Abstract
Introduction
Stomatitis is a common and potentially dose-limiting adverse event of the mammalian target of rapamycin (mTOR) inhibitor therapy. To minimize dose reductions or treatment delays that may affect therapeutic outcomes, management includes patient education, pain management strategies, and drug treatment. The aim of this study was to evaluate the effectiveness of a topically-applied galenical preparation to minimize the impact of everolimus-associated oral mucositis in patients with advanced cancer.
Methods
Patients receiving everolimus plus exemestane for advanced breast cancer or everolimus alone for advanced renal cancer were eligible for inclusion. All patients were advised on procedures to maintain good oral hygiene and directed to use a dexamethasone-containing galenical preparation at the first signs of mucositis. Questionnaires were administered at baseline, and after cycles one, two, and three to evaluate the presence, duration, and intensity of oral mucositis.
Results
Of the 19 patients included in the study (mean age 66 years; 16% male), mucositis developed in 10.5%, 47.4%, and 52.6% of patients after the first, second, and third cycles of everolimus, respectively. The median time to development of mucositis was 18.0 days, and the median time to mucositis resolution was 30.0 days. After the first, second, and third cycles of therapy, 5.3%, 10.5%, and 10.5% of patients required interruption of everolimus therapy; however, no dosage reductions for mucositis were necessary.
Conclusions
Patient education and the provision of an effective galenical preparation can minimize the effect of mTOR inhibitor-related mucositis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The orally administered mammalian target of rapamycin (mTOR) inhibitor, everolimus, has clinical activity against a range of malignancies and is currently indicated for the treatment of advanced solid tumors, including renal cell carcinoma and hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer recurring or progressing after nonsteroidal aromatase inhibitor therapy [1–3]. Everolimus is generally well tolerated, with an adverse event profile consisting of manageable side effects such as rash, diarrhea, fatigue, infections, stomatitis, noninfectious pneumonitis, and metabolic abnormalities, mostly mild-to-moderate in severity [1, 2, 4, 5].
As everolimus has the potential for prolonged use, it is important that physicians are able to recognize, monitor, and effectively manage its adverse events to ensure patient adherence, optimize health-related quality of life, and minimize toxicity. Stomatitis, or more specifically, oral mucositis, is one of the most common and potentially dose-limiting adverse events associated with mTOR inhibitor use, including everolimus [6, 7], with an incidence as high as 60% reported in trials [1, 2, 8, 9]. mTOR inhibitor-related mucositis is distinct from typical chemotherapy-related stomatitis, often presenting as distinct, painful, ovoid, superficial, well-demarcated ulcers with a grayish-white pseudomembrane and accompanied by erythema, edema, a burning sensation, and occasional bleeding [8, 9]. The stomatitis normally develops on mobile, less keratinized mucosa (inner lip, ventral and lateral surfaces of the tongue, and the soft palate) and not on keratinized mucosa (hard palate, gums, and back of the tongue). Mucositis often develops in the first 2 weeks after starting therapy, and symptoms may resolve or level off at approximately 6 weeks [10, 11].
Part of the management strategy of mTOR-related oral mucositis includes patient education in oral hygiene, diet modifications, and pain management strategies. Interventions to prevent or treat mTOR inhibitor-induced mucositis may include a range of topical preparations, such as corticosteroids and anti-inflammatories, along with supportive treatments such as local anesthetics and topical antimicrobial agents [6, 7, 9, 10]. Nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen may be useful for pain relief in persistent mucositis, and systemic or intra-lesional treatment with high-dose corticosteroids may be necessary in severe or refractory cases. The use of weak or strong opioids is seldom necessary [6, 7, 9, 10, 12].
This retrospective study aimed to evaluate the impact of a dexamethasone-containing topical galenical for the management of oral mucositis related to the use of an mTOR inhibitor in patients with metastatic breast or renal cancer.
