Abstract
Oropharyngeal cancers caused by human papillomaviruses (HPV) have a different epidemiology, prognosis, genetic mutational landscape, response to treatment, and outcome when compared to HPV-negative cancers. In this review, a summary of our current understanding of HPV in head and neck cancer and the important advances that have shown HPV to be an etiological agent are discussed. HPV-positive and HPV-negative tumors are compared discussing clinicopathological factors, prognosis, outcome following treatment, and the molecular and genetic differences. Currently, the standard of care for oropharyngeal cancer is both surgery and post-operative radiotherapy with or without cisplatin or concurrent chemo-radiotherapy. The latter is used more often, especially in cancers of tonsil and base of tongue. However, there is increased interest in trying to de-intensify treatment and in the development of new treatments to target the underlying different molecular pathways of HPV-positive cancers. The current clinical trials involving surgery, chemotherapy, and radiation therapy are discussed. The new targeted treatments are also summarized. Although there is currently is no evidence from prospective studies to support a change in the treatment algorithm, the treatment options for patients with HPV-positive disease are likely to change in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
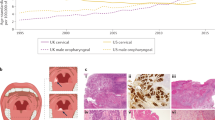
Squamous cell carcinoma (SCC) is the most common histological cancer type to affect the mucosal surfaces of the upper aero-digestive tract, accounting for 89% of cancer types [1]. Whilst the incidence of SCC in most of the major sites in the head and neck have reduced or remained static, the incidence of oropharyngeal SCC has increased [2]. In a United States (US) study examining data from the Surveillance, Epidemiology, and End Results (SEER) programme, the incidence of oropharyngeal cancers increased by 1.3% for tongue base cancers and by 0.6% for cancers of the tonsil for each year between 1973 and 2004 [3]. In contrast, the incidence of oral cavity cancers has declined by 1.9% every year during this period [3]. This has been seen worldwide, particularly in the developed world [4]. This is demonstrated in Fig. 1, adapted from the data supplement from Chaturvedi et al. [4].
The net drift percentage (net drift represents the net sum of the linear trend in period and cohort effects from age-cohort-period models) in oropharyngeal and oral cavity cancers among men stratified by age (1983–2002) for selected countries [4]. Black square oropharynx, white square oral cavity. Adapted with permission from Chaturvedi et al. [4]
Head and neck SCC has been associated with tobacco smoking, and the cancer incidence in these anatomical sites has mirrored smoking rates [5]. There has been a decreasing trend in tobacco usage in the developed world and decreasing rates of head and neck cancer in all mucosal sites, except for the oropharynx [6].
Human papillomavirus (HPV) was first identified as a possible etiological agent in oral SCC in 1983 [7]. The oncogenic potential of HPVs in squamous epithelium has been understood for many years following work in uterine cervix and ano-genital squamous cell cancers [8]. A causal link between HPV and oropharyngeal cancer was shown in a study by Gillison et al. [9], which added further support to the epidemiological and molecular evidence for HPV as the etiological factor in the increasing incidence of oropharyngeal SCC [10]. The International Agency for Research on Cancer in 2007 added HPV type 16 as a cause of oropharyngeal carcinoma [11].
Importantly, oropharyngeal cancers due to HPV have a different epidemiology, prognosis, response to treatment, and outcome. The implications of this for therapy are under investigation, and this subset of patients may be able to undergo treatments with less toxicity. It may also allow for targeted therapies related to the different underlying molecular genotype. This review presents a summary of HPV related oropharyngeal carcinoma and highlights potential therapeutic options that may become available for these patients. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
HPV and Oropharyngeal SCC
HPVs comprise 150 small non-enveloped DNA viruses that have double-stranded and circular genomes [12]. They share a similar segment in their genome called the L1 gene that encodes for the major capsid protein. They can be divided into mucosal and cutaneous types. They are further classified into high-risk and low-risk viruses depending on their ability to induce cancer. HPV 16 is a high-risk virus that has been associated with up to 90% of HPV-related head and neck cancer in mucosal surfaces. The other HPV genotypes have a prevalence of less than 5% in oropharyngeal tumors [13, 14].
The life cycle of HPV viruses is closely related to the differentiation of the squamous cell it infects [12]. Initially the virus targets and infects the basal squamous cells at the deep aspect of the skin or mucosa. It will gain access after trauma or erosion of the superficial layers. A low virus genome copy count of approximately ten is maintained in these cells. The virus is able to take over the cell’s replication machinery [15] and maintain the cell's ability to synthesize DNA, which is usually lost as the cell differentiates. As the cell starts differentiation, the productive phase of virus replication is started, where up to 1000 viral genome copies are produced along with expression of the viral L proteins [12]. Mature virus progeny particles are then released into the uppermost layers of the epithelium.
The tonsil epithelium has a specialized morphology. The tonsil has multiple crypts, and these have specialized stratified squamous non-keratinizing epithelium and patches of reticulated sponge-like epithelium [16]. These perform a function in antigen recognition as part of the immune system [16]. HPV is thought to access the oropharyngeal mucosa via the tonsillar crypts [17], through the specialized porous membrane. The mechanism of viral entry into the cell is not fully understood, but is thought to involve α6β4 integrins [18] and cell surface heparin sulfate proteoglycans (HSPGs) [19]. Once a cell is infected, the natural history of infection is not well understood in the oropharynx. The proportion of infections that enter an acute, chronic, or latent phase or are cleared by the host’s immune system is not clear [12]. However, it is thought that most oral HPV infections are cleared within a year [20].
A prevalence study in the US as part of the National Health and Nutrition Examination Survey (NHANES) in 2009–2010 [21] showed the prevalence of oral HPV infection among men and women aged 14–69 years was 6.9% and of HPV type 16 was 1.0%. A more recent systematic review showed a prevalence of 4.5% in 4070 subjects for any HPV type [22] and prevalence of 1.3% for HPV 16. The implication of HPV 16 infection was shown in a case control study in which an odds ratio of 14.6 [95% confidence interval (CI), 6.3–36.6] was seen in patients with newly diagnosed oropharyngeal cancer compared to cancer-free controls [23].
Following infection with HPV there is a latency period and a stepwise progression towards genomic instability [12] before cancer can develop. The viral genes and proteins E6, E7, and also E5 are involved in carcinogenesis. The key step is the integration of the viral genes into the host genome usually as an epitome [24].
There are two common molecular mechanisms by which the virus causes genomic instability. Firstly, the E6 protein binds to host cells’ p53 and with the cellular ubiquitin ligase E6-associated protein (E6AP) causes degradation of the cellular p53 protein [25]. This impairs cellular apoptosis providing a step towards allowing cells to become immortalized.
