Abstract
Atezolizumab is a monoclonal antibody targeting the programmed death ligand 1 (PD-L1) that was approved in 2017 in the USA and Europe for the second-line treatment of advanced or metastatic non-small cell lung cancer (NSCLC). This review article describes the practical clinical issues associated with atezolizumab treatment in NSCLC using a combination of four illustrative cases and a narrative literature review. The first two cases highlight the importance of tumor mutational status when making treatment decisions. A 62-year-old man with epidermal growth factor receptor (EGFR)-mutated, PD-L1-positive, stage IV lung adenocarcinoma received treatment with second-line atezolizumab + bevacizumab, carboplatin, and paclitaxel (BCP) after first-line osimertinib. In the second case, a 63-year-old man with stage IVb lung adenocarcinoma with anaplastic lymphoma kinase (ALK) translocation received sixth-line treatment with atezolizumab + BCP. The two final cases both had extensive metastases. A 55-year-old woman with EGFR-mutated lung adenocarcinoma received second-line treatment with atezolizumab + BCP after development of multiple metastases, followed by atezolizumab + bevacizumab until last follow-up. A 42-year-old man with PD-L1-positive pulmonary adenocarcinoma (negative for EGFR mutations) developed liver and brain metastases after several lines of therapy. He underwent holocranial radiation and received atezolizumab + BCP, which resulted in a decrease in all measurable and evaluable tumoral lesions. These illustrative cases indicate that the type and number of mutations may influence treatment response to atezolizumab, and that atezolizumab may provide clinical benefit in patients with high disease burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Targeted immunotherapies, such as atezolizumab, have improved overall survival in patients with advanced or metastatic non-small cell lung cancer (NSCLC) in clinical trials. |
Understanding how to treat patients on the basis of their targetable oncogenic mutations or those usually excluded from clinical trials (e.g., with treated brain metastases or high disease burden) is important for oncologists in the real-world clinical setting. |
Four cases of treatment with atezolizumab plus bevacizumab, carboplatin, and paclitaxel in patients with NSCLC after at least one previous line of targeted therapy are described to illustrate these clinical issues. |
What was learned from the study? |
The presented cases suggest that both the number and type of mutations may be relevant for guiding treatment decisions in NSCLC, and that patients with high disease burden or brain metastases may benefit from atezolizumab-containing therapy. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13521968.
Introduction
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer and has a high rate of mortality [1, 2]. According to global estimates, lung cancer was the most commonly diagnosed cancer in 2018 (11.6% of all new cases) and the leading cause of cancer-related mortality (18.4% of all cancer deaths) [3]. In a US analysis of patients with NSCLC, the median overall survival (OS) time after diagnosis was only 13 months, and the estimated 4-year survival rate was between 22% and 28% [1]. However, OS has been improving over recent decades as a result of novel treatments [1, 2], including targeted therapies and immunotherapies.
One such novel treatment is atezolizumab, a monoclonal antibody targeting the programmed death ligand 1 (PD-L1) [4]. Atezolizumab was approved in the USA and Europe for the second-line treatment of advanced or metastatic NSCLC in 2017, based on the results of the phase II POPLAR study [5] and the phase III OAK study [6], in which atezolizumab was associated with significantly better survival compared with docetaxel. Since its approval, the IMpower150 study has demonstrated that atezolizumab in combination with bevacizumab, carboplatin, and paclitaxel (BCP) was more effective than BCP alone as first-line therapy in previously untreated patients with metastatic non-squamous NSCLC [7].
The current review describes the practical clinical issues associated with atezolizumab + BCP in patients with NSCLC, using a combination of illustrative case presentations and a narrative literature review.
Cases Illustrating Role of Mutational Status
Patient 1
A 62-year-old man with no relevant medical or surgical history was diagnosed with stage IIIC lung adenocarcinoma (cT3cN3cM0) in January 2018. He was a former smoker with a 15 pack-year history. Molecular testing showed an L858R point mutation in epidermal growth factor receptor (EGFR) exon 21 and a primary T790M mutation in EGFR exon 20. The tumor was PD-L1 positive, with a tumor proportion score (TPS) of 80% on immunohistochemistry (pharmDx 28–8; DAKO, Glostrup, Denmark).
