Furthermore, a cross-sectional study (LACOG 0420) surveying investigators and research coordinators (n = 90) from research centers in Latin America demonstrated that oncology clinical trials have been significantly affected during the pandemic in Latin America [37].
The true cost of the COVID-19 pandemic on the global oncology community will remain unknown for many years, likely until the pandemic is over. Some countries will experience a greater impact of COVID-19, particularly those with a high prevalence of SARS-CoV-2 infection and those with poorer healthcare infrastructure/oncology management systems. Oncology services in countries with virtual elimination of the virus were able to quickly resume/catch-up following lockdown periods [17], whereas recovery in other countries will be an extremely long process. It is important that individual countries report data regarding changes to oncology management so that responses to the current pandemic can be adapted and improved, and lessons learned in the event of a future pandemic.
A useful change to clinical practice reported in the current study that could be retained in the post-COVID-19 period is the use of telemedicine alternated with face-to-face appointments in selected cases during surveillance or active treatment stages (e.g., for patients living far from cancer centers and during follow-up periods). Implementation of telemedicine networks between hospitals, patients, and their caregivers using “home-specialized” health teams may allow for drug administration with continuous monitoring of treatment adverse effects, as well as some examinations (e.g., blood analysis) and/or procedures. Moreover, such a strategy would reduce hospital visits, improve healthcare logistics, and decrease the risk of nosocomial infections and psychological stress caused by lengthy hospital stays [38]. Another benefit of the COVID-19 pandemic may be an improved awareness of the importance of seasonal influenza vaccination in patients with cancer, who are more prone to secondary and opportunistic infections. In addition, it may be useful to retain the increased preference for oral over intravenous formulations of cancer therapies, as well as the use of quarterly zoledronic acid and chemical ovarian suppression instead of monthly, the greater use of genomic platforms/signatures and molecular profiling to better guide systemic treatment, and the increased use of metronomic chemotherapy in metastatic disease.
Inclusion of 129 medical oncologists from throughout the country, including 108 medical oncologists working in the public health system, is a positive feature of the current study, ensuring that the data are representative of the national reality in Portugal, reflecting the national health system’s breast cancer management during the COVID-19 pandemic. Since the majority of medical oncologists in Portugal work in the National Health Service, and with a reported 293 medical oncologists, specialists, and consultants working in the National Health Service in 2019 [25], this suggests that we captured data from almost one third of Portugal’s medical oncologists, representing an excellent response rate from this source. Furthermore, according to the information provided to the authors by the administrative staff of the Portuguese Society of Senology (SPO), there are 36 registered breast cancer centers in Portugal, with a median of three medical oncologists per center.
However, this study also has some important limitations. For example, the observational nature of the study design and the fact that surveys are subject to recall and response bias are considerable limitations. Also, most respondents were relatively young specialists (i.e., aged < 40 years) and with a median of only 5 years of clinical practice, possibly indicating selection bias which may have influenced the results. Furthermore, given the heterogeneous hospital organizations and COVID-19 burden across Portugal, it would have been interesting to compare practices between the various regions of Portugal; however, small sample sizes for many regions precluded this. Finally, another possible limitation of the study is the absence of a survey conducted early in the pandemic in Portugal (i.e., March 2020), which would have permitted comparison with the current survey conducted later in the pandemic.
Although several similar studies have reported the impact of the COVID-19 pandemic on the management of cancer patients [11,12,13,14,15,16,17,18,19,20], our survey characterizes a real-world scenario with regard to breast cancer management during the earlier stages of the COVID-19 pandemic in Portugal. Our survey also focused on the first year of the pandemic, as no local standardized practices were in place to manage the issues that arose, and may be regarded as a framework to prepare for future situations like the current pandemic. Since some specialized breast cancer centers in Portugal are not considered for referral and hospitalization of COVID-19 patients, it would be interesting to examine differences in clinical practices between these and centers where COVID-19 patients are treated. A follow-up survey would be useful to evaluate further changes to clinical oncology practices over time as our healthcare system adapts to a new era in which evolving COVID-19 variants will emerge. Within this context, it would be useful to understand variations between countries with respect to COVID-19 vaccination practices, including the potential role of booster doses and seasonal vaccination in high-risk patients, such as those with active cancer [39]. Of note, COVID-19 vaccination was only available for priority high-risk patient groups at the time of this survey, including frontline healthcare professionals, armed forces, and patients aged ≥ 65 years, which excluded most patients with breast cancer. Therefore, collection of data related to COVID-19 vaccination (including the primary scheme and booster vaccination) should be included in a future survey.
