Abstract
Cardiac involvement from amyloidosis is of growing interest in the overall literature. Despite cardiac amyloidosis (CA) has been considered for a long time a rare disease, the diagnostic awareness is increasing mainly thanks to the improvement of diagnostic softwares and of imaging techniques such as cardiac magnetic resonance (CMR). Some authors have observed an increase of prevalence rate of CA; moreover it’s often underestimated because clinical manifestations are aspecific. The interstitial infiltration of the left ventricle has been extensively studied, while the involvement of the right ventricle (RV) has been less investigated. Involvement of the RV, even in the absence of pulmonary hypertension or clearly left ventricle infiltration, plays an important role as prognostic factor and is useful to achieve an early diagnosis. Therefore, the use of fast and low-cost diagnostic methods such as ultrasound strain of the right ventricle could be used to recognize cardiac amyloidosis early. Herein the importance of evaluating the right ventricular involvement, which can predict the most severe course of the disease also without overt clinical manifestations. The role of imaging, in particular of echocardiography, CMR, and scintigraphy is here reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The attention of the cardiology community to cardiac amyloidosis (CA), once considered to be rare, has been recently growing thanks to advances in non-invasive diagnosis, new diagnostic algorithms, and specific treatments [1].
Multimodality imaging, including advanced Doppler-echocardiography (DE) techniques, cardiac magnetic resonance (CMR) and myocardial scintigraphy (MS) can support the clinical suspicion, and allow CA early diagnosis and treatment, so improving the outcome [2].
Sistemic amyloidosis is subdivided in different forms. The most common are those secondary to light chain deposition (AL-CA) and those linked to transthyretin, further subdivided into a variant form (ATTRv-CA), caused by genetic disorders and mostly found in young people, and a wild type form (ATTRwt-CA), found in the elderly with prevalence increasing with age [3, 4].
Establishing the actual prevalence of CA is challenging. Its relative rarity, phenotypic heterogeneity and lack of dedicated epidemiological studies are the main reasons for this [5]. CA prevalence seems to be currently 40–70 in 100,000 people. However, it is hypothesized that heart involvement is frequently underestimated in clinical studies but more detected in autopsy examinations, especially for ATTRwt-CA [3; 6]. To date, it is known that the cardiac involvement in systemic amyloidosis is almost invariable in the ATTRwt-CA, variable depending on the type of genetic mutation in the ATTRv-CA and present in almost half of patients with AL-CA [6]. Some authors have observed an increase in prevalence rate between 2000 and 2012, particularly from 2006, with 4756 new cases of cardiac amyloidosis in 2012 in the United States (US) [7].
Cardiac involvement in systemic amyloidosis has phenotypical heterogenicity: clinical manifestations are not specific, and main findings are misdiagnosed with those of the most common heart diseases, such as heart failure or aortic stenosis [8, 9]. Therefore, CA should be always considered in the differential diagnosis of new-onset heart failure syndromes in patients with age > 65 yrs without known cardiovascular risk factors [7]. Moreover, in the presence of left ventricular (LV) thickness ≥12 mm, many clinical or instrumental red flags should rise the suspicion of CA, including sensory and autonomic dysfunction, peripheral polyneuropathy, hypotension or normotension if previously hypertensive, bilateral carpal tunnel syndrome, rupture of the biceps tendon, skin bruising and proteinuria, decreased QRS voltage to mass ratio, pseudo Q waves, AV conduction disease, late gadolinium enhancement (LGE) on magnetic resonance (MR) or reduced longitudinal strain with apical sparing on echocardiography [10].
The distinction between the different forms of systemic amyloidosis and the recognition of cardiac involvement is quite complex. Advanced clinical laboratory and multimodality imaging (MMI) expertise are requested. In particular, hematological exclusion of AL-CA is preliminary and of primary importance [11].
CA is expected in the elderly, and its prevalence increases with age [12, 13].
Left ventricular interstitial involvement has been extensively studied, while the right side has been less investigated [14]. Indeed, a recent ESC position paper on CA reports only the tricuspid annular plane systolic excursion (TAPSE) as a possible diagnostic point and a negative red flag [10] for CA prognosis.
Despite depicting similar morphological characteristics, the nature of myocardial damage, the extent of myocardial infiltration and the disease progression differ significantly between AL and both ATTR forms [4].
However, the amyloidotic right ventricular involvement plays an important role as a prognostic factor independently from the presence of pulmonary hypertension and helps achieve an early diagnosis of the disease [15,16,17,18]. Right ventricular dysfunction can arise late compared to the involvement of the left ventricle [15], although some authors suggest that the right ventricle may co-occur or precede the left [19, 20].
Pathophysiology and clinical features of the right involvement from CA
CA is an infiltrative cardiomyopathy characterized by segmental amyloid infiltration of the myocardium with a progressive increase in wall thickness [21] that starts from the subendocardial layer localized at the basal portions of the ventricles (especially longitudinal myocardial fibres) and then progresses toward the mid-ventricular portion in a transmural pattern, sparing the apical segments until the very late stages of the disease ('apical sparing') [22]. This myocardial deposition causes reduced contractility in systole and abnormal relaxation in diastole [21]. The progressive reduction of the ventricular cavities dimensions in favour of the increase of the wall thickness and the overall reduction of the longitudinal systolic function with an initial sparing of the circumferential and radial ones are among the main reasons for the relative preservation of the ejection fraction in CA [4].
Atrial and RV myocardial dysfunction may be caused by the primary infiltrative process and overload phenomena. As stated, the RV is commonly involved in AL-CA and ATTR-CA, and when present, depicts a poor prognosis [15,16,17,18]. Indeed, increased LV filling pressure, a distinctive feature of LV involvement in CA, induces RV chronic overload due to elevated pulmonary pressures and increased RV afterload [17]. Moreover, pulmonary arterial hypertension can also occur with a direct deposit of amyloid fibrils in the walls of the pulmonary vessels [23]. Thus, increased RV afterload leads to RV remodelling (initial hypertrophy and subsequent progressive dilatation) and reduced RV performance [15, 24]. Beyond that, tricuspid regurgitation (TR) is a common feature of CA due to primary valvular involvement by amyloid deposition and/or RV remodelling (progressive tricuspid annular dilation, papillary muscle displacement) induced by increased LV filling pressure [17].
