Abstract
The introduction of tyrosine kinase inhibitors (TKI) for the treatment of metastatic non-small cell lung cancer (NSCLC) harbouring sensitizing epidermal growth factor receptor (EGFR) gene mutations revolutionized the diagnostic and treatment algorithm of this subset of patients almost two decades ago. Since then, a number of trials have evaluated the role of TKI therapy in early-stage disease, with encouraging disease-free survival (DFS) results but lack of a survival advantage. ADAURA, a phase III trial evaluating 3 years of adjuvant osimertinib versus placebo in patients harbouring EGFR mutations with completely resected stage IB–IIIA NSCLC, recently reported a profound DFS benefit (hazard ratio 0.21), favourable quality of life and reduction in the risk of brain metastases. These results led to osimertinib’s fast track approval by the US Food and Drug Administration, with this drug thus becoming the first EGFR-TKI approved for the treatment of early-stage disease. However, the key endpoint of overall survival remains immature and questions around indication (i.e. stage, need for adjuvant chemotherapy), optimal treatment duration, biomarkers of response and cost-effectiveness remain to be answered. In this article, we critically appraise the findings of ADAURA and discuss future challenges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Osimertinib is the first epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) approved by the US Food and Drug Administration for the treatment of completely resected stage IB-IIIA EGFR-mutant non-small cell lung cancer, with the fast-track approval based on the results of the ADAURA trial which showed an unprecedented disease-free survival benefit. |
However, survival data is largely immature. With prior evidence suggesting that adjuvant EGFR-TKIs prolong disease-free survival (DFS) but not survival, the question is whether we can now rely on DFS to adopt adjuvant osimertinib in the clinic. |
Findings from ADAURA also allow reflection on other relevant topics of this field, such as optimal treatment indication and sequence, biomarkers for response, patterns of relapse, quality of life and cost-effectiveness. |
Introduction
One third of patients with non-small cell lung cancer (NSCLC) present with resectable disease [1]. However, despite surgical intervention, prognosis remains poor, with 66% of patients with stage IB and 36% with IIIA disease alive at 5 years [2]. (Neo)adjuvant chemotherapy is recommended in stage II–III NSCLC but only produces an absolute benefit of 5% in 5-year disease-free survival (DFS) and overall survival (OS) [3]. Over the last decade, the treatment of metastatic NSCLC has drastically improved, largely due to the adoption of targeted therapies for mutation-driven lung cancer and immune-checkpoint inhibitors [4]. These successful advances have only recently started to permeate clinical practice in the adjuvant setting [5] where cisplatin—vinorelbine chemotherapy had remained the only treatment for decades. This paradigm has been challenged by the US Food and Drug Administration (FDA) approval of adjuvant osimertinib for patients with completely resected NSCLC harbouring epidermal growth factor receptor (EGFR) gene mutations, based on the preliminary results of the ADAURA trial [6, 7]. In 2021, the European Medicines Agency (EMA [EU]) and the Medicines and Healthcare products Regulatory Agency (MHRA [UK]) also adopted a positive opinion and expanded osimertinib marketing authorization to the adjuvant setting [8, 9].