Conclusions
Our data indicate that medical oncologists in Portugal have implemented many changes to their practices to optimize breast cancer care and reduce the risk of infection in patients with breast cancer during the COVID-19 pandemic. Although most of the changes were reasonable responses to the current healthcare emergency, the true impact of the COVID-19 pandemic on patient outcomes remains unknown. Remarkably, the majority of survey respondents continued to work within a breast cancer unit, and the response rate was higher than expected, allowing for the collection of an adequate number of responses to provide a representative picture of the impact of the COVID-19 pandemic on breast cancer management in Portugal.
References
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
World Health Organization. Listings of WHO’s response to COVID-19. 2020. https://www.who.int/news/item/29-06-2020-covidtimeline.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–42.
Murthy S, Gomersall CD, Fowler RA. Care for critically ill patients with COVID-19. JAMA. 2020;323(15):1499–500.
Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50.
Desai A, Sachdeva S, Parekh T, Desai R. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol. 2020;6:2.
Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–7.
Rolston KV. Infections in cancer patients with solid tumors: a review. Infect Dis Ther. 2017;6(1):69–83.
Yu J, Ouyang W, Chua ML, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108–10.
Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901.
Jafari A, Rezaei-Tavirani M, Karami S, Yazdani M, Zali H, Jafari Z. Cancer care management during the COVID-19 pandemic. Risk Manag Healthc Policy. 2020;13:1711.
Jazieh AR, Chan SL, Curigliano G, Dickson N, Eaton V, Garcia-Foncillas J, et al. Delivering cancer care during the COVID-19 pandemic: recommendations and lessons learned from ASCO global webinars. JCO Glob Oncol. 2020;6:1461–71.
Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. 2020;1(6):565–7.
Luker GD, Boettcher AN. Impact of COVID-19 on clinical care and research in cancer imaging: where we are now. London: Radiological Society of North America; 2021.
Hawrot K, Shulman LN, Bleiweiss IJ, Wilkie EJ, Frosch ZAK, Jankowitz RC, et al. Time to treatment initiation for breast cancer during the 2020 COVID-19 pandemic. JCO Oncol Pract. 2021;17(9):534–40.
Gurney JK, Millar E, Dunn A, Pirie R, Mako M, Manderson J, et al. The impact of the COVID-19 pandemic on cancer diagnosis and service access in New Zealand-a country pursuing COVID-19 elimination. Lancet Reg Health West Pac. 2021;10:100127.
Millar E, Gurney J, Beuker S, Goza M, Hamilton MA, Hardie C, et al. Maintaining cancer services during the COVID-19 pandemic: the Aotearoa New Zealand experience. Lancet Reg Health West Pac. 2021;11:100172.
Amador M, Matias-Guiu X, Sancho-Pardo G, Contreras Martinez J, de la Torre-Montero JC, PeñuelasSaiz A, et al. Impact of the COVID-19 pandemic on the care of cancer patients in Spain. ESMO Open. 2021;6(3):100157.
Blay JY, Boucher S, Le Vu B, Cropet C, Chabaud S, Perol D, et al. Delayed care for patients with newly diagnosed cancer due to COVID-19 and estimated impact on cancer mortality in France. ESMO Open. 2021;6(3):100134.
Minichsdorfer C, Jeryczynski G, Krall C, Achhorner AM, Caraan A, Pasalic S, et al. Impact of COVID-19 lockdown on routine oncology versus emergency care at a high volume cancer centre. Eur J Clin Invest. 2021;51(8):e13623.
Lee JB, Jung M, Kim JH, Kim BH, Kim Y, Kim YS, et al. Guidelines for cancer care during the COVID-19 pandemic in South Korea. Cancer Res Treat. 2021;53(2):323.
