Abstract
Management of pediatric head trauma requires a delicate balance between accuracy and safety, with a dual emphasis on prompt diagnosis while minimizing radiation exposure. Ultrasonography (US) shows promise in this regard. A case study involving a 10-month-old infant with acute right parietal swelling revealed the utility of US in detecting a corresponding hypoechoic lesion, along with an underlying suspected fracture line of the vault and subdural hematoma. Subsequent CT confirmed the fracture, while MRI confirmed the subdural hematoma. At one-month follow-up, MRI demonstrated hematoma reabsorption, while US revealed a bone callus in its advanced phase. Although US is not yet standard practice for pediatric head trauma, its ability to detect fractures in infants suggests its potential role: when a fracture is evident on US, it may serve as an indication to perform neuroimaging. Potentially, adoption of US could contribute to mitigation of children’s exposure to ionizing radiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head traumas in children represent a common reason for visits to the Emergency Room (ER). Their incidence varies across countries, with an estimated 47–280 per 100.000 children presenting to the ER each year due to traumatic brain injury worldwide. The risk of skull fracture and brain injury after head trauma is inversely proportional to age: identifying skull fractures in young children is crucial as they are known risk factors for brain injury [1].
Due to its high resolution and ability to detect subtle fractures, Computed Tomography (CT) has become widely utilized for diagnosing (or excluding) skull fractures in children. Currently, head CT is the most common CT scan performed in the pediatric population. However, considering that children are more sensitive to ionizing radiations and have a higher relative risk of cancer, physicians are striving to minimize the use of X-rays and CT scans. Alternative solutions include the development of clinical guidelines to reduce unnecessary exposure, and the adoption of alternative imaging methods including magnetic resonance imaging (MRI) and ultrasonography (US) [2].
US is a noninvasive tool that is superior to CT and MRI in detecting the dura’s status. Moreover, it is preferred over MRI in the ER due to its higher availability. Rabiner et al. stated the following benefits for point-of-care ultrasound (POCUS): (a) early diagnosis of a patient and early consultation; (b) reduction in CT scan request; (c) suitability when access to CT scan is limited; and (d) serving as a triage tool in disaster area with difficult conditions [3].
Case presentation
We describe the case of a 10-month-old infant presenting at the ER with an acute parietal swelling over the past 12 h. The medical history revealed no incidents of accidental falls or head trauma. The physical examination revealed a soft, right parietal swelling measuring approximately 2 × 1 cm. The overlying skin was intact, and there was no apparent pain upon palpation. Neurological examination was normal. While awaiting for skull CT in sedation, a cerebral US revealed “a hypoechoic lesion (fluid corpuscular material) at the subcutaneous level corresponding to the swelling, along with a suspected fracture line of the vault and subdural hematoma” (Fig. 1; Online Resource 1).
Point-of-care Ultrasound performed using a very-high frequency (18–5 MHz) linear array transducer. The longitudinal view reveals a linear skull fracture (long arrow), accompanied by a small subdural hematoma (short arrows), corresponding to the clinically apparent parietal swelling. The image is obtained in the coronal plane, providing a visualization of the fracture and its spatial relationship with the surrounding tissues
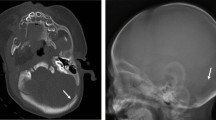
These findings were confirmed by a skull CT in sedation that revealed “the presence of hematoma in the right parietal area, a compound fracture of the underlying skull, and an associated layer of subdural hematoma with a maximum thickness of 4 mm along the adjacent convexity.” (Fig. 2).
Based on the neuroradiological findings, the neurosurgical consultant recommended in-hospital monitoring for the next 48 h. A brain MRI was also performed, confirming the presence of “subdural blood in the right parietal area, with a maximum thickness of about 3 mm, exerting minimal imprint on the adjacent parenchyma. A compound fracture of the right parietal cranial theca with associated cephalohematoma with posterior blood level was identified.” After demonstrating good general conditions, the patient was discharged with a protective helmet and scheduled for follow-up (Fig. 3). One month later, a follow-up brain MRI revealed reabsorption of the thin right parietal subdural blood layer. On US, we observed the presence of the bone callus in its advanced phase (Fig. 4; Online Resource 2).
