Abstract
Introduction
The purpose of this study was to evaluate the absolute bioavailability (BA) of AG-221 following a single oral dose of 100 mg AG-221 and an intravenous (IV) dose of ~ 100 μg AG-221 containing approximately 300 nCi of [14C]-AG-221.
Methods
This was a phase 1, open-label study. Six subjects who met all of the inclusion criteria and none of the exclusion criteria were enrolled in the study. After an overnight fast of at least 10 h, the subjects received an oral dose (coated tablet) of 100 mg of AG-221 at 0 h on dosing day. Four hours after the oral dose, the subjects received 100 μg AG-221 containing ~ 300 nCi of [14C]-AG-221 administered as an IV bolus. Blood samples were collected and analyzed for plasma concentrations of AG-221 and [14C]-AG-221 using a validated liquid chromatography with tandem mass spectrometry (LC–MS/MS) system and high-performance liquid chromatography (HPLC) fractionation followed by accelerator mass spectrometry analysis (AMS), respectively. Safety was evaluated throughout the study.
Results
The absolute BA after a 100-mg oral dose of AG-221 was measured as 57.2%. While the total clearance was 1.37 L/h, ~ 1/60 of the liver blood flow in a typical 70-kg human subject, the first-pass extraction was estimated to be less than 2%, assuming that the total clearance was entirely due to liver metabolism. Thus, the fraction of the AG-221 dose absorbed was at least 50%. AG-221 was safe and well tolerated when given under fasted conditions in a single 100-mg dose as a coated tablet with a microtracer [14C]-AG-221 solution, as few drug-related treatment-emergent adverse events (TEAEs) were reported. No clinically significant changes or findings were noted in the clinical laboratory evaluations, vital sign measurements, and electrocardiograms (ECGs) performed during this study.
Conclusions
In healthy subjects under fasting conditions, the absolute BA following oral administration of a 100-mg AG-221 tablet was 57.2%. AG-221 was safe and well tolerated in healthy male subjects when administered as a single 100-mg film-coated tablet plus 100 µg [14C]-AG-221 given intravenously.
Trial Registration
ClinicalTrials.gov identifier, NCT02443168.
Funding
Celgene Corporation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
AG-221 (enasidenib, IDHIFA®) is a novel, first-in-class, selective, oral, small-molecule inhibitor of the isocitrate dehydrogenase-2 (IDH2)-mutant protein, one of multiple genetic mutations that drive hematologic malignancies such as acute myeloid leukemia (AML) and solid tumors, making it a highly targeted therapeutic candidate for the treatment of both liquid and solid tumors [1,2,3]. The compound has been demonstrated to reverse histone and DNA hypermethylation and induce differentiation in IDH2-mutant-positive leukemia cell models as well as to reduce 2-hydroxyglutarate (2-HG, an oncometabolite synthesized by mutated IDH2 proteins) levels by > 90% in vitro and in vivo in subjects with cancers that harbor IDH2 mutations [1,2,3]. AG-221 is approved by the US Food and Drug Administration (FDA) for the treatment of adult patients with relapsed or refractory AML with an IDH2 mutation as detected by a FDA-approved test [4,5,6]. AG-221 efficacy was established from an open-label, single-arm, multicenter study, where it showed a combined response (i.e., complete response (CR) and complete response with partial hematologic recovery (CRh)) rate and duration of 23% and 8.2 months, respectively, as well as a rate of conversion from transfusion dependence to transfusion independence of 34%. The most common adverse reactions (≥ 20%) included nausea, vomiting, diarrhea, elevated bilirubin, and decreased appetite [4,5,6].
