Abstract
Purpose of Review
This article aims to review the relevant updates in pathogenesis, diagnostics, clinical manifestations, and treatments of tick-borne diseases involving the nervous system, with special emphasis on emerging viral and bacterial infections transmitted by deer ticks (Ixodes scapularis).
Recent Findings
Neuroborreliosis includes a wide array of peripheral and/or central nervous system syndromes, of which diagnosis depends on a combination of clinical gestalt, serum serologic testing, and CSF cellular and serologic analysis. Powassan virus may cause life-threatening neuroinvasive disease and diagnosis that is based on CSF serology with treatment being largely supportive. Neurologic manifestations of anaplasmosis and babesiosis are rare but have been documented in case reports. Diagnosis is dependent on serum molecular and microscopic and serologic testing, and the pathophysiologic mechanisms of these neurologic syndromes are not fully understood. Borrelia miyamotoi is an emerging pathogen and, in immunocompromised patients, can cause severe meningoencephalitis.
Summary
Ixodes scapularis-borne infections such as Lyme disease, anaplasmosis, babesiosis, Powassan virus, and Borrelia miyamotoi disease can have significant neurologic manifestations. Due to the potential for poor outcomes, physicians must have a working knowledge of these pathogens, and a high index of suspicion is required to screen for these diseases in patients with the correct risk factors. Future research will help realize the full range of neurologic manifestations of these pathogens and also clarify their underlying pathophysiologic mechanisms, which will aid in developing new methods of diagnosis and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tick-borne diseases (TBD) are widely recognized as one of the most common vector-borne diseases on a global scale [1]. According to Rodino et al., TBDs account for 77–95% of annual reported vector-borne disease in the USA [2]. TBDs are especially concerning because ticks can transmit multiple pathogens to humans during a blood meal. Examples of this include bacteria (ex: Borrelia burgdorferi, Anaplasma phagocytophilum, Borrelia miyamotoi, Ehrlichia chaffeensis, Francisella tularensis, Rickettsia rickettsii), viruses (ex: Heartland virus, Powassan virus), and parasites (ex: Babesia microti, Babesia duncani) depending on one’s geographic location and the type of tick encountered [2, 3]. This can make diagnosis and management especially challenging, as infections with many of these pathogens can present with non-specific flu-like symptoms and may overlap with one another prior to the onset of any distinguishing features [4]. Maintaining a high index of suspicion for multiple tick-borne pathogens is of paramount importance, as certain TBDs have a wide spectrum of clinical severity ranging from asymptomatic disease to life-threatening, multi-organ involvement, sepsis or death [2].
Neurologic manifestations can be a serious complication of many TBDs, as patients may develop severe neurologic deficits, life-threatening meningoencephalitis, and possibly long-term and permanent sequelae [5,6,7]. While certain TBDs such as Lyme disease are well known to have neurologic manifestations, recent studies have revealed novel neurological syndromes in diseases such as Lyme disease and other tick-borne infections and also added to the current understanding of how certain tick-borne infections affect the nervous system [5, 8,9,10, 11•]. Given the rising threat of tick-borne disease, clinicians must maintain a high index of suspicion in order to prevent poor outcomes [6, 9, 11•].
In the USA, Ixodes scapularis, also known as the black-legged deer tick, is rapidly becoming a major threat to public health. Studies show that I. scapularis has expanded its geographic range into the upper Midwest, northeast, and mid-Atlantic states, bringing the pathogens it harbors to new territories. To date, I. scapularis has four nationally notifiable diseases: Lyme disease, anaplasmosis, Powassan virus disease, and babesiosis, and studies have shown increases in the incidence of all four diseases in recent years. Additionally, while not yet a notifiable condition, B. miyamotoi, which causes relapsing tick-borne fever, is also harbored by I. scapularis. This pathogen appears to follow a similar geographic distribution to Lyme disease and is gaining momentum as an emerging infection [12].
Given the looming threat posed by I. scapularis-borne diseases, this review will focus on the relevant nervous system manifestations, underlying pathophysiology, diagnostic modalities, and current therapeutics of Lyme disease, babesiosis, anaplasmosis, B. miyamotoi disease, and Powassan virus, all of which can be transmitted by the vector I. scapularis.