Methods
Patients and Treatment Plan
Eligible patients were receiving everolimus treatment for metastatic breast cancer or metastatic renal cancer between April 3, 2012 and March 17, 2015 at the Unit of Medical Oncology of the Hospital “Annunziata” of Cosenza, Italy. At this hospital, patients receiving everolimus attend a day service clinic dedicated to oral therapies, which allows constant monitoring of appropriate laboratory and clinical parameters and the early detection of toxicities. All patients receiving everolimus were advised on procedures to maintain good oral hygiene, such as using mouthwashes devoid of alcohol (e.g., sodium bicarbonate), flossing after every meal, the careful use of toothpaste and a soft bristle toothbrush, avoidance of products containing hydrogen peroxide, iodine, and derivatives of the thymus, and the avoidance of spicy, fatty or very salty foods. Patients were also educated on how to prevent possible outbreaks of infection (such as periodontal disease and granulomas).
To be included in the analysis, patients with breast cancer received everolimus if they (1) had metastatic disease; (2) were hormone receptor (estrogen receptor, progesterone receptor) positive; (3) had received two previous lines of chemotherapy; (3) had disease progression during or after previous treatment with a non-steroidal aromatase inhibitor; (4) had no symptomatic visceral metastases; (5) were menopausal; (6) had not received everolimus previously; and (7) had normal liver function. Patients with renal cancer were required to be already on treatment with everolimus for the indication of renal cancer.
All breast cancer patients had undergone one or more cycles of chemotherapy or hormonal therapy (not including exemestane). Patients with renal cell carcinoma were given treatment with everolimus after they had presented with disease progression during or after treatment with vascular endothelial growth factor (VEGF)-targeted therapy. Everolimus was prescribed at the standard dose of 10 mg orally once daily, which could be reduced in response to toxicity. Everolimus treatment was suspended only in the case of serious toxicity (≥grade 2). Patients with metastatic breast cancer who were receiving concomitant exemestane received everolimus 10 mg orally once daily plus exemestane 25 mg orally once daily.
Patients underwent a battery of complete blood chemistry tests with particular attention to complete blood count and cholesterol, triglycerides, and blood glucose levels. Patient evaluations to monitor cancer response/disease progression included clinical assessment, computed tomography, and ultrasound mammography.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Galenical Formulation
A topical galenical for the management of mucositis was prepared independently by the pharmacist at our hospital pharmacy according to the procedure and standards of the Italian edition of the European Pharmacopoeia of the Council of Europe, the Italian Society of Pharmacists, and Good Manufacturing Practice. The galenical formulation consisted of dexamethasone 0.024 g, sweet almond oil 1 g, rose honey 15 g, glycerol 15 g, and hydroxyethylcellulose 1.2 g. The resulting odorless, brown, semi-solid, non-sterilized, microbial-controlled preparation was manufactured for application to the oral mucosa. The preparation was stored in a container made of bakelite and kept refrigerated at 2–8 °C.
Patients were instructed to use the galenical preparation at the first appearance of oral mucositis; three times a day for the first 7 days, then twice daily for the next 7 days, then once a day for the following 2 weeks. Application was by a Garrison disposable (bandages, tongue depressors).
Study Endpoints
The primary objectives of the analysis were to evaluate the presence, duration and intensity of oral mucositis, both reported by the patient and evaluated by the clinician, during the first three cycles of therapy with everolimus, by assessing duration and severity of symptoms, and to evaluate the usefulness of the topical galenical for the management of oral mucositis in these patients undergoing treatment with everolimus.
Evaluation and Treatment of Mucositis
Data were collected using four specific questionnaire forms at predetermined time points during the first three cycles of everolimus treatment (see Supplementary Material for the four questionnaires used in the study). The written questionnaires were completed on cycle 1 day 1 (baseline), after the first cycle of everolimus treatment (cycle 1 day 8), and after the second and third (last observation) cycles. The questionnaires recorded patient demographics, characteristics of the patients at baseline (primary tumor, staging, metastatic site, performance status, line of treatment, presence/absence of mucositis, systemic analgesic therapy for background disease or mucositis-related pain), time to appearance and objective and subjective assessments of mucositis severity, time to resolution and/or reduction in grade of mucositis, any everolimus dose interruptions required, and the percentage of patients discontinuing everolimus due to mucositis.