Extracellular infiltration can also affect other structures, such as atrioventricular or semilunar valves [8], or the atria wall, leading to re-entry circuits, arrhythmias, stasis and possible thrombosis [25,26,27].
Isolated right or left atrial CA is related to atrial natriuretic peptide overproduction and has been found in about 50% of patients by Sukhacheva et al. [28, 29].
Moreover some authors suggested that an increased thickness of the crista terminalis (CT), or interatrial septum (IAS), may represent an early sign of ATTR-CA [30].
MMI has been shown to be necessary for precise and early diagnosis of CA [1] and useful in the study of right heart sections [2]. All imaging methods have the same target: identifying the cardiac infiltration and its consequence on cardiac morphology and function. Understanding each technique’s properties and limits and selecting the optimal imaging modality to achieve each goal is the cornerstone of the multimodality application [31].
Echocardiographic features of right CA
Echocardiographic morpho-functional assessment of right ventricular function has a diagnostic role in the early recognition of RV involvement in CA and in discriminating CA among other cardiomyopathies with hypertrophic phenotype (e.g. hypertension, Anderson-Fabry disease, hypertrophic cardiomyopathy). In addition to concentric ventricular thickness, CA shows a restrictive flow pattern of ventricular inflow, especially for advanced disease [32] combined with a typical infiltrative strain pattern (bull’s eye) [33]. It also plays a pivotal role in CA prognosis.
Transthoracic echocardiography (TTE)
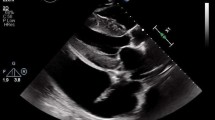
Transthoracic echocardiography (TTE) is the first-line examination of patients with suspected or confirmed CA due to its wide availability (Fig. 1).
Echocardiographic findings of right cardiac amyloidosis (CA). Parasternal long (a) and short (b, c) axis, four chamber apical (D) view of echocardiographic imaging of right heart in patient with CA characterized by the presence of concentric right (green arrows in a, d) ventricular thickness, hypertrophy of the septum (orange arrow in d), right atrial dilatation (white arrows in a–d), reduced right ventricular cavity (green spot in a, d) and sparkling spots in the right ventricle (yellow stars in a, d) and septum (yellow star in d)
RV wall thickening and impaired RV function are common findings in patients with CA. AL-CA patients with preserved LVEF show impaired tricuspid annular plane systolic excursion (TAPSE) and RV longitudinal strain (RV-LS) in comparison with control patients [34]. On the other hand, ATTRv-CA patients show significant RV enlargement, increased pulmonary arterial pressure (sPAP) and lower values of TAPSE compared to healthy controls [35].
Uzan et al. have evaluated the role of RV echocardiographic assessment in discriminating AL amyloidosis from other causes of unexplained LV hypertrophy (LVH) and assessed whether RV dysfunction predicts overall mortality in this setting [20].
However, there are many structural and functional differences between AL-CA and ATTR-CA. AL-CA is associated with slightly increased wall thickness while ATTR-CA is predominantly characterized by increased LV and RV mass and more systolic dysfunction. The discrepancy between the two types of amyloid cardiomyopathy on imaging and the clinical course reinforces that AL-CA is not a simple infiltrative disorder and should be more accurately characterized as both toxic and infiltrative cardiomyopathy [36, 37].
Strain analysis
The assessment of ventricular systolic function with the strain method is widely used in clinical practice in patients with CA. Indeed, strain imaging has been shown to play an important role, mainly when applied in the study of LV, in the early diagnosis, differential diagnosis and prognostic stratification of patients with CA [1].
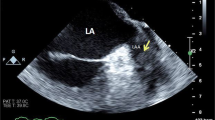
An example of strain imaging of the right ventricle in a patient with cardiac amyloidosis is shown in Fig. 2.
Imaging of right CA. Tissue doppler (a) and strain imaging (b) of the right ventricle in patient with cardiac amyloidosis showing reduced velocity peaks (yellow arrow in a), reduced TAPSE (Tricuspid Annular Plane Systolic Excursion, green arrow in b) and bull’s eye (white arrow in b). 4D imaging (c, d) showing reduced volume cavity of right ventricle, reduced TAPSE and EF (red arrow in c, d)
Several authors have applied strain imaging in the evaluation of the RV. A reduced longitudinal right ventricular free wall strain (FWRVLS) (with a cut-off of − 21.2%) discriminates AL amyloidosis from patients with other diseases. In AL amyloidosis, FWRVLS is significantly associated with mortality and is the only independent echocardiographic predictor of overall mortality, even when adjusted for Mayo staging and global left ventricular longitudinal strain [20]. Urbano-Moral JA et al. have highlighted the close correlation between the left and right ventricular strain in patients with AL-CA. In those patients, both the ventricles are involved with reduced systo-diastolic function, and this reduction is strictly correlated with an increase of brain natriuretic peptide (BNP) [38]. Moreover, Moñivas Palomero et al. have found an apex-to-base strain gradient in all forms of CA (reduced basal and mid-ventricular deformation in the presence of apical strain values) with a pattern similar to the classic 'apical sparing' and ‘bull’s eye’ pattern previously described in the LV. In this study, it also has been described that different sparing pattern gradients derived from speckle-tracking echocardiography are specifically related to the subtype. Indeed, the RV apical ratios (ARs) were higher in patients with AL-CA than in patients with ATTRwt-CA, ATTRv-CA and controls [39]. The same strain pattern has been described by Arvidsson et al. in ATTR-CA patients [14]. This feature was absent in patients with hypertrophic cardiomyopathy (HCM) and aimed at the differential diagnosis between the two forms.
In addition to the RV, the right atrial (RA) deformation has been recently evaluated in the literature. A reduction of total and active emptying fractions of the atria and a reduction of circumferential, segmental and longitudinal strain have been found in 16 patients affected by AL-CA undergoing 2D two-dimensional echocardiography (2DSTE) and three-dimensional speckle-tracking echocardiography (3DSTE) when compared to the healthy control group. This study suggests that right atrial involvement by the infiltrative process determines the progressive reduction of its contractile properties and the consequent dilatation [40].