Methods
Information gathered during this review was analyzed and presented as a theory of nervous system manifestations of TBD. Exclusion criteria included articles in a language other than English or Spanish and unpublished reports or data. Searches of literature were performed using the MEDLINE/PubMed electronic database that emphasized literature largely published within the 10 years but did include several highly relevant studies published prior to that. To retrieve information for this review, the following search terms, either alone or in combination, were used: “Tick-Borne Diseases”, “nervous system”, “meningitis”, “meningoencephalitis”, “Lyme disease”, “neuroborreliosis”, “anaplasmosis”, “Borrelia miyamotoi”, “Powassan virus”, “babesiosis” and “cerebral malaria”. Cerebral malaria was included in this search given the relationship between malarial disease and babesiosis and to try to understand if any similar pathophysiologic mechanisms exist between both diseases.
Lyme Disease
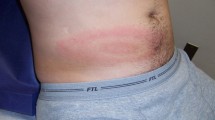
Lyme disease, which is caused by the bacteria Borrelia burgdorferi sensu lato (s.l.) complex, has been reported to be the most common tick-borne disease in North America and Europe, with the predominant culprit pathogen in the USA being Borrelia burgdorferi sensu stricto and in Europe being Borrelia garinii and Borrelia afzelii [11•]. Typical early symptoms of Lyme disease include erythema migrans and nonspecific symptoms such as fever, headache, fatigue, and myalgia. If untreated, Lyme disease can progress to its later stages, and patients may develop neurologic symptoms as the peripheral and/or central nervous system (CNS) are affected [2, 11•]. This stage is often referred to as “neuroborreliosis” and is estimated to occur in 10–20% of North American cases caused by Borrelia burgdorferi sensu stricto [11•]. The most common manifestation of neuroborreliosis is polyradiculitis, which refers to inflammation of the spinal nerve roots and/or cranial nerves, but other sequelae also include lymphocytic meningitis, parenchymal inflammation of brain or spinal cord, stroke, peripheral neuropathy, and/or encephalopathy [5, 11•]. One of the earliest described sequelae of neuroborreliosis is Bannwarth syndrome, which in Europe is the second most common presentation of Lyme disease after erythema migrans. Bannwarth syndrome (also known as Garin-Bujadoux-Bannwarth syndrome) refers to a severe polyradiculitis that leads to debilitating, burning radicular pain and/or cranial nerve palsies, typically involving the facial nerve. In 75% of Bannwarth cases, patients may develop flaccid paralysis or segmental sensory disturbances in 1–4 weeks after the onset of the radicular pain [5]. Bannwarth syndrome has largely been reported in Europe, with few cases seen in North America, which is believed to be due to different species of Borrelia inhabiting the different continents [11•]. With regards to direct parenchymal involvement during neuroborreliosis, the spinal cord is documented to be the most affected, resulting in myelitis with gait and bladder abnormalities, but encephalitis has been reported as well [5, 13]. Stroke has also been documented in case series and is suspected to be due to possible Borrelia-induced vasculitis [5].
The mechanism by which B. burgdorferi can enter the nervous system is highly complex and not yet completely understood. The first access point to the central nervous system for spirochetes is through the cerebral spinal fluid (CSF) via the bloodstream or through the peripheral nerves, as studies have shown that spirochetes can be recovered from human CSF 14-18 days post tick bite [11•]. Following this, the pathogen gains an access point for the central nervous system tissues and for the meninges. The spirochete may upregulate the release of many pro-inflammatory cytokines such as CXCL13 from neural cells, which are postulated to play a major role in the early stages of neuroborreliosis [11•, 14]. Also, studies suggest that the hypervariability of the OspC (outer surface protein) protein, which allows B. burgdorferi to invade the host and hide from the human immune system, and even tissue-specific expression of Osp proteins may play a role in the development of neuroborreliosis [11•].
The understanding of later stages of neuroborreliosis is not as well understood. Studies on the transcriptomic CSF profile of patients with neuroborreliosis pre and post treatment suggest that later stages of neuroborreliosis may be related to alteration of the molecular pathways and transcriptome, with less of an emphasis on the inflammatory pathways, but further studies are required to elucidate the complex pathophysiologic mechanism [11•].