Secondly, the E7 protein binds and inactivates the retinoblastoma (Rb) protein. Rb regulates the activity of E2F (a transcription factor that regulates cell cycle progression) [24]. By inactivating Rb, levels of E2F are increased which promotes cell cycle progression. This occurs by allowing increased expression of p16, a cyclin-dependent kinase inhibitor that functions as a checkpoint inhibitor [26]. Further investigation has shown both of these proteins to be multifunctional [27]. The E6 protein has α-helix-binding partners and PDZ-binding partners, which have been shown to contribute to tumorigenesis in other tissues and are likely to be implicated in head and neck cancer carcinogenesis [27]. These protein interactions have been reported to involve at least 30 different cellular substrates [28], with effects on polarity/tumor suppression, signal complex scaffolds, TGF-β signaling, PI3K/AKT signaling, tight junction assembly, and a number of other cellular functions.
Both the detection of HPV DNA and the identification of p16 as a surrogate for infection have been correlated with outcome in these patients [29]. The E5 protein is thought to increase epidermal growth factor receptor (EGFR) recycling to the cell surface, and this has been seen in cervical cancers [30], but its role in head and neck cancer is yet to be defined.
There are a number of different ways to detect HPV infection, but there is no worldwide consensus on which is best. Each detection method has its own associated strengths and weaknesses, and methods vary throughout the world [31]. The main aim is to identify transcriptionally active high-risk HPV. Methods vary from routine histology, polymerase chain reaction (PCR) for viral DNA or RNA, in situ hybridization to immunohistochemistry. The identification of high-risk HPV DNA and the over-expression of p16 (a surrogate marker for infection) are important in diagnosing HPV-related tumors [32].
Comparison of HPV-Positive and HPV-Negative Tumors of the Oropharynx
Clinicopathological Characteristics
Following the application of highly sensitive HPV detection methods and a rigorous definition of active HPV transcription, the vast majority of HPV-positive tumors have been shown to be located in the oropharynx, mostly in the tongue base and tonsil [31], and rarely in other head and neck sites. The correlation of HPV-related tumors and high-risk sub-sites in Waldeyer’s ring is an important and significant difference between HPV-positive and HPV-negative tumors. Two large meta-analyses have shown HPV infection is strongly associated with tonsillar and base of tongue sites [33, 34]. However, it is important to understand that the testing method can be a source of heterogeneity when comparing studies [33]. In contrast, HPV-negative tumors do not have a predilection to a particular sub-site in the head and neck [35] and field cancerization is more common.
In modern series, approximately 50% of oropharyngeal cancers have detectable HPV DNA on testing [36], with some reporting higher rates, depending on the studied population. In a United Kingdom (UK) series, HPV-related cancers were identified in 70% of the oropharyngeal tumors [37]. The high-risk HPV 16 subtype is found in 90% of these cancers with other high-risk subtypes 31, 33, and 18 identified in the other cases [36]. In comparison, transcriptionally active high-risk HPV DNA is rarely seen in other sites in the head and neck and in oropharyngeal sub-sites such as the soft palate and posterior pharynx [37].
The investigation of population-level data has been possible due to the difference in tumor sub-sites between HPV-positive and HPV-negative cancers [3]. In a 2008 analysis of SEER data from 1973 to 2004, patients with tumors that were likely to be HPV-positive by sub-site were significantly younger, 61.0 versus 63.8 years, P < 0.001 [3]. This was also seen in a series of 193 patients, in which patients with HPV-positive cancers on DNA PCR were significantly more likely to be less than 55 years old [38]. Age was also identified as an important difference between HPV-positive and HPV-negative tumors in a study of clinical correlates from Sweden [39]. Patients with HPV-related cancers, identified by DNA PCR, were younger, with a mean age of 59 years (range 42–78) compared to patients with HPV-negative tumors, who had a mean age of 66 years (range 45–89).
Overall population trends in the US SEER data have shown the incidence of oropharyngeal squamous cell cancer is higher in men compared to women and more common in black people compared to other races [3]. However, when HPV-related cancers are examined these trends are different. HPV-related oropharyngeal cancers have been seen specifically in white men and an increasing incidence trend for men compared to women in other races [3]. There is also an association between educated middle class patients and HPV-positive cancers [38].
Tobacco use is a well-known risk factor for head and neck cancer and has a synergistic effect with alcohol [40]. They have been identified as major risk factors for head and neck and oropharynx carcinoma in a large multicenter consortium study including 25,500 patients [41]. However, studies investigating patients with HPV-positive tumors report patients are more likely to be non-smokers [42], and overall tobacco use is lower compared to patients with HPV-negative tumors. Cohorts of patients with HPV-positive tumors consist of about 30% nonsmokers compared with less than 5% in the HPV-negative groups [43]. This relationship has been reported in many studies [44]. However, smoking in patients with HPV 16 infection has recently been examined in the NHANES survey in the US [45]. In the 6887 participants, bivariable analysis reported tobacco use was associated with HPV 16 infection. Therefore, there is more to understand about the interaction of tobacco and HPV infection. The effect of tobacco use on prognosis and outcomes is discussed below.
Infection of the uterine cervical and ano-genital region with high-risk HPV is transmitted through sexual contact [8]. Therefore, the mode of infection of high-risk HPV subtypes in the oropharynx has been investigated with regard to sexual behaviors. The transmission of oral HPV is not fully understood [20], but there is strong evidence for sexual transmission. In this hospital-based case–control study of 240 patients with head and neck cancer and 322 controls, self-reported sexual behaviors were associated with HPV-related cancers. The increasing numbers of lifetime vaginal or oral sex partners, participation in casual sex, infrequent use of barrier protection during vaginal or oral sex, and having had a sexually transmitted disease in the past was associated with HPV-positive tumors. In contrast, no sexual behavior was associated with patients who had HPV-negative cancers of the head and neck.
In a case–control trial of 100 patients with oropharyngeal cancer and 200 non-cancer controls, multivariate logistical regression was used to identify risk factors. It found that more than 26 sexual partners and more than six oral sexual partners were independent risk factors for HPV-related oropharyngeal cancer [23]. Further support for a sexual exposure etiology is found in the increasing incidences of herpes simplex 1 and 2 genital infections and genital wart infections in recent birth cohorts. These are observed as surrogate markers for oral sex, risky sexual behavior, and HPV exposure [46].
Clinical presentations are also different in HPV-related oropharyngeal cancers. They present with smaller primary tumor lesions, but larger and cystic cervical nodal disease [47]. Therefore, patients often present with nodal disease, without typical head and neck cancer risk factors. The primary tumor is often a low T stage [48] and can be small or not detectable by clinical examination or radiographic investigation. The cystic nodal masses can cause errors in sampling with malignant cells in solid components of the metastasis being missed.
There are also differences in the histopathology between HPV-positive tumors and HPV-negative tumors. HPV-positive tumors are more likely to be non-keratinizing and undifferentiated. In a study of 253 tumors a basaloid or poorly differentiated SCC subtype was associated with viral genome in the tumors cells [9]. However, the more aggressive histological features of HPV-related carcinomas are not related to prognosis or outcomes following treatment.