Singulane et al. noted alterations in RA size and strain, suggesting an intrisic atriopathy, which could be independent from typical CA left pattern. Larger RA dimensions were more frequent in patient with ATTR-CA [41].
Tissue doppler imaging
Limited data are available about TDI. A 5-year cohort study conducted on 249 patients who presented cardiac involvement from AL-CA, which measured some right heart echocardiographic parameters such as TAPSE, tissue systolic velocity and strain rate, showed an early right ventricular impairment that was useful to stratify the prognosis of the patients, confirming the importance of evaluating these parameters in the diagnosis of patients with AL-CA [31, 42].
Tissue doppler imaging (TDI) of the RV in a patient with CA is shown in Fig. 2a.
Other TDI findings were a reduced septal E′ [43] and tissue velocity peak of the RV [44]. Similar findings have been found in the ventricular wall of patients with familial amyloidotic polyneuropathy, confirming the importance of their assessment during the diagnostic evaluation. These functional alterations appear before the anatomical myocardial damage shown by two-dimensional transthoracic echocardiography [45].
Prognostic value of DE findings
The assessment of RV involvement by amyloidosis has demonstrated the importance of stratifying the prognosis like other cardiomyopathies [46]. Several authors have shown that a TAPSE value lower than 17 mm was associated with poor survival in patients with AL-CA [17]. Furthermore, in patients with all CA forms, a low TAPSE value was an independent predictor factor of major adverse cardiovascular events (MACE) such as acute HF, heart transplant or death [18].
In a recent study by Tjahjadi et al., FWRVLS was independently associated with all-cause mortality with worse short- and long-term survival during follow-up (FWRVLS < 16%). [47], thus suggesting its routine use during the evaluation of patients with CA [20, 47]. In addition, the RV TDI evaluation showed an early contractile alteration that appeared to be an independent predictor of death, thus allowing a valid and early instrument for prognostic stratification [34, 42].
A reduced RV contractile function secondary to amyloid infiltration affects not only the clinical prognosis and hospitalization rate but also the functional capacity and quality of life. A multivariate analysis conducted in 72 patients with ATTR-CA undergoing a thorough clinical and imaging assessment with complete cardiopulmonary evaluation and echocardiography has shown that the tricuspid S wave by TDI and TAPSE was closely correlated to the VE/VCO2 slope and therefore were independent predictors of reduced functional capacity for physical exercise and ventilation [48].
Other imaging modalities
Cardiac magnetic resonance (CMR)
In recent years CMR emerged as a second-level, multiparametric, non-invasive tool for the cardiac amyloidosis evaluation. Unlike echocardiography, CMR allows an accurate evaluation of biventricular volumes and function especially of the right ventricle because it is independent from the acoustic window and has higher spatial resolution (Fig. 3).
Cardiac magnetic resonance (CMR) of right CA. The CMR images show a case of CA characterized by the presence of concentric biventricular hypertrophy; in particular the yellow arrows show the diffuse thickening of the right ventricular wall (a–c) and the white arrow shows an area of late gadolinium enhancement (LGE) (d)
In particular it represents the reference standard in terms of accuracy and reproducibility of ventricle volumes quantification and for the assessment of regional and global systolic function [49].
CA is characterized by an hypertrophic and restrictive phenotype, dilated atria with walls thickening (Fig. 3). Right ventricle involvement is variable. Diagnosis of cardiac amyloidosis is not possible on the basis of ventricular hypertrophy. The differential diagnosis in such patients includes hypertrophic cardiomyopathy (HCM), infiltrative cardiomyopathy and storage disease. Magnetic resonance gives structural imaging and tissue characterization that are always incremental to information obtained on echocardiography. The administration of a extracellular contrast agents such as gadolinium chelates, as in the late gadolinium enhancement (LGE) method and contrast-enhanced T1-weighted imaging for the estimation of extracellular volume (ECV), reveals pathognomonic features of cardiac amyloidosis with good diagnostic accuracy [50].
These features are also present in amyloid involvement of the RV [51] and some authors suggested how LGE-CMR may detect early cardiac alterations in patients with normal right and left ventricular wall dimensions [52].
Cardiomyocytes are closely packed together in the normal heart, and their intracellular space makes up the majority (about 85%) of the myocardial volume. The extracellular space enlarges in cardiac amyloidosis as a result of increased amyloid deposition which determines the increases in native T1 mapping and ECV values and causes a global, circumferential subendocardial LGE with non-coronary artery distribution (Fig. 3d) [53].
Indeed among the 10 red flags we find diffuse late gadolinium enhancement on CMR with subendocardial or transmural distribution [10].
It should be considered that renal involvement is frequent in patients with different forms of CA and that LGE should not be used in these [54].
CMR has also been used for the global and regional strain study of the right ventricle, demonstrating utility in the differential diagnosis of CA and hypertrophic cardiomyopathy; CA subtypes had similar strain patterns [55].
In addition Steen et al., using the right ventricular strain obtained by the fast strain-encoded cardiovascular magnetic resonance, distinguished patients with chronic non-ischemic heart failure due to dilated, hypertrophic cardiomyopathy or cardiac amyloidosis [56].
The study of the right atrial strain and strain rate performed by CMR also proved to be useful in distinguishing amyloid from hypertrophic cardiomyopathy [57]. Similary, the strain evaluation of biatrial chambers and right ventricle confirmed to be promising in early CA detection [58].
Scintigraphy
In suspected cardiac amyloidosis, 99mTc-bisphosphonate bone scintigraphy is indicated for a noninvasive diagnosis of ATTR‐CA differentiating it from the amyloid light-chain amyloidosis, in association with echocardiography/CMR [10].
Quantification of intensity of 99mTc-bisphosphonate uptake is crucial for the diagnosis of ATTR-CA by bone scintigraphy.
The exam consists of a static image one or 3 h after the bone-tracer injection and the interpretation is usually performed by quantitative or semi-quantitative analysis (absent, local, or diffuse uptake) [59].