Point-of-care Ultrasound performed using a very-high frequency (18–5 MHz) linear array transducer. An advanced stage of the bone callus is evident. The bone callus (arrow) appears as a hyperechoic line, similar to the surrounding normal bone cortex, but the underlying reverberation artifact (short arrows) are absent. Of note, the aforementioned artifact stops abruptly exactly at the transitional zone from the normal bone cortex to the prominence of the callus
Discussion and conclusions
In current practice, head CT stands as the gold standard diagnostic test for assessing skull fractures and intracranial bleeding subsequent to head trauma. The algorithm outlined in the PECARN guidelines is extensively utilized to determine the necessity of CT scans in children with head trauma [4]. The PECARN study identifies three age-independent predictors, including loss of consciousness, altered state of consciousness (GCS < 15), and high-energy dynamics. Additionally, there are three variable predictors that are age-dependent: changes in behavior, hematoma of the scalp in the 'non-frontal' area, and palpable fracture of the vault in children < 2 years. For older children, repeated vomiting, worsening headache, and suspected fracture of the base are considered as predictors. Subsequently, the patients are categorized into three groups based on the described variables: high risk, requiring immediate CT; low risk, necessitating observation; and moderate risk, where the decision between CT and observation depends on specific clinical features and physician experience.
Although skull US is not yet commonly used and is not included in clinical guidelines, its adoption could potentially assist clinicians in decision-making for cases of moderate risk, potentially reducing the need for CT scans. US can be used to detect skull fractures, particularly in cases where the location of the injury is clear, and the skin is intact. When the fracture is evident on US, it serves as an indication to perform a CT scan to rule out intracranial injuries [5]. Several are the expected advantages of US. First, it can be performed rapidly, enabling earlier detection of a skull fracture as a marker for suspected intracranial injury and facilitating neurosurgical consultation. Second, unlike CT and MRI, US does not require sedation in young children [3]. Finally, and of utmost importance, US has the potential to reduce CT use and minimize ionizing radiation exposure in children. The estimated lifetime risk of cancer from a head CT is substantially higher for children than for adults due to a longer latency period and greater sensitivity of developing organs to radiation. However, it is crucial to note that intracranial injury may occur without skull fracture, and clinicians must also rely on clinical judgment or decision rules for obtaining CT scan, regardless of the presence or absence of a skull fracture in US.
In a multicenter, prospective, and observational study, Parri et al. determined the accuracy of POCUS in identifying skull fractures in children younger than 2 years of age with local signs of head trauma and they showed The sensitivity of POCUS for skull fractures was 80 of 88 (90.9%; 95% CI 82.9–96.0) and the specificity for skull fractures was 23 of 27 (85.2%; 95% CI 66.3–95.8). None of the 8 patients with false-negative POCUS results had clinically important TBIs. [6].
In a systematic review/meta-analysis conducted by Gordon et al., to determine the operating characteristics of POCUS skull studies in the diagnosis of fractures in pediatric head trauma patients, six studies comprising 393 patients with a low risk of bias were selected. The pooled sensitivity was 91%, and specificity was 96%, resulting in a pooled positive likelihood ratio of 14.4 and negative likelihood ratio of 0.14. Using the weighted prevalence of skull fractures across the studies as a pretest probability (31%), a positive skull ultrasound would increase the probability to 87%, whereas a negative test would decrease the probability of a skull fracture to 6%. To achieve a post-test probability of a skull fracture of ~ 2%, a negative skull ultrasound in a patient with a pretest probability of ~ 15% would be required [7].