Comparison of Outcomes
Survival and therapeutic response was prospectively studied as part of the Eastern Cooperative Oncology Group (ECOG) 2399 protocol in which patients with stages III and IV head and neck cancers were treated with induction chemotherapy [49]. Following assessment of response, responders received chemo-radiation and non-responders received either surgical resection or chemo-radiation. Patients with HPV-positive disease had a higher response rate to induction chemotherapy and chemo-radiotherapy. They also had a better overall survival at two years of 95% (95% CI 87–100%) compared with 62% (95% CI 49–74%) in HPV-negative tumors [49].
There has been a large number of retrospective studies demonstrating patients with HPV-positive tumors of the oropharynx have a better prognosis than patients with HPV-negative tumors [50]. However, most of these are small or the study design does not allow control for confounding factors. As previously discussed, patients with HPV-positive tumors tend to be younger, healthier, non-smokers, and from educated backgrounds.
The landmark study, performed as part of the Radiation Therapy Oncology Group (RTOG) 0129 trial planned to assess potential confounders by including a larger number of participants so additional factors could be controlled for [51]. HPV status was identified as the major independent determinant of overall survival after controlling for age, race, tumor and nodal stage, tobacco exposure, and treatment assignment [51]. At 3 years, there was a 58% reduction in the risk of death (hazard ratio 0.42, 95% CI 0.27–0.66) between patients with HPV-positive tumors and HPV-negative tumors. This has been reproduced in a number of other studies [29, 33, 49, 52]. Figure 2 shows the Kaplan–Meier curve for overall survival stratified by HPV status from this study [49]. The use of tobacco was also identified as an independent factor in the prognosis of patients with HPV-positive and HPV-negative tumors. Smoking has also been correlated with a worse outcome in other studies [49]. However, each additional pack-year was seen to decrease survival and following the incorporation of the tumor, node, metastasis (TNM) stage with HPV status, and smoking history stratification was possible. Low-, intermediate, and high-risk groups were identified [51]. Low-risk patients include HPV-positive patients with either less than 10 smoking pack-years or more than 10 smoking pack-years, but N0–N2a nodal disease. The intermediate group consists of patients with HPV-positive patients with more than 10 smoking pack-years and N2b–N3 nodal disease and patients with HPV-negative tumors with less than 10 smoking pack-years and T2–3 primary tumors. The high-risk group includes patients with HPV-negative tumors with less than 10 smoking pack-years, but T4 primary tumors or had more than 10 smoking pack-years. Figure 3 shows a summary of the overall survival for these groups [53].
Risk classification for oropharynx cancer according to HPV status for OS [53]. Low-risk patients include HPV positive patients with either less than 10 smoking pack-years or more than 10 smoking pack-years, but N0–N2a nodal disease. The intermediate group consists of patients with HPV-positive patients with more than 10 smoking pack-years and N2b–N3 nodal disease and patients with HPV negative tumors with less than 10 smoking pack-years and T2–3 primary tumors. The high-risk group includes patients with HPV-negative tumors with less than 10 smoking pack-years, but T4 primary tumors or had more than 10 smoking pack-years. HPV human papillomavirus, OS overall survival. Reproduced with permission from Chau et al. [53]
This trend has been shown in meta-analysis of studies worldwide. In a meta-analysis reporting on 5681 patients, the prevalence of HPV tumors was 22%, and this was associated with an improved survival with a hazard ratio of 0.42 (95% CI 0.27–0.57) [29].
Factors that Predict Outcome
The implication of HPV status on outcome is evolving. The traditional TNM classification system [54] has been reported to be less effective in predicting cancer-specific mortality in oropharynx cancers [55]. Prognostic factors used in HPV-negative tumors such as margin status, lymphovascular invasion, pN status, and extra-capsular spread were not predictive in HPV-positive tumors in a study reported by Iyer et al. [56]. The prognostic factors found in patients that were treated with initial surgery and then postoperative radiotherapy are shown in Tables 1 and 2. It shows distinct differences between patients with HPV-positive tumors and HPV-negative tumors. The Kaplan–Meier survival curves are also shown in Fig. 4.
Kaplan–Meier plots showing impact of prognostic factors on DSS in p16-positive and p16-negative patients. a pT classification, b pN classification, c margin status, d ECS. 5-year DSS and P values based on log rank test [56]. DSS disease-specific survival, ECS extra-capsular spread. Reproduced with permission from Iyer et al. [56]
Molecular (Genomic) Comparison
The reason why HPV-positive and HPV-negative cancers behave differently is due to the difference in the mutational landscape of these cancers. In two studies performing whole sequencing of exons (all known protein-encoding genes) in head and neck cancers published in 2011 [57, 58], dominant roles were seen for tumor-suppressor pathways including p53, Rb/INK4/ARF, and Notch in disease pathogenesis. However, only a small number of oncogene-activating mutations were identified. The vast majority of the tumors in these studies were HPV negative (28/32 and 80/92). These studies found fewer genes were mutated per tumor in the HPV-associated tumors as compared with those tumors not related to HPV [57].
TP53 mutations were not identified in any of the HPV-positive tumors in one study [57], but were seen in 78% of HPV-negative tumors. There was also an increased number in mutations in tobacco-related cancers compared to non-tobacco-related cancers.
The Cancer Genome Atlas Network (TCGA) recently published findings on the assessment of 279 patients with head and neck cancer, assessing for somatic genomic alterations [59]. This study has shown a difference between HPV-positive tumors and HPV-negative tumors. Helical domain mutations of the oncogene PIK3CA, novel alterations involving loss of TRAF3 and amplification of the cell cycle gene E2F1 are seen in HPV-positive tumors. In comparison, HPV-negative tumors demonstrated loss-of-function TP53 mutations and CDKN2A inactivation with copy number alterations of 3q26/28 and 11q13/22. Figure 5 is a graphical description of the results of the TCGA study comparing gene alterations in 279 HPV-positive and HPV-negative tumors. In general mutations found in head and neck cancers involve tumor-suppressor genes rather than oncogenes. This makes targeting a specific pathway more difficult.
A graphical description of the results of The Cancer Genome Atlas (TCGA) Network study comparing somatic alterations and altered protein expression that represent plausible therapeutic targets in HPV-positive and negative tumors [59]. Important genes are shown with their associated alteration (key below graph depicts gene aberration). HPV-positive tumors showed loss of TRAF3, activating mutations of PIK3CA, and amplification of E2F1. HPV-negative tumors contained amplicons on 11q with CCND1, FADD, BIRC2, and YAP1, or concurrent mutations of CASP8 with HRAS, targets for cell cycle death, and NF-kB. Reproduced with permission from [59]
The molecular alterations can be divided into the following pathways:
-
1.
p53 and pRb pathways (cell cycling/limitless replication). HPV-negative tumors have p53 mutations present in 86% of patients [59] and have an association with outcome [60], whereas only 3% of HPV-positive tumors have similar p53 mutations [59].