A visible myocardial uptake equivalent to or greater than that in the bone, particularly in the ribs, or a contralateral heart–lung ratio (H/CL) less than 1.5 are currently used as diagnostic criteria for patients with ATTR-CA [60]. A poor survival rate is associated with a H/CL ratio of 1.6 [61].
Based on a simple visual grading system of the delayed planar image (3 h), Perugini and colleagues categorised myocardial amyloid absorption in no cardiac uptake (grade 0), mild cardiac uptake less than bone (grade 1), heart uptake greater than bone, but uptake in bone remains clearly visible (grade 2), and substantial cardiac uptake with absent signal in bone (grade 3) [62].
This technique has been validated, and it appears to be sensitive enough to detect early ATTR-CA amyloid deposits in asymptomatic individuals with normal echocardiography and CMR [63] but it has limited value for the detection of right ventricular involvement.
A Perugini grade 2 or 3 demonstrated great sensitivity of > 99% for ATTR-CA but a poorer specificity of 82–86% [64]. However the test's specificity rises to 100% if serum and urine tests for AL amyloidosis are negative.
Prognostic value of CMR and scintigraphy findings
Beyond the diagnostic skills, CMR has an important prognostic value; in particular the presence of LGE and ECV are associated with worse prognosis for the onset of myocardial dysfunction, cardiac arrhythmias and progression to heart failure [50]. A sistematic review performed by Rana et al. marked an increased all-cause mortality due to amyloid deposition in right and left ventricle studied by CMR [65].
In a retrospective analysis conducted by Wan et al. on 61 patients with suspected CA who were subjected to CMR, right ventricular end-systolic volume index (RVESVi) and right ventricular late gadolinium enhancement (RV-LGE) were independent predictors of mortality in CA [66].
Moreover, RV LGE and right global longitudinal strain obtained with CMR confirmed to be promising predictors for CA [67].
The prognostic value of 99mTc-bisphosphonate bone scintigraphy is given by its ability to distinguish the ATTR-CA the AL-CA which is characterized by a worse prognosis [59]. Furthermore major adverse cardiac events, acute heart failure, and higher mortality are all linked to greater myocardial retention of the various bone tracers [60].
Role of biopsy
The biopsy is crucial for CA and allows its definitive diagnosis through an invasive strategy, in association with echocardiographic or CMR findings suggesting right or left CA; this can be obtained by direct biopsy of myocardial tissue, or through the biopsy study of extracardiac tissues (abdominal adiposity). The biopsy is, therefore, helpful in the case of equivocal or non-diagnostic cardiac imaging findings [10]. Congo red staining of abdominal adipose tissue has a sensitivity and specificity of up to 70–90% in subjects with AL-CA [68] and sensitivity of 15 and 45% in ATTRwt-CA and ATTRv-CA, respectively [69]. The biopsy, in particular the endomyocardial analysis, is not free from complications [70]. A noninvasive diagnosis of right CA can be reached by positive scintigraphy, typical echocardiographic or CMR findings and absence of serum or urinary evidence of light chains [10]. Although the risk of complications is reduced if expert operators, the biopsy should be performed by trained clinicians and inappropriate centres with high experience.
Conclusions
Multimodality imaging has been proposed for many cardiovascular disorders to ameliorate the diagnostic performance and management of patients affected. CA represent the paradigm of multimodality application in the recent era; the conscious use of the different imaging methods has permitted to diagnose ATTR-CA non-invasively, to diagnose AL-CA early, stratify the patient’s risk and monitor the performance of therapy in this setting.
Although the right ventricular involvement has been investigated less extensively than the left ventricle, its detection seems fundamental, especially in prognostic stratification. Indeed, when present, it is associated with the worst prognosis, even in the absence of pulmonary hypertension. It has been assumed that right myocardial damage usually arises late. However, new evidence demonstrates that sometimes the involvement is contemporary or even unilateral, such as complicating CA diagnosis and clinical identification. It is therefore advisable that in patients with clinical suspicion of CA clinicians should also evaluate the right portions of the heart even in the absence of evident anatomical damage.
In this regard, transthoracic echocardiography and more advanced ultrasonographic methods (f.e. strain imaging or tissue doppler imaging) represent a good, simple, non-invasive and practical tool for a first diagnostic assessment and prognostic stratification of such patients, if performed by skilled operators and after adequate training. MS has a limited value for the recognition of RV involvement but is pivotal for diagnosing ATTRwt-CA. DE and CMR can both offer advanced data, as strain analysis, but CMR is certainly more accurate, although less available and more expensive than DE. Thus, a confirmation with second CMR imaging and scintigraphy is essential because it sometimes could help avoid invasive diagnostic approaches such as endomyocardial biopsy, which remains the gold standard for a definitive diagnosis of CA.
Data availability
Data not available / The data that has been used is confidential.