To understand the context and relevance of this approval it is necessary to look back at recent treatment developments in metastatic and adjuvant EGFR-mutant (EGFRmt) NSCLC. Several first- and second-generation EGFR tyrosine kinase inhibitors (EGFR-TKIs) (erlotinib, gefitinib, afatinib) have been shown to be superior to platinum-based chemotherapy in metastatic EGFRmt NSCLC which, in turn, spurred clinical trials to evaluate their role in the adjuvant setting [10,11,12,13]. In the RADIANT trial [10], the subgroup of EGFRmt patients with completely resected stage IB-IIIA NSCLC and randomized to erlotinib for 2 years after adjuvant chemotherapy (A-ChT) derived a DFS benefit (hazard ratio [HR] 0.61, 95% confidence interval [CI] 0.38–0.98; P = 0.039). SELECT [11], a single-arm trial in the same setting showed a 2-year DFS of 88% for erlotinib, which is superior to historical series with A-ChT. The CTONG-1104/ADJUVANT [12] trial randomized patients with EGFRmt NSCLC stage II–IIIA (N1–N2 disease) to receive gefitinib for 2 years versus cisplatin/vinorelbine and met its primary endpoint of a 3-year DFS benefit in favour of gefitinib (HR 0.56, 95%CI 0.4–0.79; P = 0.001), but longer follow-up failed to show a difference in OS (HR 0.92, 95%CI 062–1.37; P = 0.686) [14]. The EVAN trial [13] had a similar design, only with erlotinib as EGFR-TKI of choice, and only included patients with stage IIIA NSCLC (76% with N2 disease); the 2-year DFS favoured erlotinib (81.4% vs. 44.6%; P = 0.0054) and OS data is awaited. Overall, these trials indicate that EGFR-TKIs consistently produce DFS benefit but lack survival advantage [15] or, in other words, these drugs may delay but they do not prevent cancer recurrence, which thus far has precluded their incorporation into routine clinical practice.
More recently, the availability of osimertinib, a more potent third-generation EGFR-TKI, based on the results from the FLAURA study, has remodelled the treatment algorithm of metastatic EGFRmt NSCLC [16]. This trial demonstrated the survival advantage of osimertinib over first-generation EGFR-TKIs (HR 0.79, CI 0.6–0.9; P = 0.046), thus becoming the first-line treatment of choice in this setting. The success of osimertinib relies on its design, which allows for: (1) inhibition of sensitizing EGFR mutations (point mutations in exon 21 and deletions in exon 19); (2) the ability to overcome the main mechanism of resistance to first-generation EGFR-TKIs (T790M mutation in exon 20) [17]; and (3) good central nervous system (CNS) penetration, which is a recognized site of treatment failure in NSCLC treated with other EGFR-TKIs.
Not surprisingly, the success of osimertinib has reactivated the interest in adjuvant EGFR-TKIs, leading to the ADAURA trial which recently reported an unprecedented DFS benefit in patients with completely resected stage IB-IIIA NSCLC treated with 3 years of adjuvant osimertinib versus placebo. Despite the OS data being immature, several regulatory agencies have expedited approval of osimertinib as the first adjuvant EGFR-TKI, with mixed responses from experts in the field [18, 19]. Here we discuss the design of and results from ADAURA, the strengths and weaknesses and future challenges. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
ADAURA: Design and Results
ADAURA is a phase III, double-blind, placebo-controlled study that randomized (1:1) 682 patients with completely resected non-squamous stage IB-IIIA (as per the American Joint Committee on Cancer [AJCC] staging system, 7th edition) NSCLC with EGFR exon 19 deletions (del) or L858R mutations (alone or in combination with other EGFR mutations) to receive 80 mg of osimertinib or placebo for up to 3 years. A-ChT was allowed as per investigator’s decision, and the maximum interval between surgery and randomization was 26 weeks in patients who received A-ChT and 10 weeks in those who did not receive A-ChT. Peri-operative radiotherapy was not allowed. The primary endpoint was to detect a HR = 0.70 DFS difference in patients with stage II-IIIA disease according to investigator’s assessment. The secondary endpoints were DFS in overall population (IB-IIIA), OS, health-related quality of life (HRQoL) and safety. The planned data cutoff date for the primary event-based analysis was February 2022, but an independent data monitoring committee recommended unblinding the trial early following review of the efficacy data. The following data correspond to the unplanned interim analysis as of January 2020. At the time of unblinding, the trial had completed enrolment and all patients were followed up for at least 1 year. Notably, the fact that interim results have been published will not affect outcome as patients and investigators remain unblinded to study treatment, thus protecting the osimertinib arm against bias.