Our case report underscores the successful diagnosis of a skull fracture and brain injury in an infant using US. Although the existing body of literature is scarce, US is emerging as a valuable imaging modality for diagnosing skull fractures in infants, providing a radiation-free alternative to CT scans. Further research is warranted to explore the full potential of US in diagnosing various types of head traumas in pediatric patients.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- CT:
-
Computed Tomography
- US:
-
Ultrasonography
- ER:
-
Emergency Room
- MRI:
-
Magnetic Resonance Imaging
- PECARN:
-
Pediatric Head Injury/Trauma Algorithm
- POCUS:
-
Point-of-Care Ultrasound
References
Osmond MH, Klassen TP, Wells GA, Correll R, Jarvis A, Joubert G et al (2010) CATCH: a clinical decision rule for the use of computed tomography in children with minor head injury. CMAJ 182:341–348. https://doi.org/10.1503/cmaj.091421
Brenner DJ, Hall EJ (2007) Computed tomography–an increasing source of radiation exposure. N Engl J Med 357:2277–2284. https://doi.org/10.1056/NEJMra072149
Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW (2013) Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics 131:e1757-1764. https://doi.org/10.1542/peds.2012-3921
Tsze DS, Kuppermann N, Casper TC, Barney BJ, Richer LP, Liberman DB et al (2023) Stratification of risk for emergent intracranial abnormalities in children with headaches: a pediatric emergency care applied research network (PECARN) study protocol. BMJ Open 13:e079040. https://doi.org/10.1136/bmjopen-2023-079040
Dehbozorgi A, Mousavi-Roknabadi RS, Hosseini-Marvast SR, Sharifi M, Sadegh R, Farahmand F et al (2021) Diagnosing skull fracture in children with closed head injury using point-of-care ultrasound vs. computed tomography scan. Eur J Pediatr 180:477–484. https://doi.org/10.1007/s00431-020-03851-w
Parri N, Crosby BJ, Mills L, Soucy Z, Musolino AM, Da Dalt L et al (2018) Point-of-care ultrasound for the diagnosis of skull fractures in children younger than two years of age. J Pediatr 196:230-236.e2. https://doi.org/10.1016/j.jpeds.2017.12.057
Gordon I, Sinert R, Chao J (2021) The utility of ultrasound in detecting skull fractures after pediatric blunt head trauma: systematic review and meta-analysis. Pediatr Emerg Care 37:e1701–e1707. https://doi.org/10.1097/PEC.0000000000001958
Funding
Open access funding provided by Università degli Studi di Modena e Reggio Emilia within the CRUI-CARE Agreement. No funding to declare.
Author information
Authors and Affiliations
Contributions
RF, and FM contributed to the conception and design and wrote the first draft of the manuscript. IG, LL and GP analyzed and interpreted the patient data. All authors helped to review the article and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent for publication
We obtained written informed consent was from parents for publication of this case report and accompanying images. All methods were performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Online Resource 1 Point-of-care Ultrasound using a very-high frequency (18-5 MHz) linear array transducer in the acute phase of post-traumatic injury. The misalignment of the cortical bone is coupled with the periosteal bulging and subdural hematoma. Supplementary file1 (AVI 47574 kb)
Online Resource 2 Point-of-care Ultrasound using a very-high frequency (18-5 MHz) linear array transducer in the advanced phase of the bone callus formation. The subdural hematoma is no longer visible. The bone callus appears as a hyperechoic line, similar to the surrounding normal bone cortex, but the underlying reverberation artifact are absent. Of note, the mentioned artifact stops abruptly exactly at the transitional zone from the normal bone cortex to the prominence of the callus. Supplementary file2 (AVI 28463 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Filice, R., Miselli, F., Guidotti, I. et al. Identifying skull fractures after head trauma in infants with ultrasonography: is that possible?. J Ultrasound (2024). https://doi.org/10.1007/s40477-024-00907-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40477-024-00907-7