References
Palmiero G, Vetrano E, Rubino M, Monda E, Dongiglio F, Lioncino M, Di Fraia F, Caiazza M, Verrillo F, Capodicasa L, Cerciello G, Manganelli F, Catalano M, D’Arienzo D, De Rimini ML, Ascione R, Golino P, Caso P, Ascione L, Limongelli G (2022) The role of new imaging technologies in the diagnosis of cardiac amyloidosis. Heart Fail Clin 18(1):61–72. https://doi.org/10.1016/j.hfc.2021.07.014. (Epub 2021 Oct 25, PMID: 34776084)
Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, Fontana M, Gheysens O, Gillmore JD, Glaudemans AWJM, Hanna MA, Hazenberg BPC, Kristen AV, Kwong RY, Maurer MS, Merlini G, Miller EJ, Moon JC, Murthy VL, Quarta CC, Rapezzi C, Ruberg FL, Shah SJ, Slart RHJA, Verberne HJ, Bourque JM (2021) ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2-evidence base and standardized methods of imaging. Circ Cardiovasc Imaging 14(7):e000029. https://doi.org/10.1161/HCI.0000000000000029. (Epub 2021 Jul 1, PMID: 34196223)
Bajwa F, O’Connor R, Ananthasubramaniam K (2021) Epidemiology and clinical manifestations of cardiac amyloidosis. Heart Fail Rev. https://doi.org/10.1007/s10741-021-10162-1. (Epub ahead of print, PMID: 34694575)
Martinez-Naharro A, Hawkins PN, Fontana M (2018) Cardiac amyloidosis. Clin Med (Lond) 18(Suppl 2):s30–s35. https://doi.org/10.7861/clinmedicine.18-2-s30. (PMID:29700090; PMCID:PMC6334035)
Rubin J, Maurer MS (2020) Cardiac amyloidosis: overlooked, underappreciated, and treatable. Annu Rev Med 27(71):203–219. https://doi.org/10.1146/annurev-med-052918-020140. (PMID: 31986086)
Wechalekar AD, Gillmore JD, Hawkins PN (2016) Systemic amyloidosis. Lancet 387(10038):2641–2654. https://doi.org/10.1016/S0140-6736(15)01274-X. (Epub 2015 Dec 21, PMID: 26719234, 3)
Gilstrap LG, Dominici F, Wang Y, El-Sady MS, Singh A, Di Carli MF, Falk RH, Dorbala S (2019) Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among feefor-service medicare beneficiaries in the United States. Circ Heart Fail. 12(6):e005407. https://doi.org/10.1161/CIRCHEARTFAILURE.118.005407. (Epub 2019 Jun 7, PMID:31170802; PMCID: PMC6557425)
Nitsche C, Scully PR, Patel KP, Kammerlander AA, Koschutnik M, Dona C, Wollenweber T, Ahmed N, Thornton GD, Kelion AD, Sabharwal N, Newton JD, Ozkor M, Kennon S, Mullen M, Lloyd G, Fontana M, Hawkins PN, Pugliese F, Menezes LJ, Moon JC, Mascherbauer J, Treibel TA (2021) Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol. 77(2):128–139. https://doi.org/10.1016/j.jacc.2020.11.006. (Epub 2020 Nov 9, PMID: 33181246; PMCID: PMC7805267)
Hahn VS, Yanek LR, Vaishnav J, Ying W, Vaidya D, Lee YZJ, Riley SJ, Subramanya V, Brown EE, Hopkins CD, Ononogbu S, Perzel Mandell K, Halushka MK, Steenbergen C Jr, Rosenberg AZ, Tedford RJ, Judge DP, Shah SJ, Russell SD, Kass DA, Sharma K (2020) Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. JACC Heart Fail. 8(9):712–724. https://doi.org/10.1016/j.jchf.2020.04.007. (Epub 2020 Jul 8, PMID: 32653448; PMCID: PMC7604801)
Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, Burazor I, Caforio ALP, Damy T, Eriksson U, Fontana M, Gillmore JD, Gonzalez-Lopez E, Grogan M, Heymans S, Imazio M, Kindermann I, Kristen AV, Maurer MS, Merlini G, Pantazis A, Pankuweit S, Rigopoulos AG, Linhart A (2021) Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 42(16):1554–1568. https://doi.org/10.1093/eurheartj/ehab072. (PMID:33825853; PMCID:PMC8060056)
Writing Committee; Kittleson MM, Ruberg FL, Ambardekar AV, Brannagan TH, Cheng RK, Clarke JO, Dember LM, Frantz JG, Hershberger RE, Maurer MS, Nativi-Nicolau J, Sanchorawala V, Sheikh FH (2023) ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023 Mar 21;81(11):1076–1126. https://doi.org/10.1016/j.jacc.2022.11.022
Ternacle J, Krapf L, Mohty D, Magne J, Nguyen A, Galat A, Gallet R, Teiger E, Côté N, Clavel MA, Tournoux F, Pibarot P, Damy T (2019) Aortic stenosis and cardiac amyloidosis: JACC review topic of the week. J Am Coll Cardiol 74(21):2638–2651. https://doi.org/10.1016/j.jacc.2019.09.056. (PMID: 31753206)
Imbriani M, Taino G, Panigazzi M, Capodaglio EM, Oddone E (2019) Active population aging, ICDICF clinical model and occupational and rehabilitation medicine. G Ital Med Lav Ergon 41(2):140–146 (Italian, PMID: 31170344)
Arvidsson S, Henein MY, Wikström G, Suhr OB, Lindqvist P (2018) Right ventricular involvement in transthyretin amyloidosis. Amyloid 25(3):160–166. https://doi.org/10.1080/13506129.2018.1493989. (Epub 2018 Sep 7, PMID: 30193533)
Cappelli F, Porciani MC, Bergesio F, Perlini S, Attanà P, Moggi Pignone A, Salinaro F, Musca F, Padeletti L, Perfetto F (2012) Right ventricular function in AL amyloidosis: characteristics and prognostic implication. Eur Heart J Cardiovasc Imaging 13(5):416–422. https://doi.org/10.1093/ejechocard/jer289. (Epub 2011 Dec 16, PMID: 22180463)
Wan K, Lin J, Guo X, Song R, Wang J, Xu Y, Li W, Cheng W, Sun J, Zhang Q, Han Y, Chen Y (2020) Prognostic value of right ventricular dysfunction in patients with AL amyloidosis: comparison of different techniques by cardiac magnetic resonance. J Magn Reson Imaging 52(5):1441–1448. https://doi.org/10.1002/jmri.27200. (Epub 2020 Jul 20, PMID: 32691470)