On February 1, 2018, he began first-line treatment with osimertinib; the 12-week assessment showed a partial response by Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 criteria. Response continued until March 2019, when the disease progressed to stage IV with mediastinal and retroperitoneal involvement (Fig. 1a). At this time, the patient was asymptomatic and started second-line treatment with atezolizumab in combination with BCP, based on the results of the IMpower150 clinical trial [7]. After two cycles, a computed tomography (CT) scan showed partial response by RECIST 1.1 criteria (Fig. 1b), and the patient was able to receive six cycles without significant adverse events.
Patient 1: Computed tomography scan of chest on a March 25, 2019, showing mediastinal and retroperitoneal involvement, b June 6, 2019 after two cycles of atezolizumab + BCP, c October 5, 2019 indicating a maintained radiologic response, and d January 30, 2020 showing tumor progression after 13 weeks of maintenance atezolizumab + bevacizumab. BCP bevacizumab, carboplatin, and paclitaxel
After confirming that the radiologic response was maintained (Fig. 1c), he continued maintenance treatment with atezolizumab + bevacizumab. In February 2020, after 13 cycles, tumor progression was noted (Fig. 1d) and treatment was discontinued. Biopsy showed amplification and overexpression of c-MET, so the patient initiated third-line treatment with telisotuzumab vedotin as part of a clinical trial (NCT03539536).
Patient 2
A 63-year-old man was diagnosed with a stage IVb lung adenocarcinoma with a brain metastasis (T2N3M1b) in February 2013. He was a former smoker (20 pack-year history) with occupational exposure to oil and its derivatives.
On June 13, 2013, he started induction chemotherapy with cisplatin + pemetrexed, undergoing radiosurgery for the brain lesion after the first cycle. He showed a partial radiologic and metabolic response after two cycles of cisplatin + pemetrexed, and a brain magnetic resonance imaging (MRI) revealed a reduction in the size of the brain metastasis.
He received two cycles of cisplatin + pemetrexed from June 13 to June 24, 2013, followed by radical-intent chemoradiation between June 28 and September 6, 2013, consisting of a 60 Gy dose and two cycles of weekly paclitaxel + carboplatin; however, he was unable to continue this treatment because of sustained leukopenia.
A follow-up assessment on October 6, 2014, found no signs of thoracoabdominal progression. Lesions consistent with metastases were identified in the cerebellar vermis and the right centrum semiovale; radiosurgery was administered using 20.7 Gy and 20.9 Gy, respectively, at these sites.
A CT scan on January 12, 2015, showed hilar-mediastinal progression, and a biopsy of the left hilar adenopathy showed that the tumor had wild-type KRAS, was EGFR- and BRAF-mutation negative, and had an anaplastic lymphoma kinase (ALK) translocation. In February 2015, he started second-line treatment with crizotinib and had a partial response. A brain MRI performed on November 3, 2015, showed oligoprogression, for which he received radiosurgery.
On April 4, 2016, he showed pulmonary progression, and 2 weeks later, he started third-line treatment with ceritinib as part of a clinical trial, with a best response of stable disease.
A CT scan on January 30, 2018, showed an increase in the size of the space-occupying lesions in the brain. On March 6 of the same year, he initiated fourth-line treatment with alectinib for metastases, with a best response of stable disease. A further CT scan on July 23, 2018, showed left hilar adenopathy progression and left upper lobe atelectasis. He started fifth-line therapy for metastases with brigatinib on September 5, 2018, and stable disease was his best response.
The patient developed left hemiparesis on January 16, 2019. A brain MRI on January 21 showed signs of radionecrosis, but an acute or subacute ischemic event could not be ruled out. Brain progression, with an increase in the size and number of lesions, was detected in March 2019 (Fig. 2a), and he received hippocampal-sparing whole brain radiation with focal boost (45 Gy dose) on the lesions.