The pharmacokinetics (PK) of AG-221 have been studied and characterized in both healthy volunteers and patients with hematologic malignancies [7,8,9]. PK data from subjects with advanced hematologic malignancies up to the 650-mg QD dose level demonstrated high plasma exposure, a long plasma half-life of AG-221 after a single dose, high drug accumulation after multiple doses, and relatively high PK variability [4]. The mean time to Cmax (Tmax) ranged from approximately 1 to 24 h [4]. There was an approximate 50% increase in AUC0–t and AUC0–inf and a 64% increase in Cmax when AG-221 was administered under fed conditions compared with fasted conditions, and the recommended starting dose of AG-221 is 100 mg taken orally once daily with or without food [4]. Data from a human absorption, distribution, metabolism, and excretion (ADME) study showed that the overall mean recovery of total radioactivity (TRA) was approximately 82%, with approximately 73.0% and 8.4% of the TRA recovered in feces and urine, respectively, during the 504-h postdose collection period [9]. These data indicate that TRA was primarily eliminated via fecal excretion. During the 240-h postdose period (the time frame during which metabolite profiling was performed), excreted metabolites and unchanged AG-221 accounted for greater than 47.1% and 24.9% of the administered dose, respectively. Of the 47.1% of the dose accounted for by excreted metabolites over the 240-h postdose period, fecal excretion of metabolites accounted for 39.9% [9]. These results suggest that AG-221 is mainly cleared by metabolism, with the resulting metabolites eliminated primarily in the feces [9].
Assessing the BA of a formulation is a critical component of drug development because a common reason for failed drug development is poor drug BA [10]; hence, BA should be investigated as early as possible during drug development. Because AG-221 shows poor water solubility (< 0.0003 mg/mL in pH 7 buffer, data on file) and is administered as an oral dose, it is important to assess the proportion of oral AG-221 that reaches the systemic circulation in unchanged form. Knowing the absolute BA of the AG-221 tablet formulation will assist in understanding the overall disposition of the drug and interpreting other AG-221 PK data. In addition, this information will aid the development of new formulations for other indications, e.g., liquid or suspension formulations for pediatric tumor indications.
With that in mind, we performed a phase 1, open-label study to evaluate the absolute BA of AG-221 in healthy adult male subjects. The primary objective was to evaluate the absolute BA of AG-221 following a single oral dose of 100 mg AG-221 and an IV dose of ~ 100 μg AG-221 containing approximately 300 nCi of [14C]-AG-221, and the secondary objective was to evaluate the tolerability and safety of AG-221 after a single 100-mg oral dose of a AG-221 tablet followed by an IV dose of 100 μg AG-221 containing a microtracer of [14C]-AG-221 in healthy male subjects.
Methods
Ethics
This study was conducted in accordance with the applicable US CFR governing the protection of human subjects, financial disclosure by clinical investigators, institutional review boards (IRBs), Investigational New Drug applications, applications for FDA approval to market a new drug, and radioactive drugs for certain research uses. Before the start of the study, the study protocol, informed consent form (ICF), and any other appropriate documents were submitted to the IRB (Salus Institutional Review Board, 2111 West Braker Lane, Suite 400, Austin, TX 78758, USA) with a cover letter or form listing the documents submitted, their dates of issue, and the site for which approval was sought. The investigator obtained ICFs from the subjects prior to any study-related procedures. During this study, there were no notable departures regarding compliance with applicable international good clinical practice (GCP) standards. The study was also in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Study Design
This was a single-center, open-label study conducted in six healthy adult males. Each subject participated in a screening phase (day −21 through −2), a baseline visit, a treatment phase, and a follow-up phone call (7–10 days after the last assessment).
Prior to the start of the study, subjects underwent routine screening procedures and eligible subjects returned to the study center on day −1 for baseline assessments. Subjects who still qualified for participation were administered the study drug on the morning of day 1 and were domiciled at the clinical site from day 1 until day 3.