Mucositis pain was assessed using the mTOR inhibitor-associated stomatitis (mIAS) scale, specifically developed to assess mTOR inhibitor-related stomatitis [13]. The scale comprises subjective (patient-rated) and objective (clinician-rated) classification criteria and includes duration of mTOR inhibitor-related ulceration and severity of associated pain, and persistence of lesions and pain. The subjective grading criteria range from grade 0 (no pain) to grade 3 (pain score of ≥6 on a 10-point scale). The objective grading criteria range from grade 0 (no visible lesion) to grade 3 (lesion/s persisting for ≥7 days). MTOR inhibitor dose-modification should be considered only when both subjective and objective assessments are grade 3, indicating persistent lesions with significant pain despite the use of analgesics or other palliative care [13].
Statistical Analysis
Statistical aggregate analysis of the collected data was used to describe the time course of mucositis development, severity and resolution, treatment interruptions due to mucositis, and the usefulness of the topical galenical for the management of mucositis pain and symptoms.
Results
Patient Characteristics
Data from 19 patients (mean age 66.1 years; 84.2% female) were analysed. The majority of baseline characteristics and previous treatments were balanced between the study population (Table 1). However, the primary tumor site was the breast in only the female patients included in the analysis (all were receiving everolimus plus exemestane) while the primary tumor site was the kidney in the three male patients (everolimus only). Metastatic site was bone (42.1%), viscera (31.6%) or both (26.3%). Everolimus was second- or third-line treatment in 79.0% of patients. The majority of men had an Eastern Cooperative Oncology Group (ECOG) performance status of 2, while most women had an ECOG performance status of 1. At baseline, 25.0% of women were receiving pain therapy, primarily with NSAIDs. No men were receiving analgesics at baseline.
Time Course of Mucositis Development and Resolution
On day 8, after the first cycle of everolimus, two patients (10.5%) had mucositis, which was moderately painful in one patient and severe in the other (Table 2), and with a mucositis degree of grade 2 in one patient and grade 3 in the other. After the second and third cycles, nine (47.4%) and 10 (52.6%) patients, respectively, had mucositis. Mucositis pain was assessed by patients as mild in two cases after the second cycle, while four and six patients after the second and third cycle had moderate mucositis-associated pain, and three and four patients had severe pain after the second and third cycles, respectively. Clinician-assessed grade 3 mucositis (visible oral and/or pharyngeal ulcerations persisting for at least 7 days) was observed in three patients after the second cycle and in four patients after the third cycle. Mucositis was grade 1 or 2 in the other cases (Table 2).
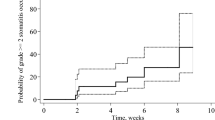
Patients received everolimus administered orally by continuous daily dosing. A Kaplan–Meier survival analysis showed that the estimated time to the development of mucositis was a mean of 29.6 (median 18.0) days (Fig. 1). A Cox regression analysis using gender, age, performance status, tumor size, node status and treatment line as covariates, found that only tumor size significantly influenced time to mucositis onset (Table 3): time to development of mucositis was significantly higher in patients with bigger tumor size (T = 4) than in patients with the smallest cancers. It must be noted here that, due to the small sample size, this latter analysis should be considered exploratory.
In one of the patients (50.0%) who had mucositis at day 8 of the first cycle, mucositis resolved during the second cycle of therapy (Fig. 2). All the patients with mucositis at the second cycle had the event resolve during the third cycle of therapy. The Kaplan–Meier survival analysis showed that the estimated time to resolution of mucositis was a mean of 31.4 (median 30.0) days (Fig. 2). The only patient who did not have resolution of mucositis during the third cycle of therapy experienced severe pain and grade 3 mucositis at day 8. No variation of mucositis severity was observed at the second cycle of therapy in this patient, but at the third cycle mucositis pain was decreased to moderate severity and the degree of mucositis was grade 2.
Interruption of Therapy
Only one patient (5.3%) required suspension of everolimus treatment on day 8 of therapy due to everolimus-induced mucositis. After each of the second and third cycles two patients (10.5%) had to suspend everolimus treatment. No everolimus dose reductions for mucositis were necessary.