Patient 2: brain magnetic resonance imaging scan results on a March 11, 2019, showing an increase in the size and number of brain lesions, and b March 22, 2020, after 4 cycles of atezolizumab + BCP followed by 13 cycles of maintenance atezolizumab + bevacizumab, and chest computed tomography scan results on c March 11, 2019, prior to, and d March 22, 2020, after atezolizumab-based treatment
On May 8, 2019, he began sixth-line treatment with atezolizumab + BCP. After four cycles (ending on August 8, 2019), he started maintenance treatment with atezolizumab + bevacizumab, achieving a partial brain response. In March 2020, he had received 13 cycles of maintenance treatment, and he showed stable disease as assessed by brain MRI (Fig. 2b) and CT scans of the chest (Fig. 2c, d), abdomen, and pelvis.
CASES Illustrating Management of High Disease Burden
Patient 3
A 55-year-old non-smoking woman without known morbidity was diagnosed with a stage IIIB lung adenocarcinoma (cT4N2) in June 2016. She received treatment with external radiation therapy (dose administered, 66 Gy) concomitantly with cisplatin and vinorelbine (three cycles). A follow-up CT scan showed partial response and the multidisciplinary Committee on Thoracic Tumors recommended surgical intervention. She underwent video-assisted thoracic surgery consisting of an upper right pulmonary lobectomy with systematic hilar and mediastinal lymph node dissection on October 5, 2016. Molecular analysis of the excised tumor showed EGFR exon 19 deletion.
In March 2018, disease progression was detected in lung, pleura, and bone, and in subcutaneous tissue and the lymphatic system. On March 27, she received palliative and decompressive radiation therapy of the lumbar spine (L5) at 8 Gy. Subsequently, in April 2018, she started treatment with afatinib at 40 mg/day, and had a partial response. Afatinib treatment continued until July 2019, when imaging identified disease progression in the liver and bone, and a sacral soft tissue mass (Fig. 3a). Analysis of liquid and sacral mass biopsy did not detect a resistant T790M mutation on EGFR exon 20.
At this time, the patient’s Eastern Cooperative Oncology Group (ECOG) performance status was 2, and she was negative for PD-L1 (TPS 0%). On August 7, 2019, on the basis of the results of the IMpower150 clinical trial [7], she began second-line treatment with atezolizumab + BCP. After three cycles she showed a partial response (Fig. 3b), and was able to complete six cycles of treatment. However, the patient developed febrile neutropenia and sepsis due to central catheter-related infections in the second cycle leading to hospital admission; grade 2 sensory neuropathy that reverted to grade 1 after discontinuing paclitaxel and carboplatin; and grade 1 asthenia. She then continued treatment with atezolizumab + bevacizumab. By June 2020, she had completed 14 cycles of treatment without relevant toxicities, but treatment was interrupted at that time because a CT scan showed progression of the liver and bone metastases.
Patient 4
A 42-year-old man was diagnosed with stage IIIB pulmonary adenocarcinoma (cT4N2M0) in June 2018; at this time, he had an ECOG performance status of 0. Molecular analysis showed the tumor was negative for EGFR mutations and ALK fusions, with PD-L1 expression on 30% of cancer cells. He was a smoker at the time of diagnosis, smoking one pack daily for 20 years (20 pack-years).
On August 22, 2018, treatment with cisplatin, gemcitabine, and paclitaxel was started, for a total of four 21-day cycles. Treatment was well tolerated, with the exception of nausea and asthenia (both grade 1). Repeat CT scans performed in September 2018 after two treatment cycles, and again in November after four treatment cycles, revealed decreasing tumor volume, with mediastinal contact still present; at this time, results of a brain MRI were normal.
In December 2018, the man underwent a right lower lobectomy; the right lower pulmonary lobe was found to have well-differentiated adenocarcinoma with an acinar pattern, without evidence of vascular invasion, and no visceral pleural infiltration. Surgical resection margins were not affected. TNM staging at this point was ypT1cN0M0. He subsequently received consolidative radiation therapy on the mediastinal bed; he had a performance status of 0 and no complications.