On day 1, subjects received oral and IV doses of AG-221 in a fixed sequence: a 100-mg oral dose of AG-221 (1 × 100-mg tablet) was given after an overnight fast of 10 h and administered with 240 mL of room-temperature, noncarbonated water. Food and beverages (including water) were withheld from the subjects until at least 2 h following oral dosing. During the fasting period, water was allowed ad libitum except from 1 h prior to dosing until 2 h following dosing [excluding any water given with the investigational product (IP)]. Four hours after the oral dose, subjects were administered a 5-mL IV solution containing 100 µg of [14C]-AG-221 (~ 300 nCi) in 0.25% DMSO/2.5% polysorbate 20/0.9% sodium chloride over approximately 2 min. The IV solution was compounded from [14C]-labeled AG-221 and unlabeled AG-221 powders.
Subjects were discharged from the unit upon completion of the 48-h PK blood draw on day 3. Subjects returned to the unit for additional PK blood draws on days 5, 8, 11, 15, 18, 22, and 29.
The study design is presented in Fig. 1.
Blood Collection for PK Analysis
Blood samples (~ 3 mL) were collected at the following time points for analysis of AG-221 concentrations: pre-dose (oral, 0 h), 0.5, 1, 2, 3, 4 (before IV dose), 4.083 (5 min after IV dose), 4.25 (15 min after IV dose), 4.5, 5, 6, 7, 8, 10, 14, 18, 24, 48, 96, 168, 240, 336, 408, 504, and 672 h (after the oral dose).
Safety Assessment
All subjects who received at least a single dose of AG-221 were included in the safety assessments, which included adverse event (AE) reporting, a review of concomitant medications/procedures, clinical laboratory safety tests, 12-lead ECG recordings, physical examinations (PEs), and vital sign measurements.
Subjects were monitored for safety throughout the study. All AEs and concomitant medications were assessed and recorded throughout the study from the time the ICF was signed until study completion, and when made known to the investigator within 28 days after the last dose of IP [as well as serious adverse events (SAEs) that were made known to the investigator at any time thereafter and were suspected of being related to IP].
Bioanalytical Methodology
Concentrations of AG-221 from oral administration were analyzed using a validated method. The lower limit of quantification (LLOQ) for AG-221 in human plasma was 1.0 ng/mL, with linearity demonstrated to 1000 ng/mL for AG-221. The interassay coefficient of variance (%CV) values based upon the accepted calibration standards across the range were ≤ 6.4% for AG-221. Interassay precision values of AG-221, based upon the %CV of the QC samples, were ≤ 6.7%. Interassay accuracy values of AG-221, based upon the percent relative error (%RE) of the QC samples, ranged from − 2.5 to 7.0% for all QC levels. All plasma samples were analyzed by Covance (West Trenton, NJ, USA).
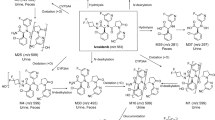
[14C]-AG-221 and AG-221 (Fig. 2) were from Nitto Denko Avecia, Inc. (8560 Reading Road, Cincinnati, OH 45215, USA) and Ash Stevens (18655 Krause Street, Riverview, MI 48193, USA), respectively. The specific radioactivity was 2.5 µCi/mg and radiochemical purity by HPLC was greater than 99%. The LLOQ for [14C]-AG-221 in human plasma was 0.00966 ng-Eq/mL, with linearity demonstrated to 5.00 ng-Eq/mL. The sample analysis was conducted at Accium BioSciences, Inc. (Seattle, WA, USA).
PK Analyses
The following plasma [14C]-AG-221 and nonlabeled AG-221 PK parameters were estimated by noncompartmental methods using actual elapsed time from dosing:
AUC0–t: area under the plasma concentration–time curve (AUC) from time 0 to time t, where t is the time of the last measurable concentration
Cmax: maximum observed plasma concentration
AUC0–inf: AUC from time 0 extrapolated to infinity
Tmax: time to Cmax obtained directly from the observed concentration versus time data
t1/2: terminal-phase elimination half-life
CL: total plasma clearance when dosed intravenously
CL/F: apparent total plasma clearance when dosed orally
Vz/F: apparent total volume of distribution when dosed orally
Vss: volume of distribution when dosed intravenously
Fabs (%): absolute BA of AG-221
Statistical Analyses
The PK population included all subjects who received at least one dose of AG-221 and had at least one measurable datum. PK analyses were performed on data from the PK population. The safety population included all subjects who received at least one dose of AG-221. For subjects who withdrew or were discontinued from the study, all available PK and safety data (including follow-up) were listed and summarized to the point of withdrawal/discontinuation.