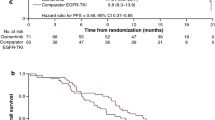
Patients’ baseline characteristics (including tumour stage) were well balanced between the two groups. With 33% of data maturity, DFS in patients with stage II-IIIA disease was not reached in the osimertinib arm (38.8 months to not reached [NR]) versus 20.4 months (16.6–24.5) in the placebo arm (HR 0.17, 95% CI 0.12–0.23; P < 0.0001). DFS in stage IB-IIIA (29% maturity) was not reached with osimertinib (NR-NR) versus 28.1 months (22.1–35.8) with placebo (HR 0.21, 95% CI 0.16–0.28; P < 0.0001). The rate of locoregional-only and distant recurrence (distant only or with locoregional disease) in the overall population also favoured osimertinib over placebo; 7% and 4% versus 18% and 28%, respectively. OS data are immature, with 29 patients dead (9 in the osimertinib group and 20 in the placebo group).
The benefit with osimertinib was seen consistently across all predefined subgroups: type of EGFR mutation (HR 0.12 in exon 19 del, HR 0.35 in L858R), race (HR 0.22 in Asians, HR 0.17 in non-Asians), disease stage (HR 0.39 in stage IB, HR 0.17 in stage II and HR 0.12 in stage IIIA) and use of A-ChT (HR 0.16 with A-ChT and HR 0.23 without A-ChT). An exploratory analysis [20] on A-ChT reported that 66% of the ADAURA population received platinum-based A-ChT for a median of 4 cycles. As expected, the percentage of patients treated with A-ChT was higher amongst younger patients (66% < 70 years versus 42% ≥ 70 years) and those with higher tumour stages (76% in stage II-IIIA versus 26% in stage IB).
The subgroup analysis on CNS recurrence was reported in the 2020 edition of the European Society of Medical Oncology (ESMO) meeting [21]. With a median follow-up of 22 months, a total of 45 patients had CNS recurrence (6 in the osimertinib arm and 39 in the placebo arm). The estimated probability of CNS recurrence at 18 months was lower with osimertinib (< 1% vs. 9%). Median CNS DFS was NR (95% CI 39.0 to not calculable [NC]) with osimertinib vs. 48.2 months with placebo (HR 0.18, 95% CI 0.10–0.33; P < 0.0001).
The safety analysis included 337 patients with osimertinib and 343 with placebo. The median duration of treatment exposure was 22.5 months for osimertinib and 18.4 months for placebo. Overall, the toxicity profile of osimertinib was consistent with that reported in previous trials [17, 22]; the most common adverse events (AEs) included diahorrea (46%), paronychia (25%), dry skin (23%), pruritus (19%), cough (18%) and stomatitis (17%). All-grade AEs, ≥ grade 3 AEs and serious AEs (SAEs) related to ‘any cause’ were similar between arms: 97, 20 and 16% (osimertinib arm) versus 89, 14 and 13% (placebo arm), respectively. As expected, all-grade AEs, AEs ≥ grade 3 and SAEs ‘possibly causally related’ were higher in the osimertinib arm (90%, 10 and 3%, respectively) compared to the placebo arm (55, 3 and 1%, respectively). Interstitial lung disease occurred in 3% of patients treated with osimertinib (all of them grade 1–2). QTc prolongation occurred in 7% of patients receiving osimertinib (in 3/22 patients grade 3) and 1% with placebo (1/4 patients grade 3). No fatal adverse events were reported with osimertinib and one such event occurred in the placebo arm due to a pulmonary embolism. Dose reductions and trial discontinuation were required in 9% and 11% of patients in osimertinib arm and in 1% and 3% of patients in placebo group, respectively.
Beyond treatment safety, another key consideration of long-term therapy with osimertinib is quality of life. HRQoL was measured by the Short Form-36 (SF-36) health survey at baseline, 12 and 24 weeks, then every 24 weeks until treatment completion or discontinuation. Time to deterioration (TTD) was defined as time from randomization to first confirmed clinically important worsening/death. Compliance rates for completion of the SF-36 health survey were high (≥ 85%) across all visits and were balanced between arms. The SF-36 scores differed from baseline to week 96 in favour of placebo; however, the difference was not clinically meaningful. TTD was comparable between both arms [23].