In October 2019, a follow-up examination revealed a de novo pulmonary micronodule in the control CT scan, but this was not seen on a positron emission tomography–CT scan. In February 2020, repeat CT scans revealed a growth on the left perihilar pulmonary nodule and a new right-hand micronodule. Metastases were seen in several liver segments. As a result of mild dizziness, a central nervous system (CNS) MRI scan was performed in January 2020, revealing the presence of brain metastases (Fig. 4a).
Holocranial radiotherapy was administered, finishing at the beginning of February 2020. After 4 weeks, a repeat MRI did not show any significant changes in the size of the brain lesions. One month after discontinuing holocranial radiotherapy, in the second week of March 2020, he began treatment with four 21-day cycles of atezolizumab + BCP, followed by atezolizumab + bevacizumab until disease progression or toxicity. After two cycles, there were no notable toxicities, and an assessment performed at the end of April 2020 revealed a decrease in all measurable and evaluable tumoral lesions (Fig. 4b). By December 2020, 40 weeks after the start of atezolizumab + BCP, the patient was still in partial response and was being managed on chemotherapy-free maintenance treatment with atezolizumab + bevacizumab.
Compliance with Ethics Guidelines
Data on these patients were collected in accordance with the Helsinki Declaration of 1964 and its later amendments concerning human and animal rights. All patients provided written informed consent to all the diagnostic and therapeutic procedures, for the use of their medical images, and for inclusion in this manuscript. Ethical committee approval was not required, as per Spanish law.
Discussion
EGFR mutations are present in 10–20% of Caucasian patients and about 50% of Asian patients with NSCLC [8]. The L858R point mutation in EGFR exon 21 identified in patient 1 at diagnosis is one of the most common “classical” EGFR mutations, along with a deletion in exon 19 [8]. Together, these mutations represent 85% of EGFR mutations. These activating mutations make the tumor sensitive to tyrosine kinase inhibitors (TKIs) and are an indication for choosing a TKI agent as first-line therapy [9], as occurred in patient 1. Indeed, current NSCLC guidelines note the importance of molecular tumor analysis in determining first-line therapy [9, 10]. In Europe, testing for mutations or rearrangements of ALK, c-ros oncogene 1 (ROS1), and EGFR is considered mandatory, and testing for BRAF V600E mutations is important in countries where BRAF/MEK inhibitors are approved. Other tests include those for human epidermal growth factor receptor 2 (HER2) and MET exon mutations and for fusion genes in RET and NTRK1, which are all are considered evolving biomarkers [10]. The US National Comprehensive Cancer Network guidelines recommend the biomarker test panel for non-squamous NSCLC should, at a minimum, include EGFR mutations, BRAF mutations, ALK fusions, ROS1 fusions, and PD-L1 expression [9].
A primary T790M mutation in EGFR exon 20 is a resistance mutation and more likely to coexist with an L858R mutation than with an exon 19 deletion [11]. While osimertinib has been shown to be effective in patients with a primary T790M mutation, as shown in patient 1, most patients with such mutations progress within 1 year of starting osimertinib [11]. Patient 1 also had high expression of PD-L1 as shown by a TPS of 80%. This suggests that this patient is a good candidate for immunotherapy, but guidelines recommend the use of targeted therapy first-line (before immunotherapy) in patients with sensitizing mutations because the response rate is likely to be higher [9]. Once targeted therapy fails, immunotherapy is indicated.
ALK rearrangements are the driving mutations responsible for the development of NSCLC in 3–7% of patients, and, as observed in patient 2, these patients have a higher risk of developing brain metastases than those with other NSCLC subtypes [12]. The first-generation ALK inhibitor crizotinib, used for second-line treatment in patient 2, has antitumor advantages over chemotherapy in this subtype of patients; however, all patients eventually progress because of drug resistance [10]. Furthermore, the amount of crizotinib that crosses the blood–brain barrier is negligible, which limits its use in patients with brain metastases [10]. In patients with crizotinib-resistant ALK-rearranged NSCLC, one strategy is sequential treatment with next-generation ALK inhibitors, such as ceritinib, alectinib, or brigatinib [12], as used in patient 2.