A total of six healthy adult male subjects were enrolled in the study. No formal sample size calculation was performed. The sample size was based on empirical rather than statistical considerations. Discontinued subjects could have been replaced at the discretion of the sponsor and investigator.
The plasma concentrations and PK parameters were summarized descriptively by treatment [mean, standard deviation, percent coefficient of variation (CV%), geometric mean, geometric CV%, minimum, median, and maximum].
AEs were classified using the Medical Dictionary for Drug Regulatory Activities (version 17.1) classification system. All AEs were listed. A TEAE was defined as any AE that occurred after dosing of the first study drug or any AE that was already present pre-dose and worsened in either intensity or frequency following dosing. If the onset date/time of an AE was missing, the AE was defined as treatment emergent. TEAEs were summarized by frequency, severity, and relationship to study drug. The frequency of TEAEs (the number of TEAEs and the number of subjects experiencing a TEAE) were tabulated by system organ class and preferred term. In the by-subject analyses, a subject who suffered the same event more than once within the same level of summarization counted only once within the same treatment. AEs leading to death or to discontinuation from treatment and serious AEs were listed separately.
Results
Demographic and Other Baseline Characteristics
Six subjects were enrolled in this study. All the subjects enrolled completed the study. A summary of the demographics is presented in Table 1. All enrolled subjects satisfied the inclusion and exclusion criteria, with no clinically significant abnormalities prior to first-dose administration, and the investigator approved all the subjects for study participation.
Of the subjects, 1 (16.7%) and 5 (83.3%) were Hispanic or Latino and not Hispanic or Latino, respectively. The majority of the subjects were white (83.3%), with age ranging between 28 and 49 years, body weight ranging between 66.3 and 101.1 kg, and body mass index ranging between 22.4 and 32.4 kg/m2.
Quantification of [14C]-AG-221 by AMS
A total of 120 plasma samples were analyzed to determine the concentrations of [14C]-AG-221 originating from an IV dose of [14C]-AG-221 using the validated HPLC-AMS method. The LLOQ for [14C]-AG-221 in human plasma was 0.00966 ng-Eq/mL, with linearity demonstrated to 5.00 ng-Eq/mL. Interassay precision values of [14C]-AG-221, based upon the %CV, were ≤ 7.72 % (Table 2). Interassay accuracy values (%RE) of [14C]-AG-221 ranged from 1.40 to 8.64% for all QC levels (Table 3), indicating a well-validated bioanalytical method to qualify [14C]-AG-221 from IV microtracer dosing.
Absolute BA
Figure 3 illustrates dose-normalized mean plasma concentration–time profiles of AG-221 after a single oral dose of 100 mg and 100 μg of an IV [14C]-AG-221 dose containing ~ 300 nCi of [14C]-AG-221. Unlabeled AG-221 administered orally was quantified for 672 h post-dose using HPLC/MS/MS, whereas 14C-labeled AG-221, administered via IV microdosing, was quantifiable for 408 h post-dose using HPLC/AMS. After IV administration, the AG-221 PK profile showed two distinct phases: a rapid distribution phase followed by a slower elimination phase. However, after oral administration, the initial distribution phase was not apparent. The PK parameters for AG-221 after a single dose of 100 mg AG-221 and an IV dose of 100 μg [14C]-AG-221 containing 300 nCi of [14C]-AG-221 are are summarized in Table 4.