-
2.
EGFR pathway (the most studied growth factor signaling pathway in head and neck cancers). EGFR expression has been reported in more than 90% of tumors and high EGFR levels are associated with poor prognosis, but its role in head and neck cancer seems to be more complex than first thought [61]. Current data on the interaction of HPV status and EGFR expression is inconsistent [62] and EGFR pathway alterations were rarely seen in HPV-positive cancers [59].
-
3.
TGFb pathway (growth factor signaling). This is an inhibitor of growth pathways and through cellular SMAD proteins [portmanteau of mothers against decapentaplegic (MAD) and the Caenorhabditis elegans protein SMA, from genesma for small body size] controls a number of cell cycle-dependent kinase inhibitors [35]. It has also been linked with nuclear factor-κB, which provides an important survival signal to cells [63]. These have been linked with metastasis and invasion [64].
-
4.
PI3K–PTEN–AKT pathway (evading apoptosis). Activating mutations in PI3K as well as inactivating mutations of PTEN have been found, both of which lead to AKT activation. There are a number of downstream mediators such as MYC, mTOR, and MDM2 that are part of this pathway and they have been implicated in head and neck cancers [35]. These appear to feature in over 50% of HPV-positive tumors and in 30% of HPV-negative tumors [59].
Treatment Approaches in HPV-Positive Disease
There are major differences in the risk factors, demographics, clinical behavior, response to treatment, and molecular patterns of HPV-positive tumors compared to HPV-negative tumors [51, 56]. Treating these as different disease entities may allow more tailored treatment, limiting toxicity [65].
At present, HPV-positive and HPV-negative head and neck cancers are treated the same. Current treatment is based on the TNM stage of pathology, patient preferences, co-morbidity, and the treating physician’s experience [40]. The standard of care for oropharyngeal cancer is either surgery and post-operative radiotherapy with or without cisplatin or concurrent chemo-radiotherapy. The latter is used more often, especially in cancers of the tonsil and base of tongue [66, 67].
The approaches to treating patients with HPV-positive oropharyngeal carcinomas can be classified into:
-
1.
prevention;
-
2.
modification of current techniques;
-
3.
new targeted therapies.
Prevention using available vaccinations is discussed below. To identify the current clinical trials and treatments under investigation in HPV-positive tumors a search of the current trials database (https://clinicaltrials.gov/) was performed (April 30, 2015) using the search terms ‘HPV’ and ‘human papillomavirus’ in any field. Out of the 683 trials returned in the search, 46 trials were identified as related to head and neck cancers. These clinical trials are summarized into surgical trials, trials using modification of standard radiotherapy/chemotherapy techniques, and new agents and immune therapies.
Prevention
Prevention of a virally related malignancy can be divided into primary and secondary prevention [68]. Primary prevention methods focus on preventing persistent infection with HPV. Secondary prevention describes methods of early identification of pre-cancerous lesions or early stage cancers. The papilloma virus’ self-assembly of the L1 major capsid protein has allowed for a prophylactic HPV vaccine [69]. The vaccines generate neutralizing antibodies to the highly visible immunogenic target. There are two available vaccines, a bivalent HPV 16/18 vaccine (Cervarix®, GlaxoSmithKline Biologicals) and a quadrivalent HPV 6/11/16/18 vaccine (Gardasil®, Merck Sharp and Dohme). There have been a number of trials supporting the efficacy of these for women in uterine cervical, vaginal and vulvar related diseases [70]. The evidence for efficacy of vaccination in prevention of oropharyngeal disease is limited. A proof of concept study in Costa Rica made use of a randomized controlled trial (RCT) looking at effectiveness of vaccination in cervical HPV and tested at the end of the protocol for oral HPV infection in 5834 women [71]. A significant reduction in infection was found in the treatment arm compared to control. Further progress has been hampered by the limited uptake of vaccination and the different disease profile of HPV infection between the two sites. Cervical HPV infection is associated with age of sexual debut whilst the risk of oral HPV infection appears to last longer, requiring a prolonged immune response from vaccination. Further developments in this area included the development and production of new vaccinations targeting more HPV subtypes [72]. This new vaccine targets 6, 11, 16, and 18 and five additional oncogenic types 31, 33, 45, 52, and 58. Subtypes 31 and 45 [27] have been thought to have a small, but significant role in oropharyngeal cancers of non-HPV 16 type [13, 14].
Techniques for secondary prevention or screening have been investigated. The use of an oropharyngeal ‘pap smear’ equivalent was unfortunately not seen to be useful because cytological changes associated with dysplasia were not associated with HPV infection in patients without obvious lesions [73]. The use of HPV 16 E6 antibody serology as a blood test biomarker is under investigation. Although evidence of HPV 16 infection increases the likelihood of oropharyngeal cancer [74], a positive result has been seen in patients over 10 years before cancer development, questioning the usefulness of it in identifying treatable patients [75].
De-Intensification of Current Treatments
Surgical Trials for HPV-Positive Cancers of the Oropharynx
The use of surgery in HPV-positive oropharyngeal cancer has become focused on the application of minimally invasive techniques, including trans-oral laser surgery (TLS) and trans-oral robotic surgery (TORS). Although trans-oral surgery is not a new treatment, new surgical advances and the changing epidemiology of oropharyngeal cancer have suggested these as an alternative approach to the ‘organ preservation non-surgical’ treatments [76]. Radical radiation and chemotherapy treatments are achieving good clinical responses in patients with HPV-positive tumors [51], but the treatments are often associated with unpleasant toxicities and long-term effects [77, 78]. In the HPV-positive cohort there is a greater chance of long-term survival following treatment, in younger and healthier individuals. Offering minimally invasive surgery to reduce the long-term side effects associated with chemo-radiation is fuelling the desire to expand these techniques [79].
The role of surgery in these patients is to reduce adjuvant treatment and potentially to avoid it [80]. The use of TLS was first popularized in Europe by Steiner [81], and whilst there has been increasing experience with this technique, its use for oropharyngeal tumors has been small. It has also been limited to a few high-volume centers in the US [79] and European units mainly in France and Germany [80]. The use of the da Vinci® (Intuitive Surgical, Inc.) robot to perform robotic trans-oral surgery has gained increasing popularity and is being used increasingly throughout the world. There are no comparative prospective studies comparing minimally invasive surgery with modern chemo-radiotherapy. However, a number of retrospective cohort studies are showing promising results [82–84].
Currently the clinical trials database has six trials investigating the role of minimally invasive surgery in HPV-positive oropharyngeal disease. These are summarized in Table 3.