Pain Therapy by Cycle
Systemic pain therapy for cancer-related pain was required by four patients (21.1%) during the first and second cycles and by three patients (15.8%) during the third cycle. Pain therapy for everolimus-induced mucositis was required by two patients (10.5%) during the first cycle, six patients (31.6%) in the second cycle and eight patients (42.1%) during the third cycle (Table 4). In all cases, NSAIDs were the only analgesics prescribed for mucositis-related pain.
Discussion
All patients undergoing everolimus therapy in our study were provided with the combination of standardized suggestions for the prevention of mucositis and the use of the galenical preparation, as part of the support offered by our outpatient clinic dedicated to effectively managing and monitoring the administration of oral therapies. This allowed not only the disease patterns of everolimus-related mucositis to be identified and responded to more readily but, most importantly, in almost all cases there was no need to interrupt everolimus treatment. When mucositis occurred, the use of a novel preparation formulated with a corticosteroid (dexamethasone) and rose honey enabled rapid resolution of the mucositis with minimal need for systemic analgesic use. No patient needed opioids for mucositis pain. In those patients who required analgesics for everolimus-related mucositis, common NSAIDs (nimesulide, ketorolac, aspirin) were all that were required to control pain.
As with other mTOR inhibitors, oral mucositis can be a significant dose-limiting adverse event of everolimus therapy. For example, in three recent trials of everolimus plus exemestane in women with advanced breast cancer, 39.8–56.0% of patients experienced at least one episode of everolimus-related mucositis, which was grade 3/4 in approximately 8% of patients [1, 14, 15]. This is similar to that reported in patients with renal cell carcinoma [2]. Dose reductions because of mucositis were necessary in over 20% of patients, and everolimus treatment interruptions for a median of 7–17 days were necessary in approximately 40–60% [1, 14, 15]. In contrast, no patient in our study had a mucositis-related dose reduction, and after the first, second, and third cycles of therapy, only 5.3%, 10.5% and 10.5% of patients required treatment interruptions.
Of interest, in an interim analysis of the BRAWO study, a large, German non-interventional study, which will ultimately enroll 3000 patients with advanced breast cancer receiving everolimus plus exemestane, recommendations on stomatitis prevention similar to those in our study were given to 86.8% of patients. A total of 39.8% of patients had at least one mucositis event, which was grade 2 in 17.0% and grade 3 in 3.4%. In addition to dose reduction or treatment interruption, a range of therapeutic interventions for mucositis were utilized, alone or in combination, including non-drug mouthwashes, cooling strategies such as sucking ice, and systemic or topical drug interventions (not further specified) [14].
The use of topical or systemic treatment with corticosteroids has been shown to be successful in resolving mucositis in the majority of patients with mTOR inhibitor-related mucositis [6, 7, 9, 10, 12, 16], and our findings show that early intervention with a dexamethasone-containing galenical preparation is effective in managing mucositis. By integrating appropriate instruction on good oral hygiene designed to prevent mucositis, effective monitoring for the development of mucositis, and the provision of an effective treatment for use at the first signs of mucositis into the management plan of all patients undergoing treatment with everolimus, treatment adherence can be improved to help ensure the best therapeutic outcome. Furthermore, in some, if not all cases, a galenical preparation such as that used in our institution will be more cost-effective than proprietary formulations.
Although the retrospective study design, small patient numbers and absence of a control group (due to the fact that all patients at our institution who are receiving everolimus are put on the program described to minimize the impact of mucositis) can be considered a limitation of our analysis, our standardized approach to instructing and supporting patients undergoing everolimus therapy is a study strength, and the specially-prepared galenical formulation significantly limited the negative effects of mucositis.
Conclusions
The dose-limiting effects of everolimus-related mucositis can be minimized by successfully educating patients about the importance of oral hygiene in combination with an effective medicinal product that can be manufactured in the hospital pharmacy or other galenical laboratory and provided to the patient to be used at the first signs of mucositis.
References
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65.
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23.