Diagnosis of neuroborreliosis focuses on examining the host immune response, as studies have suggested that the bacterial burden during nervous system infection appears to be very low, and the pathophysiology is driven by the inflammatory response that occurs in response to spirochete exposure [15]. When a patient with the correct epidemiologic risk factors (i.e., living in the Northeast of the USA) presents with a syndrome concerning for neuroborreliosis, diagnosis is typically made through a combination of clinical gestalt, serum, and CSF testing [16•]. Serum testing involves two-tiered testing which begins with a screening ELISA, followed by a confirmatory immunoblot (standard two-tiered test) or a second enzyme immune assay (modified two-tier test) [17]. Results should be interpreted cautiously as it may take 3–6 weeks to develop a serologic response, and positive titers can be reflective of prior infection, general background seropositivity in the population, and are known to be highly cross-reactive [15, 16•, 18]. The diagnosis of neuroborreliosis is supported by both the CSF analysis and Borrelia-specific CSF/serum antibody index (BSAI). CSF chemistry and cell count typically reveals inflammatory signs of lymphocytic pleocytosis, elevated protein and normal glucose [15, 18]. The BSAI can be a helpful diagnostic tool as it measures the intrathecal antibody production of anti-B. burgdorferi antibodies, while correcting for blood brain barrier breakdown and diffusion of antibodies from the serum [16•]. To perform this test, samples of paired serum and CSF are collected ideally at the same time, anti-B. burgdorferi-specific IgG-class antibodies and total IgG antibodies are measured from both sources, and the quantitative levels are compared to one another. An index of >1 provides evidence that at least some of the anti-B. burgdorferi-specific IgG antibodies have been synthesized within the central nervous system, rather than being solely due to diffusion from serum, thereby supporting the diagnosis of neuroborreliosis [16•]. Multiple studies have investigated the sensitivity of BSAI, but a systematic review/meta-analysis evaluating CSF-specific antibody index reported a summary sensitivity of either 79 or 86% and a specificity of either 94 or 96% depending on the type of study evaluated [19]. One review suggested that the sensitivity of BSAI may increase to 95% in patients that had not received antibiotics and had symptoms for >6 weeks [16•]. However, results should be interpreted cautiously, as false positives can occur in scenarios such as in cross reactivity with neurosyphilis, intrathecal antibody production in chronic inflammatory conditions unrelated to Lyme disease, and increased background positivity of intrathecal antibodies. Some limitations of using BSAI in the evaluation of neuroborreliosis include variability with different specific Borrelia species, duration of symptoms prior to testing, and exposure to antibiotics [16•]. In addition, the BSAI is a laboratory-developed test that is not widely available and must be validated in each clinical laboratory; as a result, interpretive cutoffs vary across laboratories [16•]. Recent literature has also investigated the biomarker potential of the chemokine CXCL13, as European studies have shown its presence in the CSF during confirmed neuroborreliosis, and it may be present during early infection prior to the development of BSAI and decrease after antibiotic therapy [14]. However, a major limitation to its use is that this marker can also be elevated in multiple other neuroinflammatory conditions (such as viral and tuberculous meningitis, CNS lymphomas, and neurosyphilis), and levels can fall below detectable limits if antibiotics are given prior to CSF testing [14, 16•, 18]. It has been documented that the use of CXCL13 requires further improvement in its specificity at this time and is not typically offered by laboratories in the USA [14, 16•].
CSF Borrelia-specific PCR is one additional testing modality but is not routinely recommended. A recent review of studies evaluating nucleic acid amplification tests (NAATs) for the detection of Lyme Borrelia DNA in CSF revealed and reported a mean sensitivity of roughly 22.5%, although the specificity was estimated to be >99% [16•]. There are no current FDA-approved Lyme NAATs; thus, available Lyme Borrelia molecular testing modalities are considered lab-developed tests, and therefore, the specificity can be dependent on the lab performing the test [16•, 18]. Multiple studies have called into question the utility of Borrelia-specific CSF PCR in the diagnosis of neuroborreliosis, often citing the poor sensitivity as a major drawback [20,21,22]. One study evaluating two different Borrelia-specific PCRs in Lyme neuroborreliosis postulated that perhaps the poor sensitivity is due to the bacterial burden being below the detectable limit by PCR, as previous microscopic studies have demonstrated low spirochete loads in CSF despite severe symptoms of neuroborreliosis [21, 23]. Several specific settings have been suggested where molecular methods can be considered such as immunocompromised patients with poor antibody production or cases with short duration of illness where CSF antibody index testing may be negative [18, 21]. Despite this, further study is required to realize the utility of molecular methods in these settings.
For patients with Lyme meningitis, cranial neuropathy, and radiculopathy, treatment with intravenous (IV) ceftriaxone, cefotaxime, penicillin G, or oral doxycycline is currently recommended per IDSA guidelines [18, 24•]. However, in patients with parenchymal involvement of the brain or spinal cord, IV antibiotics are the favored strategy. Generally, the recommended duration of antibiotic treatment is 14–21 days for Lyme neuroborreliosis [24•]. Even after proper antibiotic therapy, about 10–20% of Lyme patients may continue having subjective symptoms (brain fogginess, arthralgia, fatigue) for more than 6 months, sometimes called Post Treatment Lyme Disease Syndrome, for which the pathogenesis is unknown to date [15, 25].