In the Sinai Robotic Surgery Trial study, de-escalation based on surgical resection and neck stage is being assessed in HPV-positive tumours (ClinicalTrials.gov identifier, NCT02072148). In a study at the University of Pennsylvania, robotic surgery is also being used to de-escalate adjuvant treatment by reducing treatment to the primary tumor bed in fully resected tumors (ClinicalTrials.gov identifier, NCT02225496). In an ECOG 3311 study, patients are randomized to either normal-dose postoperative radiation or low-dose treatment (ClinicalTrials.gov identifier, NCT01898494) following minimally invasive surgery. This is a large multicentre study in which patients are stratified following surgery. Low-risk patients receive observation post-operatively and high-risk patients receive chemo-radiotherapy. Intermediate risk patients are randomized to low-dose or normal-dose radiotherapy. Figure 6 shows the protocol for the study.
The protocol for the Eastern Cooperative Oncology Group (ECOG) 3311 study (NCT01898494). Patients are stratified with low-risk patients receiving observation post operatively and high-risk patients receive chemo-radiotherapy. Intermediate risk patients are randomized to low-dose or normal-dose radiotherapy. Reproduced with permission from: http://ecog-acrin.org/clinical-trials/e3311-educational-materials
The PATHOS trial (Post-operative adjuvant treatment for HPV-positive tumours) is a randomized multicentre trial based in the UK comparing post-operative treatment following surgery for HPV-positive disease, which is due to start soon. Depending on resection and staging information, patients will be classified as low, intermediate or high risk. Low-risk patients will not receive adjuvant treatment. Intermediate and high-risk patients will be randomized to an adjuvant treatment. The intermediate risk group will be randomised to high-dose or low-dose radiotherapy, and the high-risk group will be randomized to standard radiotherapy with or without chemotherapy (ClinicalTrials.gov identifier, NCT02215265). In the APEDT trial (post operative adjuvant therapy de-intensification trial for human papillomavirus-related, p16+ oropharynx cancer) patients with fully excised HPV-positive primary tumors will receive either radiotherapy or radiotherapy and cisplatin (ClinicalTrials.gov identifier, NCT01687413). In another study, the use of post-operative docetaxel with hyper-fractioned intensity modulated radiotherapy (IMRT) is being investigated following minimally invasive surgery with curative intent (ClinicalTrials.gov identifier, NCT01932697).
Trials Using Modification of Standard Radiotherapy or Chemotherapy Techniques in HPV-Positive Oropharyngeal Tumors
A summary of the trials discussed below can be found in Table 4.
Cisplatin Alternatives Given with Radiation
EGFR therapies are being used and investigated as an option for reducing toxicity in HPV-positive tumors. The most widely used is cetuximab, a monoclonal antibody that targets the EGFR extracellular ligand-binding domain. Following a phase III trial [85], cetuximab has been approved for use in Europe and the US in locally advanced head and neck cancer. Cetuximab has shown improved overall survival in a patient group given cetuximab and radiation over radiation alone, but the study did not test for HPV status in the tumor specimens.
To answer whether cetuximab is beneficial in HPV-positive patients, the RTOG is running a randomized trial of cisplatin versus cetuximab with radiation (ClinicalTrials.gov identifier, NCT01302834). A UK based trial called De-ESCALaTE (Determination of cetuximab versus cisplatin early and late toxicity events) is addressing a similar question comparing either cisplatin or cetuximab with radiation and focusing on toxicity outcomes (ClinicalTrials.gov identifier, NCT01874171). An Australian group TROG (Trans-Tasman Radiation Oncology Group) are also recruiting into a trial comparing cetuximab to cisplatin with radiotherapy (ClinicalTrials.gov identifier, NCT01855451). A further study investigating a cohort treated with cetuximab with pre- and post-treatment biopsies is also being carried out and will be compared to a historical series of cisplatin-treated patients (ClinicalTrials.gov identifier, NCT01663259).
Cetuximab has been shown to be effective in head and neck cancer, but recent studies investigating its use have not shown HPV a useful predictor of response in EGFR therapies [52, 86–88]. Additionally, the HPV status of tumors did not affect the response to cetuximab in vitro or in vivo in this study [62]. The findings of the genomic sequencing studies have also shown that the EGFR pathway alterations are rare in HPV-positive tumors [59] and makes the results of the above studies more important before cetuximab is used widely in HPV-positive disease.
Reducing Radiation Dose and Modulation of Radiation Dose Following Induction Chemotherapy for HPV-Positive Tumors
There are also other trials investigating different de-intensification treatments based on the response to induction chemotherapy. In an ECOG trial, induction chemotherapy with paclitaxel, cisplatin, and cetuximab will be followed by cetuximab in combination with either low-dose or standard-dose IMRT depending on the response to the induction chemotherapy (ClinicalTrials.gov identifier, NCT01084083). The Quarterback Trial (ClinicalTrials.gov identifier, NCT01706939) is also comparing a reduced radiation dose with weekly carboplatin to the standard radiation dose and weekly carboplatin in patients that have responded to induction chemotherapy.
Another trial that will de-intensify treatment based on response to induction chemotherapy is the OPTIMA trial. Nab-paclitaxel and carboplatin will be followed by response-based therapy of chemo-radiation of high or low dose or just radiation alone in stages III or IV HPV-related oropharyngeal cancer (ClinicalTrials.gov identifier, NCT02258659).
Paclitaxel and carboplatin as induction therapy before radiation therapy with concomitant paclitaxel for HPV-positive patients is undergoing evaluation in another trial that is currently recruiting (ClinicalTrials.gov identifier, NCT02048020).
Reduced Radiation Dose in HPV-Positive Patients
In a single intervention group study (ClinicalTrials.gov identifier, NCT01530997), a reduced dose of 54–60 Gy of IMRT with concurrent weekly intravenous cisplatin in HPV-positive patients will be followed by surgical resection of any clinically apparent residual primary tumor or neck disease. A biopsy of primary site and a limited neck dissection will be performed in complete responders.
A randomized trial using patients with HPV-positive oropharynx tumors is recruiting using a reduced-dose IMRT treatment with patients randomized to just radiotherapy alone or to receive concomitant cisplatin (ClinicalTrials.gov identifier, NCT02254278). In this study, treatment de-intensification for HPV-positive SCC of the oropharynx is being investigated alongside cisplatin chemotherapy with a reduced radiation in the experimental arm from 70 to 63 Gy and from 58.1 to 50.75 Gy in primary treatment volume and clinical target volumes respectively (ClinicalTrials.gov identifier, NCT01088802).
Another trial considered a reduced radiation dose to the nodal basins but it is currently suspended (ClinicalTrials.gov identifier, NCT01891695). A different group had planned to treat patients with low-risk HPV-related oropharyngeal SCC and a N0 neck with a de-intensification of radiation and chemotherapy (ClinicalTrials.gov identifier, NCT02281955), but this study has also suspended recruitment.
A meta-analysis of RCTs that performed post hoc stratification for HPV analyzed five trials. They suggested HPV-positive groups were a heterogeneous population with non-smokers demonstrating improved survival compared to smokers. They concluded that de-intensification in HPV-positive smokers had to be carefully assessed for safety [86].