Ghio S, Perlini S, Palladini G, Marsan NA, Faggiano G, Vezzoli M, Klersy C, Campana C, Merlini G, Tavazzi L (2007) Importance of the echocardiographic evaluation of right ventricular function in patients with AL amyloidosis. Eur J Heart Fail 9(8):808–813. https://doi.org/10.1016/j.ejheart.2007.05.006. (Epub 2007 Jun 22, PMID: 17586091)
Bodez D, Ternacle J, Guellich A, Galat A, Lim P, Radu C, Guendouz S, Bergoend E, Couetil JP, Hittinger L, Dubois-Randé JL, Plante-Bordeneuve V, Deux JF, Mohty D, Damy T (2016) Prognostic value of right ventricular systolic function in cardiac amyloidosis. Amyloid 23(3):158–167. https://doi.org/10.1080/13506129.2016.1194264. (Epub 2016 Jun 27, PMID: 27348696)
Mishra T, Pahuja M, Abidov A (2019) Increasingly Recognized Role of Right Ventricle Assessment in Cardiac Amyloidosis. JACC Heart Fail 7(3):277–278. https://doi.org/10.1016/j.jchf.2018.11.014. (PMID: 30819388)
Uzan C, Lairez O, Raud-Raynier P, Garcia R, Degand B, Christiaens LP, Rehman MB (2018) Right ventricular longitudinal strain: a tool for diagnosis and prognosis in light-chain amyloidosis. Amyloid 25(1):18–25. https://doi.org/10.1080/13506129.2017.1417121. (Epub 2017 Dec 20, PMID: 29260587)
Merlini G, Bellotti V (2003) Molecular mechanisms of amyloidosis. N Engl J Med 349(6):583–596. https://doi.org/10.1056/NEJMra023144. (PMID: 12904524)
Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, Marwick TH, Thomas JD (2012) Relative apical sparing of longitudinal strain using two-dimensional speckle tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 98(19):1442–1448. https://doi.org/10.1136/heartjnl-2012-302353. (Epub 2012 Aug 3, PMID: 22865865)
Hashimoto H, Kurata A, Mizuno H, Nashiro T, Hangaishi A, Kuroda M, Usuki K, Horiuchi H (2015) Pulmonary arterial hypertension due to pulmonary vascular amyloid deposition in a patient with multiple myeloma. Int J Clin Exp Pathol. 8(11):15391–15395 (PMID: 26823900; PMCID: PMC4713686)
Luz AE, Schneider U, Ehlerding G, Frenzel H, Koch KM, Kühn K (1995) Right ventricular cardiac failure and pulmonary hypertension in a long-term dialysis patient–unusual presentation of visceral beta 2-microglobulin amyloidosis. Nephrol Dial Transplant 10(4):555–558. https://doi.org/10.1093/ndt/10.4.555. (PMID: 7624004)
Giancaterino S, Urey MA, Darden D, Hsu JC (2020) Management of arrhythmias in cardiac amyloidosis. JACC Clin Electrophysiol 6(4):351–361. https://doi.org/10.1016/j.jacep.2020.01.004. (PMID: 32327068)
Ballantyne B, Manian U, Sheyin O, Davey R, De S (2020) Stroke risk and atrial mechanical dysfunction in cardiac amyloidosis. ESC Heart Fail. 7(2):705–707. https://doi.org/10.1002/ehf2.12602. (Epub 2020 Jan 21, PMID: 31965737; PMCID: PMC7160485)
Hiroshima Y, Sugaya A, Aizawa K, Kawahito K (2019) Right atrium thrombus due to cardiac amyloidosis; Report of a case. Kyobu Geka 72(11):931–934 (Japanese, PMID: 31588112)
Vergaro G, Aimo A, Rapezzi C, Castiglione V, Fabiani I, Pucci A, Buda G, Passino C, Lupón J, Bayes-Genis A, Emdin M, Braunwald E (2022) Atrial amyloidosis: mechanisms and clinical manifestations. Eur J Heart Fail 24(11):2019–2028. https://doi.org/10.1002/ejhf.2650. (Epub 2022 Aug 21, PMID: 35920110)
Sukhacheva TV, Eremeeva MV, Ibragimova AG, Vaskovskii VA, Serov RA, Revishvili ASh (2016) Isolated atrial amyloidosis in patients with various types of atrial fibrillation. Bull Exp Biol Med 160(6):844–849. https://doi.org/10.1007/s10517-016-3324-3. (Epub 2016 May 10, PMID: 27160887)
Di Bella G, Cappelli F, Licordari R, Piaggi P, Campisi M, Bellavia D, Minutoli F, Gentile L, Russo M, de Gregorio C, Perfetto F, Mazzeo A, Falletta C, Clemenza F, Vita G, Carerj S, Aquaro GD (2022) Prevalence and diagnostic value of extra-left ventricle echocardiographic findings in transthyretin-related cardiac amyloidosis. Amyloid 29(3):197–204. https://doi.org/10.1080/13506129.2022.2064739. (Epub 2022 Apr 23, PMID: 35465808)
Jurcuţ R, Onciul S, Adam R, Stan C, Coriu D, Rapezzi C, Popescu BA (2020) Multimodality imaging in cardiac amyloidosis: a primer for cardiologists. Eur Heart J Cardiovasc Imaging 21(8):833–844. https://doi.org/10.1093/ehjci/jeaa063. (PMID: 32393965)
Klein AL, Tajik AJ (1991) Doppler assessment of diastolic function in cardiac amyloidosis. Echocardiography 8(2):233–251. https://doi.org/10.1111/j.1540-8175.1991.tb01394.x. (PMID: 10149254)
Fontana M, Ćorović A, Scully P, Moon JC (2019) Myocardial amyloidosis: the exemplar interstitial disease. JACC Cardiovasc Imaging 12(11 Pt 2):2345–2356. https://doi.org/10.1016/j.jcmg.2019.06.023. (Epub 2019 Aug 14, PMID: 31422120)
Bellavia D, Pellikka PA, Dispenzieri A et al (2012) Comparison of right ventricular longitudinal strain imaging, tricuspid annular plane systolic excursion, and cardiac biomarkers for early diagnosis of cardiac involvement and risk stratification in primary systematic (AL) amyloidosis: a 5-year cohort study. Eur Heart J Cardiovasc Imaging 13(8):680–689
Licordari R, Minutoli F, Recupero A, Campisi M, Donato R, Mazzeo A, Dattilo G, Baldari S, Vita G, Zito C, Di Bella G (2021) Early impairment of right ventricular morphology and function in transthyretin-related cardiac amyloidosis. J Cardiovasc Echogr. 31(1):17–22. https://doi.org/10.4103/jcecho.jcecho_112_20. (Epub 2021 May 21, PMID: 34221881; PMCID:PMC8230159)