Soefje SA, Karnad A, Brenner AJ. Common toxicities of mammalian target of rapamycin inhibitors. Target Oncol. 2011;6:125–9.
Grünwald V, Karakiewicz PI, Bavbek SE, Miller K, Machiels JP, Lee SH, et al. An international expanded-access programme of everolimus: addressing safety and efficacy in patients with metastatic renal cell carcinoma who progress after initial vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapy. Eur J Cancer. 2012;8:324–32.
Boers-Doets CB, Epstein JB, Raber-Durlacher JE, Ouwerkerk J, Logan RM, Brakenhoff JA, et al. Oral adverse events associated with tyrosine kinase and mammalian target of rapamycin inhibitors in renal cell carcinoma: a structured literature review. Oncologist. 2012;17:135–44.
Boers-Doets CB, Raber-Durlacher JE, Treister NS, Epstein JB, Arends AB, Wiersma DR, et al. Mammalian target of rapamycin inhibitor-associated stomatitis. Future Oncol. 2013;9:1883–92.
Sonis S, Treister N, Chawla S, Demetri G, Haluska F. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer. 2010;116:210–5.
Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transpl Rev (Orlando). 2014;28:126–33.
Aapro M, Andre F, Blackwell K, Calvo E, Jahanzeb M, Papazisis K, et al. Adverse event management in patients with advanced cancer receiving oral everolimus: focus on breast cancer. Ann Oncol. 2014;25:763–73.
Rugo HS, Pritchard KI, Gnant M, Noguchi S, Piccart M, Hortobagyi G, et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2. Ann Oncol. 2014;25:808–15.
Peterson ME. Management of adverse events in patients with hormone receptor-positive breast cancer treated with everolimus: observations from a phase III clinical trial. Support Care Cancer. 2013;21:2341–9.
Boers-Doets CB, Lalla RV. The mIAS scale: a scale to measure mTOR inhibitor-associated stomatitis. Support Care Cancer. 2013;21:S140.
Fasching PA, Decker T, Schneeweiss A, Uleer C, Förster F, Wimberger P, et al. Breast cancer treatment with everolimus and exemestane for ER+ women: results of the 2nd interim analysis of the non-interventional trial BRAWO [Poster]. Ann Oncol. 2014;25:LBA9.
Jerusalem G, Mariani G, Ciruelos EM, Martin M, Tjan-Heijnen VCG, Neven P, et al. Everolimus in combination with exemestane in hormone receptor-positive locally advanced or metastatic breast cancer (BC) patients progressing on prior non-steroidal AI (NSAIs): Ballet study (CRAD001YIC04) [Poster]. Cancer Res. 2015;75:P5-19-02.
Grünwald V, Weikert S, Pavel ME, Horsch D, Luftner D, Janni W, et al. Practical management of everolimus-related toxicities in patients with advanced solid tumors. Onkologie. 2013;36:295–302.
Acknowledgments
No funding or sponsorship was received for this study. Sponsorship for article processing charges was provided by Novartis Farma, Origgio, Italy. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The authors thank Valentina Mirisola of Medi Service, Genoa, for assistance with the statistical analyses of the study results, Ray Hill, an independent medical writer, who provided medical writing support on behalf of Springer Healthcare Italia, and Simone Boniface, of Springer Healthcare Communications, who edited the manuscript post-submission. This support was funded by Novartis Farma, Origgio, Italy. SC planned the study; SM collected the data; LC and SB manufactured and stored the galenical preparation; ST, EV and SP contributed to data interpretation. All the authors critically revised the manuscript drafts and read and approved the final version of the paper.
Disclosures
S. Conforti, S. Minardi, L. Conforti, S. Turano, S. Bilotta, E. Vilardo and S. Palazzo have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/3BF6F06059C477FC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Conforti, S., Minardi, S., Conforti, L. et al. Topical Application of a Galenical Formulation for the Management of Everolimus-Induced Mucositis in Patients with Metastatic Cancer: a Retrospective Study. Oncol Ther 4, 275–286 (2016). https://doi.org/10.1007/s40487-016-0032-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-016-0032-0