New Agents and Immune Therapy
Chemotherapy
Ribavirin is a drug that is used in the treatment of hepatitis C. It targets the 4E protein and has a role in ribosome function. HPV-positive tumors have shown abnormally high levels of this protein and its utility in HPV-related cancers is under investigation in the setting of recurrent or metastatic cancers (ClinicalTrials.gov identifier, NCT02308241). It is also being evaluated as part of a phase I trial in association with induction chemotherapy including afatinib (a tyrosine kinase inhibitor) and weekly carboplatin/paclitaxel for stage IV HPV-associated oropharynx SCC (ClinicalTrials.gov identifier, NCT01721525).
A PI3K (phosphatidylinositol-3 kinase) and PLK (Polo-like kinase) signalling pathway inhibitor called rigosertib is being investigated in a phase II trial in patients with relapsed or recurrent disease (ClinicalTrials.gov identifier, NCT01807546). A phase I trial in which it is being used as initial treatment with platinum-based chemo-radiotherapy is also recruiting (ClinicalTrials.gov identifier, NCT02107235). A University of Pittsburg study is planning to use a PI3K inhibitor, BYL719, with induction paclitaxel and cisplatin for HPV-associated oropharyngeal SSC. This will be followed by surgery to the primary site and neck with post-operative risk adapted IMRT (ClinicalTrials.gov identifier, NCT02298595). In a Phase Ib study of BKM120 (a PI3K inhibitor), it will be administered with cisplatin and radiotherapy in high-risk, locally advanced SCC of the head and neck (ClinicalTrials.gov identifier, NCT02113878).
In a phase II trial at MD Anderson, a PD-1 (programmed cell death protein) blocker, nivolumab, and a new HPV-16 vaccination (ISA101) will be given to HPV-16-positive incurable solid tumors (ClinicalTrials.gov identifier, NCT02426892). A planned phase I and II study of ADXS11-001 (live-attenuated Listeria monocytogenes cancer vaccine) and MEDI4736 (anti-PD-L1 antibody) will give these medications either alone or in combination to patients with cervical or HPV-positive head and neck cancer (ClinicalTrials.gov identifier, NCT02291055).
In a Yale pilot trial ‘Window Trial 5-aza in HNSCC’, an inhibitor of DNA methylation will be evaluated in HPV-positive and HPV-negative oropharyngeal cancers (ClinicalTrials.gov identifier, NCT02178072).
The activity of a heat shock protein inhibitor, Hsp90 Inhibitor AT13387, in treating patients undergoing radiation therapy and cisplatin in HPV-positive and HPV-negative tumours is also planned (ClinicalTrials.gov identifier, NCT02381535).
Immune Therapy and Vaccines
Immune therapy and vaccines offer a different modality for the treatment of oropharyngeal SCC that has not been available before. This is because foreign viral antigens that are present in cancers cells could be amenable to targeted therapy. This is supported by the local presence of HPV16-specific T cell immunity found in HPV-16-induced SCC [89].
Genetically Modified T Cell Response
T cells can be used as an autologous transfusion in a process termed adoptive immunotherapy. In the past, melanoma and viral-associated malignancies have been responsive to this type of therapy [90] and it is being investigated for use in head and neck cancers.
In patients with recurrent HPV-positive tumors, a team is planning to investigate the role of HPV-specific T cells. These are T cells that have been modified to kill HPV-infected cells through the recognition of the E6 and E7 viral proteins. They have also been modified to prevent T cell inactivation that can be associated with these tumors (ClinicalTrials.gov identifier, NCT02379520).
The National Cancer Institute (NCI) Surgery Branch has also developed an experimental therapy using T-cell receptor immunotherapy targeting HPV-16 E6 cell surface receptors. T cells of the patients are genetically modified using a therapy called gene transfer. These cells are modified with a virus (retrovirus) to attack only the tumor cells and then transfused back into the patient (ClinicalTrials.gov identifier, NCT02280811). In this phase II trial, tumor-infiltrating lymphocytes are harvested from the patient’s tumor and then expanded before being infused back into the patient (ClinicalTrials.gov identifier, NCT01585428).
Cancer Vaccines
Antitumor vaccines aim to stimulate a host’s immune system in the treatment of cancer. Viral-induced cancers are a particular focus as they are associated with immunogenic antigens.
Microorganism vaccines In a phase I trial of a recombinant Listeria monocytogenes-based vaccine that has been modified to express HPV-16 targets (REALISTIC trial), the safety of the vaccine (ADXS11-001) in patients treated with oropharyngeal cancer is being assessed (ClinicalTrials.gov identifier, NCT01598792) and is hoped to boost a patient’s immune response to the cancer.
This listeria-based HPV vaccine is also forming part of a trial based at the Mount Sinai hospital in the US. They plan to investigate circulating and tumour-infiltrating antigen-specific T cells in HPV-16-positive oropharyngeal cancer patients undergoing TORS resection (ClinicalTrials.gov identifier, NCT02002182).
DNA vaccines DNA vaccines are also being evaluated in the treatment of head and neck cancers. The delivery method of DNA vaccines is important and a number of studies are evaluating electroporation delivery of vaccine [91]. The efficacy of DNA vaccines in a murine model has been shown [92] and there is hope for its use in humans.
This trial had planned to assess the safety of a DNA vaccine pNGVL4a-CRT/E7 (detox) administered with an electroporation device and a low dose of cyclophosamide (ClinicalTrials.gov identifier, NCT01493154), but has been terminated due to lack of funding.
A further DNA vaccine, in a phase I open-label study, plans to evaluate the safety, tolerability, and immunogenicity of INO-3106 alone or in combination with INO-9012 (an interleukin 12 vaccine). The DNA vaccines are delivered by electroporation to subjects with HPV-16-associated head and neck cancer (ClinicalTrials.gov identifier, NCT02241369).
A further trial involving the interleukin 12 vaccine (INO9012) and VGX-3100 (two separate DNA plasmids respectively encoding E6 and E7 proteins of HPV-16 and HPV-18) delivered by electroporation in subjects with HPV-16 and/or 18-positive head and neck cancer is also in a phase I trial (ClinicalTrials.gov identifier, NCT02163057).
Peptide vaccines Peptide immunomodulatory vaccines against HPV-16 and MAGE-A3 (Melanoma antigen E) have also been investigated. They have been assessed in recurrent/metastatic head and neck SCC and the results of this trial (ClinicalTrials.gov identifier, NCT00704041) from the University of Maryland have been published and show they are well tolerated and stimulate a potentially meaningful T cell and antibody response [93]. Another immunomodulatory peptide, P16_37-63-peptide, is undergoing assessment in a phase I trial (ClinicalTrials.gov identifier, NCT01462838) in Germany. A trial examining a number of patients with advanced or recurrent HPV-driven cancers were assigned to receive either HPV E6 or E7 peptide. The study has completed, but there are no results currently available (ClinicalTrials.gov identifier, NCT00019110).