As the amount administered following IV dosing was only 100 µg containing ~ 300 nCi of [14C]-AG-221, the geometric mean peak plasma AG-221 concentration Cmax (2.83 ng/mL, 31.0%) was lower following IV administration than the Cmax following 100 mg oral dosing (703 ng/mL, 16.4%). Similarly, the geometric mean systemic exposure of AG-221 as defined by AUC0–inf was lower following IV infusion of AG-221 (73.0 ng h/mL, 21.9%) than following oral AG-221 administration (41800 ng h/mL, 27.2%).
The geometric mean (geometric CV %) absolute BA (Fabs) using the IV infusion treatment as the reference treatment was calculated as 57.2% (± 10.8%). The time to peak plasma AG-221 concentration (Tmax) (median, range) was achieved at the end of infusion (0.117 h, range = 0.117–0.5 h). Following oral administration, the drug was steadily absorbed and achieved Cmax at 3.01 h (range = 3.0–24.0 h). The plasma AG-221 terminal half-life (t1/2) after oral administration (29.0 h) was comparable to that following IV administration (28.3 h). The CL/F of AG-221 following oral administration was 2.39 L/h vs. 1.37 L/h following IV administration, which is approximately 1/60 of the liver blood flow in a typical 70-kg human subject, indicating a low first-pass extraction ratio for AG-221. The Vz/F of AG-221 following oral administration was 100 L vs. 55.8 L for the Vss following IV administration, indicating that AG-221 is readily distributed out of the vasculature.
Of note, the intersubject variability of clearance expressed as the CV% of the geometric mean was 27.2 and 21.9% for CL/F and CL from oral and IV administration, respectively, and the intersubject variability of absolute BA expressed as the CV% of the geometric mean was 10.8%, indicating the relatively high accuracy and precision of the AMS bioanalytical methodology. Consistent with this, individual dose-normalized AUC0–inf values for oral and IV administration were relatively tightly clustered, suggesting relatively low intersubject variability, as illustrated in Fig. 4.
Safety
Six subjects were given the study drug AG-221 as a single 100-mg coated tablet; 4 h later, all 6 subjects were also given an IV dose of 100 µg of AG-221 containing approximately 300 nCi of [14C]-AG-221. The 100-mg AG-221 film-coated tablet plus 100 µg IV [14C]-AG-221 were well tolerated. Overall, 4 of 6 subjects (66.7%) reported 7 TEAEs and 1 of 6 subjects (16.7%) reported 1 TEAEs that were suspected of being related to the study drug. All TEAEs were mild in severity. There were no deaths reported, and no subjects experienced a SAE or discontinued due to TEAEs.
The most commonly reported TEAEs overall were general disorders and administration site conditions, with a total of 4 TEAEs reported by 3 subjects. There were 4 TEAEs related to conditions at the vessel puncture site and 1 TEAE of headache.
Sporadic out-of-range values occurred in multiple subjects on various clinical laboratory measurements. There were no clinically significant laboratory abnormalities, and no clinical laboratory values were reported as a TEAE. No marked changes in mean laboratory values over time or appreciable trends overall or within subjects were observed during the study.
Discussion
Based on the guidance from the US FDA [11], from a PK perspective, the relative fraction of an extravascularly administered dose that is absorbed into the systemic blood circulation for a drug in a given formulation compared to that for a suspension, solution, or IV dose form can be estimated from relative/absolute BA studies. Insightful PK information on distribution, elimination, the effects of formulation excipients on the absorption of the drug, dose linearity, and proportionality in the PK of active ingredients and (where appropriate) inactive ingredients can be obtained directly from data collected from BA studies. In addition, useful indirect information that is related to the properties of a drug substance before it enters the systemic blood circulation and plays an important role in the design and selection of formulations, such as the permeability, the rate of absorption, the in vivo dissolution profile, the in vivo disintegration profile for a given dosage form, and the influence of pre-systemic first-pass metabolizing enzymes and/or transports (such as P-glycoprotein, BCRP, etc.), can be further explored based on BA data. Therefore, the determination of the absolute BA in order to evaluate a drug’s biopharmaceutic properties and to establish its pharmacology profile is recommended by various health authorities [12,13,14].