European Society for Medical Oncology. Cancer patient management during the COVID-19 pandemic. 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic.
Lambertini M, Toss A, Passaro A, Criscitiello C, Cremolini C, Cardone C, et al. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: young oncologists’ perspective. ESMO open. 2020;5(2):e000759.
European Society for Medical Oncology. ESMO management and treatment adapted recommendations in the COVID-19 era: Breast cancer. 2020.
Direção-Geral da Saúde. Programa Nacional para as Doenças Oncológicas. 2020. https://www.dgs.pt/pns-e-programs/programs-de-health-prioritarios/doencas-oncologicas.aspx. Accessed September 20 2021.
Demony C. Portugal's health system on brink of collapse as COVID-19 cases surge. Reuters. 2021. https://www.reuters.com/article/us-health-coronavirus-portugal-idUSKBN29M0L3.
Tarantino P, Tolaney SM, Harbeck N, Cortes J, Curigliano G. Anthracyclines for human epidermal growth factor receptor 2–positive breast cancer: are we ready to let them go? J Clin Oncol. 2021. https://doi.org/10.1200/jco.21.01059.
Qi W-X, Shen Z, Lin F, Sun Y-J, Min D-l, Tang L-N, et al. Paclitaxel-based versus docetaxel-based regimens in metastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. Curr Med Res Opin. 2013;29(2):117–25.
Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13.
Abdel Halim II, El Ashri M, El Sadda W. Weekly paclitaxel versus standard 3-week schedule in patients with metastatic breast cancer. J Clin Oncol. 2011;29(15):627.
Rocha Paes F, Vaz Duarte Luis IM, Costa RB, Filho OM, Hughes ME, Losk K, et al. Variation in the use of granulocyte-colony stimulating factor (G-CSF) for dose dense paclitaxel: A single institution retrospective study. J Clin Oncol. 2015;33(15):561.
Thill M, Schmidt M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther Adv Med Oncol. 2018;10:1758835918793326.
European Medicines Agency. Verzenios (abemaciclib): summary of product characteristics. 2018. https://www.ema.europa.eu/en/documents/product-information/verzenios-epar-product-information_en.pdf. Accessed September 20 2021.
European Medicines Agency. Kisqali (ribociclib): summary of product characteristics. 2017. https://www.ema.europa.eu/en/documents/product-information/kisqali-epar-product-information_en.pdf. Accessed September 20 2021.
European Medicines Agency. Ibrance (palbociclib): summary of product characteristics. 2016. https://www.ema.europa.eu/en/documents/product-information/ibrance-epar-product-information_en.pdf. Accessed September 20 2021.
Poggio F, Tagliamento M, Di Maio M, Martelli V, De Maria A, Barisione E, et al. Assessing the impact of the COVID-19 outbreak on the attitudes and practice of Italian oncologists toward breast cancer care and related research activities. JCO Oncol Pract. 2020;16(11):e1304–14.
Lara Gongora AB, Werutsky G, Jardim DL, Nogueira-Rodrigues A, Barrios CH, Mathias C, et al. Impact of the COVID-19 pandemic on oncology clinical research in Latin America (LACOG 0420). JCO Glob Oncol. 2021;7:649–58.
Shirke MM, Shaikh SA, Harky A. Implications of telemedicine in oncology during the COVID-19 pandemic. Acta Biomed. 2020;91(3):e2020022.
Saini KS, Martins-Branco D, Tagliamento M, Vidal L, Singh N, Punie K, et al. Emerging issues related to COVID-19 vaccination in patients with cancer. Oncol Ther. 2021;9(2):255–65.
Acknowledgements
We dedicate this publication to our dear colleague Dr Noémia Afonso – friend, mother and wife – who recently passed away. We have lost an invaluable pillar of our scientific community. She was considered as one of the most brilliant oncologists, strongly committed to research and training in breast and gynecological cancer, and with a unique vitality and determination in favor of patients. Our commitment is to continue to preserve her legacy: keep her memory alive by perpetuating her teachings and her enthralling passion for oncology.