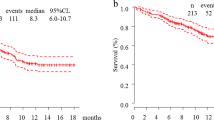
In the IMpower150 study, most patients had wild-type EGFR and ALK, but 10% of patients were EGFR-mutation positive and 8.5% were ALK-rearrangement positive [7]. IMpower150 is the only trial that has shown positive results with immunotherapy in patients with EGFR- or ALK-positive NSCLC [10]. OS in the population that included patients with these mutations receiving the atezolizumab + BCP regimen was 19.8 months, which was similar to that in the population excluding patients with these mutations (19.5 months), and significantly longer than in the BCP group (15.0 months for the whole population and 14.7 months for the EGFR and ALK wild-type population) [13]. In patients with EGFR mutations, OS was 29.4 months in the atezolizumab + BCP group and 18.1 months in the BCP group [13]. Median progression-free survival (PFS) in patients with EGFR mutations receiving the atezolizumab + BCP regimen was 9.7 months, similar to the PFS in patients with wild-type EGFR (8.3 months), and significantly longer than in the BCP group irrespective of EGFR mutation status (6.1 months and 6.8 months, respectively, in the EGFR mutation and wild-type groups receiving BCP) [7]. Similarly, median PFS was significantly longer in the atezolizumab + BCP group than in the placebo + BCP group in patients with KRAS mutations (8.1 vs. 5.8 months, respectively), as well as in those with wild-type KRAS (9.7 vs. 5.8 months) [7].
The number of mutations, as well as the type, may be relevant to treatment response to atezolizumab. Data suggest that the survival (PFS and OS) benefit of PD-L1 or PD-1 inhibitors may be more marked in patients with a high tumor mutation burden (TMB) than in those with low TMB [14,15,16], although this result was not seen in some trials [17, 18]. Recent data from randomized trials show that a blood-based assay for TMB can be a useful and valid biomarker for atezolizumab [19].
The cases of patients 3 and 4 illustrated the use of atezolizumab in patients with high disease burden, which was characterized by extensive metastases, including in the liver. In the IMPower150 study, 13% of patients had liver metastases, and atezolizumab + BCP significantly prolonged PFS and OS compared with placebo + BCP in patients with or without liver metastases [7, 20]. However, the difference in PFS and OS between the atezolizumab + BCP group and the BCP group was more marked in the cohort with liver metastases [7]. In the group with liver metastases, the PFS hazard ratio was 0.42 (median PFS 7.4 vs. 4.9 months for atezolizumab + BCP vs. placebo + BCP), whereas in the group without liver metastases the PFS hazard ratio was 0.63 (median PFS 8.3 vs. 7.0 months, respectively) [7]; the OS hazard ratio was 0.52 in the group with liver metastases (median OS 13.3 vs. 9.4 months for atezolizumab + BCP vs. placebo + BCP), and in the group without liver metastases the OS hazard ratio was 0.82 (median OS 20.4 vs. 17.0 months, respectively) [20].
In addition to liver metastases, patient 3 also had bone metastases, which are associated with significantly reduced survival in patients with NSCLC [21, 22]. Moreover, bone metastases are associated with significant pain, fatigue, and disturbed sleep [23], which may negatively affect the patient’s performance status. Indeed, this patient had an ECOG performance status of 2. The IMpower150 study excluded patients with ECOG performance status of 2 or higher [7]; however, data indicate that between one-third and one-half of patients with NSCLC have poor performance status (e.g., ECOG PS of 2 or higher) [24]. It is encouraging that patient 3, who had multiple metastatic sites including in the appendicular skeleton, was able to benefit from treatment with atezolizumab over a prolonged period before disease progression.