Traditionally, a crossover study design using either clinically relevant doses or doses that can provide sufficient plasma exposure to permit adequate characterization of the PK profiles is used to determine the absolute BA of a drug administered through a non-IV route versus an IV route [13]. Due to advances in mass spectrometry, the emergence of the concept of a microdosing/microtracer study has introduced an alternative approach for assessing the absolute BA [15,16,17,18,19]. In a microdosing/microtracer study, the subjects are administered a sub-pharmacologically active dose and blood/plasma samples are collected and analyzed for the parent drug or its metabolites. This approach first appeared in the late 1990s as a method of assessing human PK prior to full phase 1 clinical trials [15]. The sub-pharmacological dose administered in a human microdosing study normally leads to low drug plasma concentrations, so sensitive analytical technologies are needed to perform the requisite measurements over an appropriate time. For this reason, drugs are labeled with 14C, and AMS is used as it is a very sensitive analytical technique, capable of detecting drug concentrations in the femtogram to attogram (10−15 to 10−18 g) range [15]. The advantages of the 14C-microtracer approach have been well described. First, as compared to the traditional crossover study approach, a stable IV formulation and associated toxicology packages are not required by regulatory agencies [15]; secondly, the potential intrasubject variability from crossover studies is eliminated [13]. In addition, the microtracer approach provides accurate plasma concentrations from IV and extravascular doses, and allows researchers (i.e., PK, pharmacodynamics, and DMPK scientists) to obtain more detailed information about the behavior of the drug in humans (i.e., exposure and elimination) [10, 13, 20,21,22].
The primary objective of this phase 1, open-label study was to evaluate the absolute BA of AG-221 following a single oral dose of 100 mg AG-221 and an IV dose of ~ 100 μg AG-221 containing approximately 300 nCi of [14C]-AG-221 using the microtracer approach. The advantage of this approach is that accurate plasma concentrations from the IV dose (i.e., 0.1% of the oral dose of AG-221) can be determined by sensitive AMS and LC–MS/MS assays, thereby rendering the traditional crossover study design to determine the Fabs of AG-221 as a tablet formulation unnecessary. In healthy subjects under fasting conditions, the geometric mean absolute BA following oral administration of a 100-mg AG-221 tablet was 57.2%, indicating that AG-221 is well absorbed in humans. The results from this study suggest that AG-221 undergoes limited first-pass metabolism with low clearance. These results are consistent with data from a human mass balance ADME study, which showed that the recovery of total [14C] radioactivity over the 504-h post dose period was ~ 82% of the administered dose. The major route of AG-221 elimination was through the feces (~ 73.0% of the radioactive dose), while urinary excretion was found to be only a minor elimination pathway (~ 8.4% of the radioactive dose). AG-221 was the most predominant component in the systemic blood circulating.
Data from the current absolute BA study provided helpful insights into the hepatic extraction of AG-221 (first-pass effect). The geometric mean systemic plasma clearance of AG-221 following the IV administration of a 100-μg dose was 1.37 L/h and the geometric mean plasma renal clearance of TRA in the ADME study was 0.0875 L/h. The partitioning of AG-221-related radioactivity in blood cells is limited, as reflected by a mean blood-to-plasma ratio of radioactivity ranging from approximately 0.38 to 0.81 from 0.5 to 672-h post-dosing. Therefore, assuming a well-stirred model and that AG-221 is eliminated through hepatic pathways, considering the typical hepatic blood flow of 87 L/h, the hepatic extraction ratio of AG-221 was small, suggesting a low first-pass effect for AG-221. Data from the current absolute BA study also provided helpful insights into the absorption profile of AG-221. The fraction of the AG-221 dose absorbed, assessed based on the absolute BA and the hepatic extraction of AG-221, was at least 50%.