Rapezzi C, Merlini G, Quarta CC et al (2009) Systemic cardiac amyloidosis: disease profiles and clinical courses of the 3 main types. Circulation 120(13):1203–1212
Falk RH, Alexander KM, Liao R et al (2016) AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol 68(12):1323–1341
Urbano-Moral JA, Gangadharamurthy D, Comenzo RL, Pandian NG, Patel AR (2015) Threedimensional speckle tracking echocardiography in light chain cardiac amyloidosis: examination of left and right ventricular myocardial mechanics parameters. Rev Esp Cardiol (Engl Ed). 68(8):657–664. https://doi.org/10.1016/j.rec.2015.01.009. (Epub 2015 Jun 17, PMID: 26092748)
Moñivas Palomero V, Durante-Lopez A, Sanabria MT, Cubero JS, González-Mirelis J, Lopez- Ibor JV, Navarro Rico SM, Krsnik I, Dominguez F, Mingo AM, Hernandez-Perez FJ, Cavero G, Santos SM (2019) Role of right ventricular strain measured by two-dimensional echocardiography in the diagnosis of cardiac amyloidosis. J Am Soc Echocardiogr 32(7):845-853.e1. https://doi.org/10.1016/j.echo.2019.03.005. (Epub 2019 May 8, PMID: 31078369)
Nemes A, Földeák D, Domsik P, Kalapos A, Kormányos Á, Borbényi Z, Forster T (2018) Right atrial deformation analysis in cardiac amyloidosis—results from the three-dimensional speckle-tracking echocardiographic MAGYAR-path study. Arq Bras Cardiol. 111(3):384–391. https://doi.org/10.5935/abc.20180150. (Epub 2018 Aug 20, PMID: 30133551; PMCID: PMC6173351)
Singulane CC, Slivnick JA, Addetia K, Asch FM, Sarswat N, Soulat-Dufour L, Mor-Avi V, Lang RM (2022) Prevalence of right atrial impairment and association with outcomes in cardiac amyloidosis. J Am Soc Echocardiogr 35(8):829-835.e1. https://doi.org/10.1016/j.echo.2022.03.022. (Epub 2022 Apr 7, PMID: 35398489)
Miller F Jr, Bellavia D (2013) Comparison of right ventricular longitudinal strain imaging, tricuspidannular plane systolic excursion, and cardiac biomarkers for early diagnosis of cardiac involvement and risk stratification in primary systematic (AL) amyloidosis: a 5-year cohort study: reply. Eur Heart J Cardiovasc Imaging 14(1):91–92. https://doi.org/10.1093/ehjci/jes227. (PMID: 23362526)
Granstam SO, Rosengren S, Vedin O, Kero T, Sörensen J, Carlson K, Flachskampf FA, Wikström G (2013) Evaluation of patients with cardiac amyloidosis using echocardiography, ECG and right heart catheterization. Amyloid 20(1):27–33. https://doi.org/10.3109/13506129.2012.761967. (Epub 2013 Jan 22 PMID: 23339421)
Ahmad MM, Basraon J, Razzaque I, Port SC, Ammar KA (2018) The complementary nature of tissue Doppler to 99mTc-PYP imaging in diagnosis of right ventricular cardiac amyloidosis. J Nucl Cardiol 25(4):1412–1414. https://doi.org/10.1007/s12350-017-0882-3. (Epub 2017 Apr 21, PMID: 28432670)
Lindqvist P, Olofsson BO, Backman C, Suhr O, Waldenström A (2006) Pulsed tissue Doppler and strain imaging discloses early signs of infiltrative cardiac disease: a study on patients with familial amyloidotic polyneuropathy. Eur J Echocardiogr 7(1):22–30. https://doi.org/10.1016/j.euje.2005.03.004. (PMID: 15869906)
Tadic M, Kersten J, Nita N, Schneider L, Buckert D, Gonska B, Scharnbeck D, Dahme T, Imhof A, Belyavskiy E, Cuspidi C, Rottbauer W (2021) The prognostic importance of right ventricular longitudinal strain in patients with cardiomyopathies, connective tissue diseases, coronary artery disease, and congenital heart diseases. Diagnostics (Basel) 11(6):954. https://doi.org/10.3390/diagnostics11060954. (PMID:34073460; PMCID:PMC8228710)
Tjahjadi C, Fortuni F, Stassen J, Debonnaire P, Lustosa RP, Marsan NA, Delgado V, Bax JJ (2022) Prognostic implications of right ventricular systolic dysfunction in cardiac amyloidosis. Am J Cardiol 15(173):120–127. https://doi.org/10.1016/j.amjcard.2022.02.048. (Epub 2022 Apr 1, PMID: 35369931)
Bartolini S, Baldasseroni S, Fattirolli F, Silverii MV, Piccioli L, Perfetto F, Marchionni N, Di Mario C, Martone R, Taborchi G, Morini S, Vignini E, Cappelli F (2021) Poor right ventricular function is associated with impaired exercise capacity and ventilatory efficiency in transthyretin cardiac amyloid patients. Intern Emerg Med 16(3):653–660. https://doi.org/10.1007/s11739-020-02474-1. (Epub 2020 Sep 12, PMID: 32918156)
Francone M, Aquaro GD, Barison A, Castelletti S, de Cobelli F, de Lazzari M, Esposito A, Focardi M, di Renzi P, Indolfi C, Lanzillo C, Lovato L, Maestrini V, Mercuro G, Natale L, Mantini C, Polizzi G, Rabbat M, Secchi F, di Cesare E, Pontone G (2021) Appropriate use criteria for cardiovascular MRI: SIC— SIRM position paper part 2 (myocarditis, pericardial disease, cardiomyopathies and valvular heart disease). J Cardiovasc Med (Hagerstown) 22(7):515–529. https://doi.org/10.2459/JCM.0000000000001170. (PMID: 34076599)
White SK et al (2013) T1 mapping for myocardial extracellular volume measurement by CMR bolus only versus primed infusion technique. JACC Cardiovasc Imaging 6:955–962