Discussion
The impressive DFS benefit reported in ADAURA is undeniably encouraging and gives hope that the advances achieved in metastatic EGFRmt NSCLC might translate into higher curation rates in early-stage disease. The results from ADAURA were recently included in a meta-analysis evaluating four other clinical trials with adjuvant EGFR-TKI in resected stage IB-IIIA NSCLC [24]; the DFS favoured EGFR-TKIs compared to A-ChT or placebo (HR 0.38, 95% CI 0.22–0.63) and fewer adverse events were reported with EGFR-TKI compared to A-ChT (relative risk 0.28, 95% CI 0.09–0.94) but no significant OS benefit was observed (HR 0.61, CI 95% 0.31–1.22). While this analysis reinforces the concept that EGRF-TKIs induce DFS benefit, the key question of whether a third-generation EGFR-TKI can improve survival will likely remain unanswered for a few years as survival data in ADAURA is only 5% mature.
The nature of adjuvant trials does not allow for rapid conclusions, as results usually take approximately 10 years from first patient enrolment. Some argue that a strong efficacy signal in a surrogate endpoint (e.g. reduction in the risk of relapse) is sufficient to fast track drug approval to allow for timely incorporation of a promising drug into clinical practice. On the other hand, and as the main criticism to the FDA’s approval, a significant DFS improvement does not guarantee OS benefit as seen in the CTONG-1104/ADJUVANT trial; therefore, while using a more potent EGFR inhibitor may delay tumour recurrence, the inevitable re-growth of drug-resistant subclones may not be treatable [25], at the cost of no real survival benefit [19]. Hence, it would appear that the selection of DFS as endpoint in ADAURA corresponds more to a strategic decision to obtain efficacy results quickly, rather than to address a real clinical need.
Another criticism to the FDA’s approval is that tumour stage and the need for A-ChT is not specified. Although DFS improvement is observed across all subgroups, the benefit is more profound in higher tumour stages (risk of relapse is reduced by 61% and 88% in stage IB and IIIA NSCLC, respectively) and ADAURA is only powered to detect DFS benefit in stage II-IIIA NSCLC. At 24 months, 75% of patient in stage IB had not recurred, which means that almost two thirds of these patients did not benefit from adjuvant osimertinib.
Another relevant consideration is whether osimertinib may be an adequate substitute for A-ChT in patients with EGFRmt NSCLC. In the pre-specified subgroup analysis, DFS benefit was seen irrespective of the use of A-ChT, but a closer look at the HRs demonstrates that the reduction of the risk of relapse is slightly higher in patients receiving A-ChT (84% versus 77%). There is preclinical evidence to suggest that EGFR-TKIs have a synergistic effect with chemotherapy [26], and this question is currently being addressed in two clinical trials: one trial with osimertinib plus chemotherapy in the metastatic setting (FLAURA2; NCT04035486), and one trial (NEOADAURA, NCT04351555) in the neoadjuvant setting. Thus, at present there is insufficient evidence to conclude that A-ChT may be simply omitted in the treatment of patients with EGFRmt NSCLC. A real-world study on the use of A-ChT in stage IB-IIIA NSCLC in three European countries highlighted that 50% of patients did not receive A-ChT (compared to 40% in ADAURA), with the main reasons being patient decision (12.6%) and comorbidities (11.9%)[27]. Therefore, an ‘osimertinib-only strategy’ could be a reasonable option for patients otherwise not eligible for treatment with A-ChT. Further research on tumour biomarkers and minimal residual disease might help to decipher subsets of patients who require dual adjuvant therapy, allowing for a more tailored approach.