The last case presented (patient 4) had both liver and brain metastases at the time of starting treatment with atezolizumab. Adenocarcinomas are the most common tumor type to metastasize to the CNS, and 30–64% of patients with NSCLC have CNS metastases [9, 10]. Historically, patients with brain metastases have had a particularly poor prognosis [25], although this is starting to change with the increasing availability of novel agents. However, patients with untreated brain metastases are usually excluded from clinical trials, many of which also exclude patients with treated brain metastases. Indeed, both the POPLAR study and IMPower150 excluded patients with untreated CNS metastases [5, 7], with neither study reporting on patients with treated brain metastases.
Patient 4 described here received treatment of brain metastases with holocranial radiotherapy, and subsequently derived benefit from atezolizumab treatment. The positive CNS responses in this patient is consistent with findings reported in the OAK trial [6]. In this trial, which specified inclusion of patients with treated, asymptomatic supratentorial CNS metastases, subgroup analysis demonstrated a survival benefit with atezolizumab compared with docetaxel in patients with treated CNS metastases at baseline, with a hazard ratio of 0.54 [95% confidence interval (CI) 0.31–0.94] [6].
These findings for atezolizumab in patients with brain metastases are also consistent with those of a phase II study of pembrolizumab in patients with NSCLC (n = 18) or melanoma and untreated brain metastases, in which pembrolizumab demonstrated activity in brain metastases, with a response rate for brain metastasis of 33% among patients with NSCLC [26].
Although this case series provides valuable information regarding the practical clinical issues associated with atezolizumab + BCP treatment, more well-designed studies are needed to develop evidence-based recommendations on immunotherapy to guide oncologists, immunologists, and other specialists in the management of patients with mutated NSCLC.
Conclusion
As more becomes known about the importance of the various genetic mutations, rearrangements, and expression profiles in NSCLC, testing for targetable oncogenic alterations and immuno-oncology therapy biomarkers is becoming more and more essential for treatment decisions [10]. The first two patients discussed here suggest that the number and type of mutations may be relevant for guiding treatment decisions in NSCLC. To our knowledge, atezolizumab + BCP is the only immunotherapy combination that has demonstrated efficacy in patients with mutated NSCLC [7, 27], and these cases show that the results of these studies have been transferred to clinical practice. Another factor that should be considered when deciding upon a course of therapy is a patient’s performance status. However, clinical trials often exclude patients with poor performance status; hence, the second two cases described here of patients with NSCLC and high disease burden (patients 3 and 4), who benefited from atezolizumab-containing therapy over a prolonged period, are particularly encouraging. Finally, the CNS responses reported here suggest that atezolizumab + bevacizumab may be an important option for the significant unmet need of treating brain metastases in NSCLC. These observations provide valuable insights into the practical clinical issues associated with atezolizumab + BCP treatment in patients with advanced or metastatic NSCLC. These illustrative clinical cases may also help clinicians to identify patients with NSCLC who will potentially gain the most benefit from the use of atezolizumab + BCP in routine clinical practice.
References
Lou Y, Dholaria B, Soyano A, et al. Survival trends among non-small-cell lung cancer patients over a decade: impact of initial therapy at academic centers. Cancer Med. 2018;7(10):4932–42.
Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943–53.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Blair HA. Atezolizumab: a review in previously treated advanced non-small cell lung cancer. Target Oncol. 2018;13(3):399–407.
Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65.
Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301.
Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. 2020;61:167–79.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: non-small cell lung cancer. Version 3.2020. National Comprehensive Cancer Network. 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed August 3 2020
Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. European Society of Medical Oncology. 2019. https://www.esmo.org/content/download/227453/3874538/1. Accessed August 3 2020
Li W, Qiu T, Guo L, et al. Primary and acquired EGFR T790M-mutant NSCLC patients identified by routine mutation testing show different characteristics but may both respond to osimertinib treatment. Cancer Lett. 2018;423:9–15.
Golding B, Luu A, Jones R, Viloria-Petit AM. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol Cancer. 2018;17(1):52.