Conclusions
Oropharyngeal cancer has undergone an epidemic change in the last 20 years. HPV has become the leading etiological cause of oropharyngeal cancer in the developed world [4]. The differences between tumors caused by HPV and those related to tobacco smoking and alcohol are well documented. HPV tumors generally affect patients that are younger and more likely to be from a Caucasian and educated background. These patients tend to be healthier with less exposure to tobacco and alcohol, but have risk factors related to sexual behavior [44].
Patients with HPV-positive tumors present with small primary lesions that are almost exclusively in the tonsil and tongue base, but can have larger cystic nodal disease. They have a significantly improved prognosis and better response to all modalities of treatment [51]. They significantly fewer mutations with retained p53 wild type while HPV-negative tumors have an incidence of loss of function p53 mutations in 80% of tumors [60].
Increasing technological advances has allowed for more minimally invasive techniques to become available to the patients. HPV-positive patients are younger and healthier and more likely to be cured of their disease. They, therefore, have a high chance of living with side effects and toxicities of treatment. Therefore, a number of strategies are being investigated to reduce toxicities related to treatment. Surgical options promise to reduce the need for adjuvant chemotherapy and may allow for reduced radiotherapy. De-intensified radiotherapy and chemotherapy regimes based on individual risk stratification offers the hope to tailor treatment and offer a personalized treatment minimizing risks of toxicity and maintaining high cure rates.
New chemotherapy agents are currently being evaluated aimed at HPV-positive disease. PI3K (phosphatidylinositol-3 kinase) and PD-1 inhibitors hope to exploit the limited mutational genome of HPV-positive tumors and target the viral-induced carcinogenic pathways.
The immunogenic nature of HPV is also under investigation with treatments aimed at modifying host immune systems. Cancer vaccines in the form of microorganism, DNA, and peptide vaccination offer promise with significant T-cell response being induced [93].
However, the vaccination of new generations of the population against HPV infections offers the greatest potential for the prevention of virally induced cancer.
References
Cooper JS, Porter K, Mallin K, Hoffman HT, Weber RS, Ang KK, et al. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck. 2009;31(6):748–58.
Fakhry C, Cohen E. The rise of HPV-positive oropharyngeal cancers in the United States. Cancer Prev Res (Phila). 2015;8(1):9–11.
Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–9.
Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–9.
Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110(7):1429–35.
Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806–16.
Syrjanen K, Syrjanen S, Lamberg M, Pyrhonen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418–24.
zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186:131–56.
Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–20.
Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22(2):128–42.
Cancer IAfRo. Monograph volume 90: WHO. 2007. Available from: (http://monographs.iarc.fr). Cited 2015.
Rautava J, Syrjanen S. Biology of human papillomavirus infections in head and neck carcinogenesis. Head Neck Pathol. 2012;6(Suppl 1):S3–15.
Dahlgren L, Dahlstrand HM, Lindquist D, Hogmo A, Bjornestal L, Lindholm J, et al. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int J Cancer. 2004;112(6):1015–9.
St Guily JL, Jacquard AC, Pretet JL, Haesebaert J, Beby-Defaux A, Clavel C, et al. Human papillomavirus genotype distribution in oropharynx and oral cavity cancer in France-The EDiTH VI study. J Clin Virol. 2011;51(2):100–4.
Rampias T, Sasaki C, Psyrri A. Molecular mechanisms of HPV induced carcinogenesis in head and neck. Oral Oncol. 2014;50(5):356–63.
Surjan L Jr. Immunohistochemical markers of tonsillar crypt epithelium. Acta Otolaryngol Suppl. 1988;454:60–3.
Kim SH, Koo BS, Kang S, Park K, Kim H, Lee KR, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120(7):1418–25.
Kajiji S, Tamura RN, Quaranta V. A novel integrin (alpha E beta 4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J. 1989;8(3):673–80.
Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 2003;77(24):13125–35.
Chung CH, Bagheri A, D’Souza G. Epidemiology of oral human papillomavirus infection. Oral Oncol. 2014;50(5):364–9.
Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307(7):693–703.
Kreimer AR, Bhatia RK, Messeguer AL, Gonzalez P, Herrero R, Giuliano AR. Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex Transm Dis. 2010;37(6):386–91.
D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–56.
Allen CT, Lewis JS Jr, El-Mofty SK, Haughey BH, Nussenbaum B. Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope. 2010;120(9):1756–72.
Rolfe M, Beer-Romero P, Glass S, Eckstein J, Berdo I, Theodoras A, et al. Reconstitution of p53-ubiquitinylation reactions from purified components: the role of human ubiquitin-conjugating enzyme UBC4 and E6-associated protein (E6AP). Proc Natl Acad Sci USA. 1995;92(8):3264–8.
Tornesello ML, Perri F, Buonaguro L, Ionna F, Buonaguro FM, Caponigro F. HPV-related oropharyngeal cancers: from pathogenesis to new therapeutic approaches. Cancer Lett. 2014;351(2):198–205.
Strati K, Lambert PF. Human papillomavirus association with head and neck cancers: understanding virus biology and using it in the development of cancer diagnostics. Expert Opin Med Diagn. 2008;2(1):11–20.
Ganti K, Broniarczyk J, Manoubi W, Massimi P, Mittal S, Pim D, et al. The human papillomavirus E6 PDZ binding motif: from Life Cycle to Malignancy. Viruses. 2015;7(7):3530–51.
Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010;2:15.
Um SH, Mundi N, Yoo J, Palma DA, Fung K, MacNeil D, et al. Variable expression of the forgotten oncogene E5 in HPV-positive oropharyngeal cancer. J Clin Virol. 2014;61(1):94–100.
Bishop JA, Lewis JS Jr., Rocco JW, Faquin WC. HPV-related squamous cell carcinoma of the head and neck: an update on testing in routine pathology practice. Semin Diagn Pathol. 2015;32(5):344–51.
Rietbergen MM, Snijders PJ, Beekzada D, Braakhuis BJ, Brink A, Heideman DA, et al. Molecular characterization of p16-immunopositive but HPV DNA-negative oropharyngeal carcinomas. Int J Cancer. 2014;134(10):2366–72.
Hobbs CG, Sterne JA, Bailey M, Heyderman RS, Birchall MA, Thomas SJ. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31(4):259–66.
Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–75.
Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22.
Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31(6):744–54.
Upile NS, Shaw RJ, Jones TM, Goodyear P, Liloglou T, Risk JM, et al. Squamous cell carcinoma of the head and neck outside the oropharynx is rarely human papillomavirus related. Laryngoscope. 2014;124(12):2739–44.
Smith EM, Ritchie JM, Summersgill KF, Klussmann JP, Lee JH, Wang D, et al. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108(5):766–72.
Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89(3):300–4.
Shah JP, Patel SG, Singh B. Jatin Shah’s head and neck surgery and oncology. 4th ed. Philadelphia: Elsevier; 2012.