Although most EGFR mutations occur in ‘classical’ hot spots (exon del 19 and point mutation L858R in exon 21), up to 10–20% of patients with EGFRmt NSCLC harbour the so-called ‘uncommon’ mutations (for example, exon 18 G719X, exon 20 S768I and exon 21 L861Q). Because of the heterogeneity and low frequency of uncommon EGFR mutations they tend to be excluded from most prospective studies. Aside from exon 20 insertions, which are universally resistant, most uncommon variants retain a degree of sensitivity to EGFR-TKIs [28], specially to afatinib (the only EGFR-TKI approved by the FDA for uncommon EGFRmt [29]) and osimertinib [30]. Moreover, it has been reported that up to 25% of patients could harbour compound mutations where two distinct EGFR variants co-exist (either two ‘classical’, two ‘uncommon’ or a mix of both) [28, 31]. The efficacy of EGFR-TKIs in this setting depends on the specific mutant variants, such that if both variants are sensitive, treatment efficacy is comparable to that reported for the single mutation. However, if there is a mix of sensitive and resistant mutations, EGFR-TKI sensitivity is reduced. In patients with compound mutations, one of them is believed to behave as a clonal driver mutation and the other as a subclone. A similar theory has been proposed for patients with ‘de novo’ T790M mutations, which are thought to be extremely rare (< 1%)[32]. However, using highly sensitive sequencing methods, 20% of treatment-naïve patients seem to harbour T790M mutations at the subclonal level, which could negatively impact treatment response [33]. The theory is that a pre-existing subclonal T790M variant becomes the predominant driver of the cancer under selective EGFR-TKI pressure. In ADAURA, compound mutations were allowed if they co-existed with classical mutations. In the pre-specified analysis, patients with the EGFR del 19 mutation were more sensitive (greater DFS benefit) than those with the EGFR L858R point mutation, in line with prior evidence in the metastatic setting [17, 34]. At present, ADAURA has not reported outcome data of patients with compound EGFR mutations, but this could be relevant clinical information to support osimertinib over other EGFR-TKIs in the adjuvant setting, especially for patients with ‘de novo’ T790M mutations. Moving forward, the inclusion of patients with uncommon EGFR variants is urgently needed to provide prospective clinical information in this subgroup.
Two key messages from ADAURA that might favour the adoption of osimertinib in clinical practice are: (1) HRQoL is maintained despite prolonged therapy and (2) osimertinib reduces the risk of CNS relapse (HR 0.18). The fact that HRQoL is not significantly affected throughout osimertinib therapy is reassuring considering that adjuvant therapies are given to patients who are, in theory, cancer free. However, in the absence of OS advantage, the argument in favour of osimertinib relies on quality of life improvement (secondary to delay in cancer progression), hence the need to gather HRQoL data at progression and at the time of therapeutic management of first relapse. At this point, it would be expected that patients in the placebo arm will respond better to first-line osimertinib and therefore exhibit an improvement in quality of life. Moreover, this maintenance in HRQoL could also be justified by the fact that osimertinib is generally well tolerated and that 90% of ‘causally related’ AEs were grade 1–2. However, osimertinib toxicity should not be overlooked: the vast majority (90%) of patients receiving osimertinib experienced ‘causally related’ AEs, and the percentage of high-grade AEs (≥ grade 3) and SAEs was threefold higher than that reported for the placebo. Arguably most common AEs secondary to osimertinib are ‘treatable’ (for example, skin problems and diahorrea), but others might be more challenging to manage (such as interstitial lung disease and QTc prolongation). Indeed, an evaluation of the incidence, type and severity of AEs needs to be included in the decision-making process, particularly in the absence of survival benefit.
The question of CNS relapse is especially relevant for patients with EGFRmt NSCLC stage I-III in whom the risk of developing CNS metastases seems to be higher compared to patients with EGFR wild-type tumours [35] which, in turn, predicts a poor prognosis [36]. The CNS activity reported in ADAURA is consistent with the subgroup analysis from FLAURA; patients with CNS metastases treated with osimertinib had better CNS-progression-free survival and intracranial response rates compared to those treated with gefitinib or erlotinib [37]. Moreover, it is also possible that some CNS events were missed in ADAURA as brain imaging was mandatory at baseline but thereafter only indicated in the presence of symptoms. In this context, it would be also interesting to know whether the pattern of relapse in other metastatic sites which affect quality of life (e.g. bone metastases or pleural disease) differs between arms.