Socinski MA, Mok TSK, Nishio M, et al. IMpower150 final analysis: efficacy of atezolizumab and chemotherapy ± bevacizumab in first-line metastatic non-squamous non-small cell lung cancer across key subgroups Cancer Res. 2020;80:16 Suppl (Abstract CT216).
Kim JY, Kronbichler A, Eisenhut M, et al. Tumor mutational burden and efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers (Basel). 2019;11:11.
Wu Y, Xu J, Du C, et al. The predictive value of tumor mutation burden on efficacy of immune checkpoint inhibitors in cancers: a systematic review and meta-analysis. Front Oncol. 2019;9:1161.
Zhu J, Zhang T, Li J, et al. Association between tumor mutation burden (TMB) and outcomes of cancer patients treated with PD-1/PD-L1 inhibitions: a meta-analysis. Front Pharmacol. 2019;10:673.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92.
Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–508.
Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–8.
Socinski MA, Jotte RM, Cappuzzo F, et al. IMpower150: analysis of efficacy in patients (pts) with liver metastases (mets). J Clin Oncol. 2019;37(15_suppl):9012.
Landi L, D’Inca F, Gelibter A, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer. 2019;7(1):316.
Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res. 2008;466(3):729–36.
Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17(5):320–32.
Naqash AR, Stroud CRG, Butt MU, et al. Co-relation of overall survival with peripheral blood-based inflammatory biomarkers in advanced stage non-small cell lung cancer treated with anti-programmed cell death-1 therapy: results from a single institutional database. Acta Oncol. 2018;57(6):867–72.
Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43(4):795–803.
Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–83.
Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401.
Acknowledgments
We thank the patients who allowed us to report their clinical details.
Funding
Medical writing assistance and the Rapid Service Fee for this manuscript were funded by Roche.
Medical Writing Assistance
We would like to thank Catherine Rees of Springer Healthcare Communications, and Marie Cheeseman, on behalf of Springer Healthcare Communications, who wrote the outline and first draft, respectively. This medical writing assistance was funded by Roche.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Compliance with Ethics Guidelines
Data from these patients were collected in accordance with the Helsinki Declaration of 1964 and its later amendments, concerning human and animal rights. All patients provided written informed consent to all the diagnostic-therapeutic procedures, for the use of their medical images, and for inclusion in this manuscript. Ethical committee approval was not required, as per Spanish law.
Disclosures
Dr Cobo reports receiving advisory or consultancy fees from Roche, Bristol Myers Squibb, AstraZeneca, Pfizer, and Boehringer, and travel and accommodation funding from Roche, Bristol Myers Squibb, and AstraZeneca outside of the submitted work. Dr Rodríguez-Abreu reports receiving advisory or consultancy fees from Roche, Merck, Bristol Myers Squibb, AstraZeneca, Pfizer, Boehringer, and Takeda, speaker’s fees from Roche, Merck, Bristol Myers Squibb, AstraZeneca, Boehringer, and Takeda, and travel and accommodation funding from Roche, Merck, Bristol Myers Squibb, and AstraZeneca outside of the submitted work. Diego Pérez Parente is an employee of Roche Farma, Spain. Pedro Ruiz Gracia is an employee of Roche Farma, Spain. Dr González reports receiving advisory or consultancy fees from Roche, Bristol Myers Squibb, AstraZeneca, Boehringer, and Sanofi, speaker’s fees from Roche, Merck, Bristol Myers Squibb, AstraZeneca, Lilly, and Ipsen, and travel and accommodation funding from Roche, Merck, Bristol Myers Squibb, and Lilly outside of the submitted work.
Data Availability
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cobo, M., Rodríguez-Abreu, D., Pérez Parente, D. et al. Practical Issues in the Use of Atezolizumab for Patients with Non-Small Cell Lung Cancer: Case Reports and Literature Review. Oncol Ther 9, 41–53 (2021). https://doi.org/10.1007/s40487-021-00139-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-021-00139-3