Powassan Virus
Powassan virus is a tick-borne flavivirus that is known to cause severe encephalitis in the USA and is transmitted by the bite of I. scapularis vector. Studies show that it is closely related to the well-described tick-borne encephalitis virus of Europe and Asia, but much less is known about Powassan virus in comparison. It has been estimated that more than 240 cases have been reported thus far, and a prevalence of 1–3% has been reported amongst I. scapularis ticks in the northeast and north central USA [26].
Powassan virus has an incubation period of roughly 2–4 weeks. Symptoms typically begin with nonspecific fever, headache, and fatigue but can progress to severe neuroinvasive disease [26, 27•, 28•]. Clinical presentation can vary significantly, ranging from asymptomatic disease to severe neuroinvasive syndromes [28•, 29]. Other symptoms may include confusion, seizures, clonus, focal neurologic deficits, ophthalmoplegia, and spinal cord involvement leading to flaccid paralysis [26, 28•]. Some cases have also reported intraparenchymal hemorrhage and subdural hematoma [27•, 30]. Roughly 50% of cases of neuroinvasive disease can result in lasting hemiplegia, memory problems, and muscle wasting, with 10% of cases being fatal [28•].
Animal models have suggested that Powassan virus may gain access to the central nervous system by infecting the microvascular endothelial cells and pericytes of the blood-brain barrier [31]. Once inside the nervous system, the virus has direct neurotropism for glial cells and neurons throughout the CNS including in the anterior horn of the spinal cord [27•, 30]. Pathologic studies of mice infected with Powassan virus demonstrated patterns of meningoencephalitis with focal mononuclear infiltrates, perivascular cuffing, and necrotic lesions seen throughout the cerebral tissues. Powassan virus was also localized to the neurons and in the cases of infected mice who developed poliomyelitis-like syndromes, as the death of motor neurons in the brain stem and spinal cord was felt to be the mechanism of action driving these symptoms [27•].
Diagnosis is supported by multiple modalities, but evaluation typically begins with serum testing, lumbar puncture, and neuroimaging. CT imaging may be normal in the absence of intracranial bleeding, but MRI may show reversible, nonspecific T2 hyperintensities primarily in the periventricular white matter. A lumbar puncture will typically reveal a lymphocytic pleocytosis, elevated protein, with variable changes in glucose. CSF should be analyzed with ELISA-based IgM and immunofluorescence antibody assay, with positive results confirmed via neutralizing antibody testing [27•, 30]. Detection of Powassan virus nucleic acid by NAAT testing in blood, CSF, or tissue can also support diagnosis, but the virus can only be detected by PCR methods during the short-lived viremic phase that occurs early in the life cycle, often prior to when patients present with symptoms. Thus, the utility of PCR-based testing is limited, and serologic methods are heavily relied on to make a diagnosis [27•, 28•]. Diagnostic testing is only performed by state health departments and confirmed by the Centers for Disease Control and Prevention, which may contribute to the underdiagnosis of this viral infection in those cases with “aseptic meningoencephalitis” seen in clinical practice [30].
Treatment is largely supportive care for Powassan virus infection, which highlights the importance of taking preventive measures against tick exposures [28•, 31].
Anaplasmosis
Anaplasma phagocytophilum causes the syndrome Human Granulocytic Anaplasmosis (HGA) and is a gram-negative bacteria transmitted by the bite of infected I. scapularis ticks. Epidemiological studies suggest that cases of HGA are increasing in the USA, and estimated mortality rates are 0.6–1%. Typical symptoms may include a nonspecific febrile illness, headache, and myalgias, while common lab abnormalities include leukopenia, thrombocytopenia, anemia, and transaminitis [32•].
Neurologic manifestations of anaplasmosis are generally considered uncommon. Several case reports describe rare but severe neurologic syndromes such as cerebral infarction and encephalitis [33, 34, 35•]. Additionally, several peripheral nervous system manifestations have been described such as trigeminal neuralgia, demyelinating polyneuropathy, facial nerve palsies, brachial nerve plexopathies, transient hearing loss, and peripheral neuropathy [33, 35•, 36].
The pathophysiology by which HGA affects the nervous system is not well understood, but several theories exist. Direct infection of endothelial cells by A. phagocytophilum may explain manifestations such as stroke. It has also been postulated that an increase in proinflammatory cytokines may be linked to altered nerve function seen in manifestations such as trigeminal neuralgia [35•, 36]. Further study is required on this topic to elucidate the underlying mechanism driving neurologic syndromes seen in HGA.