There are two other considerations in ADAURA’s design that could also have affected patient outcome. First, the trial did not report the percentage of patients who underwent exhaustive tumour staging with mediastinoscopy, positron emission tomography-computed tomography (PET–CT) and brain magnetic resonance imaging (MRI). In fact, the trial allowed for baseline brain assessment with either CT or MRI although some guidelines favour the use of MRI in patients with tumour stage higher than IA [38]. Second, no evaluation of surgery quality was performed; only 3% of patients underwent pneumonectomy, in contrast to historical series reporting up to 30% [18]. Whether lymph nodes dissection was performed according to the International Association for the Study of Lung Cancer (IASLC) specifications is also not reported. While these factors might have contributed to incorrect tumour staging, the performance of the placebo arm does not seem to have been greatly impacted, being comparable to other trials; 2-year DFS for the placebo arm in the ADAURA, CTONG-1104/ADJUVANT and EVAN trials are approximately 50% (II-IIA), 40% (II–IIA node positive) and 45% (IIA–N2), respectively.
Beyond survival benefit, there are other questions around the optimal treatment duration and the sequence of EGFR-TKIs that have not been elucidated. The decision to stop treatment at 3 years in ADAURA is arbitrary and probably influenced by the CTONG-1104/ADJUVANT trial where DFS benefit was lost after gefitinib discontinuation at 2 years. Regardless, the median treatment duration with osimertinib reported in ADAURA is only 22.5 months (although at data cutoff 61% of patients remained on treatment), and of those who discontinued, two thirds were due to patient choice or toxicity. It is possible that a more potent EGFR-TKI, such as osimertinib, does not require 3 years of therapy to attain DFS or OS benefit. ADAURA was not designed to answer this question; however, longer follow-up of patients who discontinued treatment for reasons other than disease progression might shed some light towards this.
A deeper understanding of the mechanism involved in tumour resistance following adjuvant EGFR-TKITo is needed to accurately weigh the benefit of subjecting resected (and possibly cured) patients to 3 years of adjuvant osimertinib, as opposed to postponing osimertinib until disease progression (i.e. optimal treatment sequence). Unfortunately, most adjuvant trials have not delved into this area, and the only evidence available comes from the SELECT trial where only one of 15 patients who underwent a tumour biopsy at relapse had a T790M mutation; 71% of patients were successfully rechallenged with erlotinib. In the CTONG1104/ADJUVANT trial, patients treated with adjuvant gefitinib also responded to further EGFR-TKI therapy at relapse (response rate 55.6% with osimertinib and 42.1% with other EGFR-TKIs). In fact, survival after relapse was higher in those patients who had received adjuvant gefitinib for ≥ 18 months and also received EGFR-TKI at disease progression, probably indicting a subgroup of patients especially sensitive to EGFR inhibition. While these results seem to indicate that is possible to derive benefit from EGFR-TKI rechallenge, one main concern is that osimertinib-resistant subclones may emerge, posing a great challenge as there are as yet no other targeted therapies approved in this setting. There is hope that we will see a ‘face to face’ comparison between adjuvant versus first-line osimertinib if patients on the placebo arm are treated with osimertinib at progression. Translational studies to detect biomarkers of response are paramount to further refining treatment in this setting.
The final consideration relates to the cost-effectiveness of adjuvant osimertinib. The current annual cost of osimertinib is $204,000 which seems a relatively large sum of money in the absence of survival gain. Conversely, it could be argued that treating recurrent cancer also poses a significant economic burden. An accurate health economics evaluation will only be possible once OS data is released.
Conclusion
The reduction in the risk of relapse reported with osimertinib in patients with EGFRmt NSCLC stage IB-IIIA is truly unprecedented in lung cancer and is a major step towards incorporating targeted therapies in early-stage disease. This together with the reduction in the risk of CNS relapse, favourable toxicity and HRQoL seems to greatly favour adjuvant osimertinib. However, whether an adjuvant therapy should be adopted in the absence of mature survival data remains an area for debate. Further research is warranted to identify biomarkers to predict response and resistance to osimertinib, and to help fine-tune both adjuvant treatment and subsequent treatment options for patients with resected EGFRmt NSCLC.
References
Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest. 2003;123(6):2096–103.https://doi.org/10.1378/chest.123.6.2096.
Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. https://doi.org/10.1016/j.jtho.2015.09.009.
Pignon J-P, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–9. https://doi.org/10.1200/JCO.2007.13.9030.
Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–237. https://doi.org/10.1093/annonc/mdy275.