Winn DM, Lee YC, Hashibe M, Boffetta P, INHANCE consortium. The INHANCE consortium: toward a better understanding of the causes and mechanisms of head and neck cancer. Oral Dis. 2015;21(6):685–93.
Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92(4):805–13.
Hong AM, Martin A, Chatfield M, Jones D, Zhang M, Armstrong B, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748–54.
Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–20.
Fakhry C, Gillison ML, D’Souza G. Tobacco use and oral HPV-16 infection. JAMA. 2014;312(14):1465–7.
Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301.
Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30(7):898–903.
Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50(5):380–6.
Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–9.
Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813–20.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
Vermorken JB, Psyrri A, Mesia R, Peyrade F, Beier F, de Blas B, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol. 2014;25(4):801–7.
Chau NG, Rabinowits G, Haddad RI. Human papillomavirus-associated oropharynx cancer (HPV-OPC): treatment options. Curr Treat Options Oncol. 2014;15(4):595–610.
Compton CC, Greene FL, American Joint Committee on Cancer. AJCC cancer staging atlas: a companion to the seventh editions of the AJCC cancer staging manual and handbook. 2nd ed. New York: Springer; 2012.
Keane FK, Chen YH, Neville BA, Tishler RB, Schoenfeld JD, Catalano PJ, et al. Changing prognostic significance of tumor stage and nodal stage in patients with squamous cell carcinoma of the oropharynx in the human papillomavirus era. Cancer. 2015;121(15):2594–602.
Iyer NG, Dogan S, Palmer F, Rahmati R, Nixon IJ, Lee N, et al. Detailed analysis of clinicopathologic factors demonstrate distinct difference in outcome and prognostic factors between surgically treated hpv-positive and negative oropharyngeal cancer. Ann Surg Oncol. 2015;24:1068.
Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–7.
Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–60.
Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82.
Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357(25):2552–61.
Vokes EE, Seiwert TY. EGFR-directed treatments in SCCHN. Lancet Oncol. 2013;14(8):672–3.
Pogorzelski M, Ting S, Gauler TC, Breitenbuecher F, Vossebein I, Hoffarth S, et al. Impact of human papilloma virus infection on the response of head and neck cancers to anti-epidermal growth factor receptor antibody therapy. Cell Death Dis. 2014;5:e1091.
Mishra A, Bharti AC, Varghese P, Saluja D, Das BC. Differential expression and activation of NF-kappaB family proteins during oral carcinogenesis: role of high risk human papillomavirus infection. Int J Cancer. 2006;119(12):2840–50.
Han G, Lu SL, Li AG, He W, Corless CL, Kulesz-Martin M, et al. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest. 2005;115(7):1714–23.
Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22(5):1071–7.
Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Stage III and IV cancers of the oropharynx: results of a randomized study of Gortec comparing radiotherapy alone with concomitant chemotherapy. Bull Cancer. 2000;87 Spec No:48–53.
Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–9.
Kreimer AR. Prospects for prevention of HPV-driven oropharynx cancer. Oral Oncol. 2014;50(6):555–9.
Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89(24):12180–4.
Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl 5):F123–38.
Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329.
Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–23.
Fakhry C, Rosenthal BT, Clark DP, Gillison ML. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “pap-test equivalent” in high-risk populations. Cancer Prev Res (Phila). 2011;4(9):1378–84.
Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–24.
Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708–15.
Mydlarz WK, Chan JY, Richmon JD. The role of surgery for HPV-associated head and neck cancer. Oral Oncol. 2015;51(4):305–13.
Fein DA, Lee WR, Amos WR, Hinerman RW, Parsons JT, Mendenhall WM, et al. Oropharyngeal carcinoma treated with radiotherapy: a 30-year experience. Int J Radiat Oncol Biol Phys. 1996;34(2):289–96.
Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582–9.
Haughey BH, Hinni ML, Salassa JR, Hayden RE, Grant DG, Rich JT, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck. 2011;33(12):1683–94.
Lorincz BB, Jowett N, Knecht R. Decision management in transoral robotic surgery (tors): Indications, individual patient selection, and role in the multidisciplinary treatment of head and neck cancer from a european perspective. Head Neck. 2015. doi:10.1002/hed.24059.
Steiner W. Experience in endoscopic laser surgery of malignant tumours of the upper aero-digestive tract. Adv Otorhinolaryngol. 1988;39:135–44.
Dowthwaite SA, Franklin JH, Palma DA, Fung K, Yoo J, Nichols AC. The role of transoral robotic surgery in the management of oropharyngeal cancer: a review of the literature. ISRN Oncol. 2012;2012:945162.
de Almeida JR, Byrd JK, Wu R, Stucken CL, Duvvuri U, Goldstein DP, et al. A systematic review of transoral robotic surgery and radiotherapy for early oropharynx cancer: a systematic review. Laryngoscope. 2014;124(9):2096–102.
Kelly K, Johnson-Obaseki S, Lumingu J, Corsten M. Oncologic, functional and surgical outcomes of primary Transoral Robotic Surgery for early squamous cell cancer of the oropharynx: a systematic review. Oral Oncol. 2014;50(8):696–703.
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78.
Masterson L, Moualed D, Liu ZW, Howard JE, Dwivedi RC, Tysome JR, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50(15):2636–48.
Mirghani H, Amen F, Moreau F, Guigay J, Hartl DM. Lacau St Guily J. Oropharyngeal cancers: relationship between epidermal growth factor receptor alterations and human papillomavirus status. Eur J Cancer. 2014;50(6):1100–11.
Vermorken JB, Stohlmacher-Williams J, Davidenko I, Licitra L, Winquist E, Villanueva C, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14(8):697–710.
Kofler B, Laban S, Busch CJ, Lorincz B, Knecht R. New treatment strategies for HPV-positive head and neck cancer. Eur Arch Otorhinolaryngol. 2014;271(7):1861–7.
Kershaw MH, Westwood JA, Slaney CY, Darcy PK. Clinical application of genetically modified T cells in cancer therapy. Clin Transl Immunology. 2014;3(5):e16.
Best SR, Peng S, Juang CM, Hung CF, Hannaman D, Saunders JR, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27(40):5450–9.
Lescaille G, Pitoiset F, Macedo R, Baillou C, Huret C, Klatzmann D, et al. Efficacy of DNA vaccines forming e7 recombinant retroviral virus-like particles for the treatment of human papillomavirus-induced cancers. Hum Gene Ther. 2013;24(5):533–44.
Zandberg DP, Rollins S, Goloubeva O, Morales RE, Tan M, Taylor R, et al. A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN). Cancer Immunol Immunother. 2015;64(3):367–79.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Ashley Hay and Ian Ganly have nothing to disclose.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hay, A., Ganly, I. Targeted Therapy in Oropharyngeal Squamous Cell Carcinoma: The Implications of HPV for Therapy. Rare Cancers Ther 3, 89–117 (2015). https://doi.org/10.1007/s40487-015-0008-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-015-0008-5