Funding
Funding of the medical writing assistance, statistical analysis, and the journal’s Rapid Service Fee was provided by CUF Oncologia.
Medical Writing and/or Editorial Assistance
We would like to thank Andrea Bothwell who wrote the manuscript outline and first draft on behalf of Springer Healthcare Communications. We also thank Prof. Carina Silva (ESTEsL – Escola Superior de Tecnologias de Saúde de Lisboa) who performed the preliminary statistical analysis of this study. This medical writing assistance and statistical analysis was funded by CUF Oncologia.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
The present manuscript is the result of original work by the authors. Alpuim Costa D and Gonçalves Nobre JG conceived and designed the study; Alpuim Costa D, Gonçalves Nobre JG, Fernandes JP, Batista MV, Simas A, Gouveia H, Ribeiro LA, Brito M, Chaves A, Martins-Branco D, and Afonso N reviewed the survey prior to its release; all the authors, except for Fernandes JP and Sampaio-Alves M, disseminated the study through different channels; Alpuim Costa D, Gonçalves Nobre JG, and Sampaio-Alves M performed the statistical analysis; Alpuim Costa D, Gonçalves Nobre JG, Fernandes JP, Simas A, Ribeiro LA, Brito M, Mariano M, Savva-Bordalo J, Fernandes R, Sampaio-Alves M, Martins-Branco D, and Afonso N performed the analysis and interpretation of the data; Alpuim Costa D, Gonçalves Nobre JG, Fernandes JP, Batista MV, Simas A, Ribeiro LA, Brito M, Mariano M, Savva-Bordalo J, Fernandes R, Martins-Branco D, and Afonso N read and approved manuscript drafts; Alpuim Costa D, Fernandes JP, Ribeiro LA, and Afonso N performed the manuscript supervision.
Prior Presentation
The results of this study were previously presented at a virtual scientific meeting “Breast Cancer Sessions I”, sponsored by Pfizer.
Disclosures
Diogo Alpuim Costa has received honoraria from the Portuguese Navy, CUF Oncologia, and NTT DATA, and has served as a speaker, advisory board member, or has received research or education funding from CUF Oncologia, AstraZeneca, Hoffmann-La Roche, Merck KGaA, Novartis, Pfizer, Uriage, Daiichi Sankyo, Gilead, Merck Sharp & Dohme, Nanobiotix, Puma Biotechnology Inc., Sanofi, and Seagen Inc. Margarida Brito has participated as advisory board member for Roche, Novartis, Merck Sharp & Dohme, and Pfizer. Mário Fontes-Sousa has served as a speaker or advisory board member for Bristol Myers Squibb, Daiichi Sankyo, Gilead, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Servier. Diogo Martins-Branco received honoraria and advisory board fees from Janssen, Pfizer, Merck Sharp & Dohme, Angelini, AstraZeneca, and Novartis, meeting and travel grants from LEO Farmacêuticos, Merck Sharp & Dohme, Ipsen, Janssen, and Roche, and institutional grants from F. Hoffmann-La Roche Ltd. José Guilherme Gonçalves Nobre, João Paulo Fernandes, Marta Vaz Batista, Ana Simas, Carolina Sales, Helena Gouveia, Leonor Abreu Ribeiro, Andreia Coelho, Mariana Inácio, André Cruz, Mónica Mariano, Joana Savva-Bordalo, Ricardo Fernandes, André Oliveira, Andreia Chaves, Mafalda Sampaio-Alves, and Noémia Afonso have nothing to declare.
Compliance with Ethics Guidelines
The study was endorsed by the Portuguese Society of Senology and Portuguese Medical Association, and complied with the rules of the European Union’s General Data Protection Regulations. Since this was a survey-based study with a targeted audience of anonymized medical oncologists, local ethics committee approval was not necessary.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alpuim Costa, D., Nobre, J.G.G., Fernandes, J.P. et al. Impact of the COVID-19 Pandemic on Breast Cancer Management in Portugal: A Cross-Sectional Survey-Based Study of Medical Oncologists. Oncol Ther 10, 225–240 (2022). https://doi.org/10.1007/s40487-022-00191-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-022-00191-7