Felip E, Altorki N, Zhou C, Cet al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344–57. https://doi.org/10.1016/S0140-6736(21)02098-5.
Wu Y-L, Tsuboi M, He J, et al. Osimertinib in resected EGFR -mutated non–small-cell lung cancer. N Engl J Med. 2020;383(18):1711–23. https://doi.org/10.1056/NEJMoa2027071.
Herbst RS, Tsuboi M, John T, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J Clin Oncol. 2020;38(18_suppl):LBA5–LBA5. https://doi.org/10.1200/JCO.2020.38.18_suppl.LBA5.
European Medicines Agency. Tagrisso. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/tagrisso. Accessed 10 Feb 2022.
Medicines and Healthcare products Regulatory Agency. UK medicines regulator issues its first authorisation under Project Orbis—GOV.UK. https://www.gov.uk/government/news/uk-medicines-regulator-issues-its-first-authorisation-under-project-orbis. Accessed 10 Feb 2022.
Kelly K, Altorki NK, Eberhardt WEE, et al. Adjuvant Erlotinib versus Placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol. 2015;33(34):4007–14. https://doi.org/10.1200/JCO.2015.61.8918.
Pennell NA, Neal JW, Chaft JE, et al. SELECT: a phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol. 2019;37(2):97–104. https://doi.org/10.1200/JCO.18.00131.
Zhong W-Z, Wang Q, Mao W-M, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1):139–48. https://doi.org/10.1016/S1470-2045(17)30729-5.
Yue D, Xu S, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med. 2018;6(11):863–73. https://doi.org/10.1016/S2213-2600(18)30277-7.
Wu Y-L, Zhong W, Wang Q, et al. CTONG1104: Adjuvant gefitinib versus chemotherapy for resected N1–N2 NSCLC with EGFR mutation—final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial. J Clin Oncol. 2020;38(15_suppl):9005–9005. https://doi.org/10.1200/JCO.2020.38.15_suppl.9005.
Huang Q, Li J, Sun Y, Wang R, Cheng X, Chen H. Efficacy of EGFR tyrosine kinase inhibitors in the adjuvant treatment for operable non-small cell lung cancer by a meta-analysis. Chest. 2016;149(6):1384–92. https://doi.org/10.1016/j.chest.2015.12.017.
Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR—mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. https://doi.org/10.1056/NEJMoa1913662.
Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med. 2017;376(7):629–40. https://doi.org/10.1056/NEJMoa1612674.
Planchard D. Adjuvant Osimertinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1780–2. https://doi.org/10.1056/NEJMe2029532.
Addeo A, Banna GL, Friedlaender A. ADAURA: mature enough for publication, not for prime time. Oncologist. 2021;26(4):266–8. https://doi.org/10.1002/onco.13637.
Wu Y-L, John T, Grohe C, et al. Postoperative chemotherapy use and outcomes From ADAURA: Osimertinib as adjuvant therapy for resected EGFR-mutated NSCLC. J Thorac Oncol. 2021;S1556-0864(21)03285-8 https://doi.org/10.1016/j.jtho.2021.10.014.
Tsuboi M, Wu Y, He J, et al. LBA1—Osimertinib adjuvant therapy in patients (pts) with resected EGFR mutated (EGFRm) NSCLC (ADAURA): central nervous system (CNS) disease recurrence. Ann Oncol. 2020;31(suppl_4):1142–215.
Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. https://doi.org/10.1056/NEJMoa1913662.
Majem M, Goldman J, John T, et al. OA06.03 Patient-reported outcomes from ADAURA: osimertinib as adjuvant therapy in patients with resected EGFR mutated (EGFRm) NSCLC. J Thorac Oncol 2021;16(3):S112–3. https://doi.org/10.1016/j.jtho.2021.01.291.
Lin C, Hu F, Chu H, et al. The role of EGFR-TKIs as adjuvant therapy in EGFR mutation-positive early-stage NSCLC: a meta-analysis. Thorac Cancer. 2021;12(7):1084–95. https://doi.org/10.1111/1759-7714.13874.