Finally, subjects who received both oral and IV administration of AG-221 showed good safety profiles. All TEAEs were of mild severity and resolved by the end of the study. There were no SAEs, and no subject withdrew from the study due to a TEAE. Drug-related TEAEs consisted of gastrointestinal disorders and headache. No clinically significant changes or findings were noted in clinical laboratory evaluations, vital sign measurements, or ECGs.
A limitation of the current study is that the absolute BA of the AG-221 tablet was assessed in healthy volunteers. Although a study in healthy volunteers is likely to produce less PK variability than that in the intended patient population with potentially confounding factors such as underlying and/or concomitant disease and concomitant medications, pharmacological data collected from healthy subjects may not translate exactly to the intended patient population.
Conclusions
Based on the results from the microdosing study, the mean absolute BA after a 100-mg oral dose of AG-221 is 57.2%. While the total clearance is 1.37 L/h, ~ 1/60 of the liver blood flow in a typical 70-kg human subject, the first-pass extraction is estimated to be less than 2%, assuming the total clearance is by liver metabolism. Thus, the fraction of the AG-221 dose absorbed is at least 50%. AG-221 was safe and well tolerated when given under fasted conditions as a single 100-mg dose, either as an oral solution or as a coated tablet with a microtracer [14C]-AG-221 solution, with few drug-related TEAEs reported. There were no clinically significant changes or findings noted in clinical laboratory evaluations, vital sign measurements, or ECGs during this study.
References
Dogra R, Bhatia R, Shankar R, Bansal P, Rawal RK. Enasidenib: first mutant IDH2 inhibitor for the treatment of refractory and relapsed acute myeloid leukemia. Anticancer Agents Med Chem. 2018;18(14):1936–51.
Myers RA, Wirth S, Williams S, Kiel PJ. Enasidenib: an oral IDH2 inhibitor for the treatment of acute myeloid leukemia. J Adv Pract Oncol. 2018;9(4):435–40.
Pollyea DA, Tallman MS, de Botton S, Kantarjian HM, Collins R, Stein AS, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019. https://doi.org/10.1038/s41375-019-0472-2
Celgene Corporation. Package insert for enasidenib. Summit, NJ: Celgene Corporation; 2017.
Click ZR, Seddon AN, Bae YR, Fisher JD, Ogunniyi A. New Food and Drug Administration-approved and emerging novel treatment options for acute myeloid leukemia. Pharmacotherapy. 2018;38(11):1143–54.
Talati C, Sweet K. Recently approved therapies in acute myeloid leukemia: a complex treatment landscape. Leuk Res. 2018;73:58–66.
Li Y, Connarn JN, Chen J, Tong Z, Palmisano M, Zhou S. Modeling and simulation of the endogenous CYP3A induction marker 4β-hydroxycholesterol during enasidenib treatment. Clin Pharmacol. 2019;11:39–50.
Li Y, Liu L, Gomez D, Chen J, Tong Z, Palmisano M, et al. Pharmacokinetics and safety of enasidenib following single oral doses in Japanese and Caucasian subjects. Pharmacol Res Perspect. 2018;6(6):e00436.
Tong Z, Atsriku C, Yerramilli U, Wang X, Li Y, Reyes J, et al. Absorption, distribution, metabolism and excretion of an isocitrate dehydrogenase-2 inhibitor enasidenib in rats and humans. Xenobiotica. 2019;49(2):200–10.
Shaik N, Hee B, Liang Y, LaBadie RR. Absolute oral bioavailability of glasdegib (PF-04449913), a smoothened inhibitor, in randomized healthy volunteers. Clin Pharmacol Drug Dev. 2019. https://doi.org/10.1002/cpdd.692
US FDA. Bioavailability studies submitted in NDAs or INDs—general considerations. Silver Spring, MD: US FDA; 2019.