Mavrogeni SI, Vartela V, Ntalianis A, Vretou R, Ikonomidis I, Tselegkidou M, Paraskevaidis I, Markousis-Mavrogenis G, Noutsias M, Rigopoulos A, Kolovou G, Kastritis E (2021) Cardiac amyloidosis: in search of the ideal diagnostic tool. Herz 46(Suppl 1):9–14. https://doi.org/10.1007/s00059-019-04871-5. (English, Epub 2019 Dec 3, PMID: 31796976)
Syed IS, Glockner JF, Feng D, Araoz PA, Martinez MW, Edwards WD, Gertz MA, Dispenzieri A, Oh JK, Bellavia D, Tajik AJ, Grogan M (2010) Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging 3(2):155–164. https://doi.org/10.1016/j.jcmg.2009.09.023. (PMID: 20159642)
Rehwald WG, Fieno DS, Chen EL, Kim RJ, Judd RM (2002) Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation 105:224–229
Fontana M et al (2014) Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging 7:157–165
Eckstein J, Körperich H, Weise Valdés E, Sciacca V, Paluszkiewicz L, Burchert W, Farr M, Sommer P, Sohns C, Piran M (2022) CMR-based right ventricular strain analysis in cardiac amyloidosis and its potential as a supportive diagnostic feature. Int J Cardiol Heart Vasc. 44:101167. https://doi.org/10.1016/j.ijcha.2022.101167. (PMID: 36632287; PMCID: PMC9827025)
Steen H, Giusca S, Montenbruck M, Patel AR, Pieske B, Florian A, Erley J, Kelle S, Korosoglou G (2021) Left and right ventricular strain using fast strain-encoded cardiovascular magnetic resonance for the diagnostic classification of patients with chronic non-ischemic heart failure due to dilated, hypertrophic cardiomyopathy or cardiac amyloidosis. J Cardiovasc Magn Reson 23(1):45. https://doi.org/10.1186/s12968-021-00711-w.PMID:33823860;PMCID:PMC8025329
Eckstein J, Sciacca V, Körperich H, Paluszkiewicz L, Valdés EW, Burchert W, Gerçek M, Farr M, Sommer P, Sohns C, Piran M (2022) Cardiovascular magnetic resonance imaging-based right atrial strain analysis of cardiac amyloidosis. Biomedicines 10(12):3004. https://doi.org/10.3390/biomedicines10123004. (PMID:36551760; PMCID:PMC9775378)
Eckstein J, Moghadasi N, Körperich H, Weise Valdés E, Sciacca V, Paluszkiewicz L, Burchert W, Piran M (2022) A machine learning challenge: detection of cardiac amyloidosis based on bi-atrial and right ventricular strain and cardiac function. Diagnostics (Basel) 12(11):2693. https://doi.org/10.3390/diagnostics12112693. (PMID:36359536; PMCID:PMC9689404)
Mattana F, Muraglia L, Girardi F, Cerio I, Porcari A, Dore F, Bonfiglioli R, Fanti S (2022) Clinical application of cardiac scintigraphy with bone tracers: controversies and pitfalls in cardiac amyloidosis. Vessel Plus 6:13. https://doi.org/10.20517/2574-1209.2021.87
Bokhari S et al (2013) (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 6:195–201
Castano A et al (2016) Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol 1:880–889
Perugini E et al (2005) Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3- diphosphono- 1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 46:1076–1084
Rapezzi C et al (2011) Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretinrelatedg cardiac amyloidosis. JACC Cardiovasc Imaging 4:659–670
Falk RH, Lee VW, Rubinow A, Hood WB Jr, Cohen AS (1983) Sensitivity of technetium-99m-pyrophosphate scintigraphy in diagnosing cardiac amyloidosis. Am J Cardiol 51:826–830
Raina S, Lensing SY, Nairooz RS, Pothineni NV, Hakeem A, Bhatti S, Pandey T (2016) Prognostic value of late gadolinium enhancement CMR in systemic amyloidosis. JACC Cardiovasc Imaging 9(11):1267–1277. https://doi.org/10.1016/j.jcmg.2016.01.036. (Epub 2016 Aug 24, PMID: 27568115)
Wan K, Sun J, Han Y, Luo Y, Liu H, Yang D, Cheng W, Zhang Q, Zeng Z, Chen Y (2018) Right ventricular involvement evaluated by cardiac magnetic resonance imaging predicts mortality in patients with light chain amyloidosis. Heart Vessels 33(2):170–179. https://doi.org/10.1007/s00380-017-1043-y3. (Epub 2017 Aug 24; PMID: 28840397; PMCID: PMC5766713)
Li X, Li J, Lin L, Shen K, Tian Z, Sun J, Zhang C, An J, Jin Z, Vliegenthart R, Selvanayagam JB, Wang Y (2020) Left and right ventricular myocardial deformation and late gadolinium enhancement: incremental prognostic value in amyloid light-chain amyloidosis. Cardiovasc Diagn Ther 10(3):470–480. https://doi.org/10.21037/cdt-20-181. (PMID:32695626; PMCID:PMC7369280)
Gertz MA, Li CY, Shirahama T, Kyle RA (1988) Utility of subcutaneous fat aspiration for the diagnosis of systemic amyloidosis (immunoglobulin light chain). Arch Intern Med 148(4):929–933 (PMID: 2451487)
Siddiqi OK, Ruberg FL (2017) Challenging the myths of cardiac amyloidosis. Eur Heart J 38(24):1909–1912. https://doi.org/10.1093/eurheartj/ehx210. (PMID: 28444296)
Shah Z, Rali A, Gupta K (2019) National trends and procedural complications from endomyocardial biopsy: results from the national inpatient sample, 2007–2014. Cardiology 142(4):223. https://doi.org/10.1159/000500113. (Epub 2019 Jun 18, PMID: 31212282)
Funding
There is no financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tana, M., Tana, C., Palmiero, G. et al. Imaging findings of right cardiac amyloidosis: impact on prognosis and clinical course. J Ultrasound 26, 605–614 (2023). https://doi.org/10.1007/s40477-023-00789-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-023-00789-1