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725. https://doi.org/10.1038/s41416-019-0573-8.
La Monica S, Minari R, Cretella D, et al. Third generation EGFR inhibitor osimertinib combined with pemetrexed or cisplatin exerts long-lasting anti-tumor effect in EGFR-mutated pre-clinical models of NSCLC. J Exp Clin Cancer Res. 2019;38(1):222. https://doi.org/10.1186/s13046-019-1240-x.
Chouaid C, Danson S, Andreas S, et al. Adjuvant treatment patterns and outcomes in patients with stage IB-IIIA non-small cell lung cancer in France, Germany, and the United Kingdom based on the LuCaBIS burden of illness study. Lung Cancer. 2018;124:310–6. https://doi.org/10.1016/j.lungcan.2018.07.042.
Passaro A, Mok T, Peters S, Popat S, Ahn M-J, de Marinis F. Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non exon 20 insertions, EGFR mutations. J Thorac Oncol. 2021;16(5):764–73. https://doi.org/10.1016/j.jtho.2020.12.002.
US Food and Drug Administration. FDA broadens afatinib indication to previously untreated, metastatic NSCLC with other non-resistant EGFR mutations. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-broadens-afatinib-indication-previously-untreated-metastatic-nsclc-other-non-resistant-egfr. Accessed 9 Feb 2022.
Cho JH, Lim SH, An HJ, et al. Osimertinib for patients with non-small-cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSG-Lu15-09). J Clin Oncol. 2020;38(5):488–95. https://doi.org/10.1200/JCO.19.00931.
Kim EY, Cho EN, Park HS, et al. Compound EGFR mutation is frequently detected with co-mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol Ther. 2016;17(3):237–45. https://doi.org/10.1080/15384047.2016.1139235.
Yu HA, Arcila ME, Hellmann MD, Kris MG, Ladanyi M, Riely GJ. Poor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testing. Ann Oncol. 2014;25(2):423–8. https://doi.org/10.1093/annonc/mdt573.
Dong Y, Zhou Z, Wang J, et al. Origin of the T790M mutation and its impact on the clinical outcomes of patients with lung adenocarcinoma receiving EGFR-TKIs. Pathol Res Pract. 2019;215(5):946–51. https://doi.org/10.1016/j.prp.2019.01.045.
Ramalingam SS, Yang JC-H, Lee CK, et al. Osimertinib as first-line treatment of EGFR mutation–positive advanced non–small-cell lung cancer. J Clin Oncol. 2018;36(9):841–9. https://doi.org/10.1200/JCO.2017.74.7576.
Chang WY, Wu YL, Su PL, Yang SC, Lin CC, Su WC. The impact of EGFR mutations on the incidence and survival of stages I to III NSCLC patients with subsequent brain metastasis. PLoS One. 2018;13(2). https://doi.org/10.1371/journal.pone.0192161.
Taniguchi Y, Tamiya A, Nakahama K, et al. Impact of metastatic status on the prognosis of EGFR mutation-positive non-small cell lung cancer patients treated with first-generation EGFR-tyrosine kinase inhibitors. Oncol Lett. 2017;14(6):7589–96. https://doi.org/10.3892/ol.2017.7125/abstract.
Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR—mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(33):3290–7. https://doi.org/10.1200/JCO.2018.78.3118.
Ettinger DS, Wood DE, Aggarwal C, et al. Non-small cell lung cancer, version 1.2020: featured updates to the NCCN guidelines. JNCCN J Natl Compr Cancer Netw. 2019;17(12):1464–72. https://doi.org/10.6004/jnccn.2019.0059.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
AOF: Conceptualization, writing of original draft, and writing and editing of reviewed article. SR: Writing of original draft, and writing and editing of reviewed article.
Disclosures
Ana Ortega-Franco has nothing to disclose, Shereen Rafee has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ortega-Franco, A., Rafee, S. ADAURA: The Splash of Osimertinib in Adjuvant EGFR-Mutant Non-small Cell Lung Cancer. Oncol Ther 10, 13–22 (2022). https://doi.org/10.1007/s40487-022-00190-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-022-00190-8