European Medicines Agency. Pharmacokinetic studies in man. Guideline. Amsterdam: European Medicines Agency; 1987.
Boulton DW, Kasichayanula S, Keung CF, Arnold ME, Christopher LJ, Xu XS, et al. Simultaneous oral therapeutic and intravenous 14C-microdoses to determine the absolute oral bioavailability of saxagliptin and dapagliflozin. Br J Clin Pharmacol. 2013;75(3):763–8.
Center for Drug Evaluation and Research. Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. Guidance for industry. Silver Spring, MD: US FDA; 2000.
Lappin G, Noveck R, Burt T. Microdosing and drug development: past, present and future. Expert Opin Drug Metab Toxicol. 2013;9(7):817–34.
Burt T, Yoshida K, Lappin G, Vuong L, John C, de Wildt SN, et al. Microdosing and other phase 0 clinical trials: facilitating translation in drug development. Clin Transl Sci. 2016;9(2):74–88.
Sudo K. Microdosing for reduction of the time and resources for drug development. Drug Metab Pharmacokinet. 2007;22(5):327.
Ings RM. Microdosing: a valuable tool for accelerating drug development and the role of bioanalytical methods in meeting the challenge. Bioanalysis. 2009;1(7):1293–305.
Burt T, Vuong LT, Baker E, Young GC, McCartt AD, Bergstrom M, et al. Phase 0, including microdosing approaches: applying the three Rs and increasing the efficiency of human drug development. Altern Lab Anim. 2018;46(6):335–46.
Vishwanathan K, So K, Thomas K, Bramley A, English S, Collier J. Absolute bioavailability of osimertinib in healthy adults. Clin Pharmacol Drug Dev. 2019;8(2):198–207.
Raje S, Callegari E, Sahasrabudhe V, Vaz A, Shi H, Fluhler E, et al. Novel application of the two-period microtracer approach to determine absolute oral bioavailability and fraction absorbed of ertugliflozin. Clin Transl Sci. 2018;11(4):405–11.
Cho YS, Lim HS, Han S, Yoon SK, Kim H, Cho YL, et al. Single-dose intravenous safety, tolerability, and pharmacokinetics and absolute bioavailability of LCB01-0371. Clin Ther. 2019;41(1):92–106.
Acknowledgements
We thank the participants of the study and the site staff who made this study possible.
Funding
This study was funded by and the journal’s rapid service fee was paid by Celgene Corporation. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Xiaomin Wang is an employee of and holds equity ownership in Celgene Corporation. Jian Chen is an employee of and holds equity ownership in Celgene Corporation. Josephine Reyes is an employee of and holds equity ownership in Celgene Corporation. Simon Zhou is an employee of and holds equity ownership in Celgene Corporation. Maria Palmisano is an employee of and holds equity ownership in Celgene Corporation. Yan Li is an employee of and holds equity ownership in Celgene Corporation.
Compliance with Ethics Guidelines
This study was conducted in accordance with the applicable US CFR governing the protection of human subjects, financial disclosure by clinical investigators, institutional review boards (IRBs), Investigational New Drug applications, applications for FDA approval to market a new drug, and radioactive drugs for certain research uses.
All procedures performed in studies involving human participants were approved by the Salus Institutional Review Board, Austin, Texas, USA. They were also in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. ICFs were obtained from all individual participants included in the study.
Trial Registration
ClinicalTrials.gov identifier, NCT02443168.
Data Availability
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8845160.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Wang, X., Chen, J., Reyes, J. et al. A Phase 1, Open-Label Study in Healthy Subjects to Evaluate the Absolute Bioavailability of AG-221 by a Microtracer Approach. Oncol Ther 8, 91–102 (2020). https://doi.org/10.1007/s40487-019-0097-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-019-0097-7