The most sensitive method of diagnosis is through serum PCR, but diagnosis may also be supported by the detection of morulae inclusion bodies in neutrophils on microscopy. Serology testing for anaplasmosis can be helpful, but testing should include both acute and convalescent titers to improve sensitivity [32•, 37]. Case reports of anaplasmosis encephalitis reported CSF analysis may show a lymphocytic pleocytosis, with normal glucose and slightly elevated protein. Serological or PCR-based testing for A. phagocytophilum in the CSF are not currently approved; thus diagnosis largely depends on correlation of clinical findings, CSF analysis, and serum testing [35•].
Doxycycline is the mainstay of treatment for anaplasmosis and is typically given for 7–10 days [32•, 37]. All reported cases of neurological manifestations of anaplasmosis have improved with doxycycline [35•].
Babesiosis
Human babesiosis in the USA is predominantly caused by the parasite B. microti and is transmitted through the bite of the infected I. scapularis tick. Epidemiologic studies report that cases have continued to increase over the past two decades, and the disease continues to expand into new territories in the USA. This TBD is also especially unique as infected individuals may transmit the pathogen to unknowing recipients via blood transfusion. Clinical manifestations can range from asymptomatic presentations to severe hemolytic anemia resulting in end-organ damage, complications such as disseminated intravascular coagulation (DIC), acute respiratory distress syndrome (ARDS), congestive heart failure, and even death. Typical clinical findings include fever, splenomegaly, hemolytic anemia, thrombocytopenia, and transaminitis [38•].
Meningitis and/or encephalitis rarely occurs in patients with babesiosis; however, neurologic symptoms are not uncommon. Case reports have described patients diagnosed with babesiosis presenting with confusion, cognitive impairment, coma, speech dysfunctions, and ataxia, and usually, patients with neurological complications have severe parasitemia and poor outcomes [39, 40•]. A retrospective review of adult patients with babesiosis from 2011 to 2021 reported that out of 163 patients, 52% experienced headache, 27% had confusion/delirium, 24% had impaired consciousness, 17% had ataxia/gait disorder, and 10% had vision impairment. Six patients with symptoms such as headache, confusion, altered mental status, and hearing loss underwent lumbar puncture. None of the patients had CSF pleocytosis, one had elevated protein, and CSF studies were otherwise reported as unremarkable. The authors of this study also demonstrated that as parasitemia increased, the prevalence of confusion, delirium, and impaired consciousness increased as well. Locke et al. also acknowledged that while Lyme disease may cause neurologic symptoms, less than 1/10th of patients in their study had erythema migrans, and also, their data demonstrated no relationship between the presence of neurologic symptoms and coinfection with Lyme disease [41•].
The pathophysiology regarding neurologic symptoms of babesiosis is not known but has been theorized to occur through a mechanism similar to that of Plasmodium falciparum cerebral malaria [40•]. The current understanding of the pathophysiology underlying cerebral malaria is highly complex and not fully clear, but it is believed to occur largely through vascular occlusion via cytoadherence and also through excessive proinflammatory cytokine production [42•]. While the exact mechanism by which cytokine production influences the development of cerebral malaria is unknown, cytokines such as TNF-a, IL-6, and IL-10 are increased in cerebral malaria, and it has been theorized that perhaps certain cytokines such as TNF-a can upregulate nitric oxide (NO) production, which can diffuse across the blood-brain barrier and cause neuronal dysfunction [42•]. Animal studies have demonstrated that the genomes of Babesia bovis and P. falciparum encode proteins involved in facilitating infected red blood cells adherence to vascular endothelium, but no other species of Babesia has shown similar potential for cytoadherence [38•, 40•]. Despite this, autopsy reports from cases of severe B. microti infection and neurologic syndromes are mixed, as patients with cerebellar hemorrhage and profound disorientation did not have evidence of cytoadherence/vascular occlusion, yet retinal pathology from a patient with retinal nerve infarcts did [40•]. Despite this, excess production of pro-inflammatory cytokines is thought to be central to the mechanism of babesiosis, and similar upregulation of cytokines such as TNF-a and IL-6 has been seen in the acute stages of both babesiosis and malaria [43•]. Further study is required to elucidate the exact mechanisms by which babesiosis may affect the nervous system.
Diagnosis of babesiosis can be made through visualization of Babesia parasitic forms on Wright–Giemsa stained thin and thick blood smears. It has been reported that microscopy has an estimated sensitivity of 85% and specificity of 100%, with further increases in sensitivity when multiple smears are performed [44]. If low parasitemias are present, the protozoal forms can be missed, thus necessitating more sensitive measures of making a diagnosis [45]. PCR is the more sensitive modality for the detection of Babesia, with one study reporting a sensitivity and specificity of 100% and 97.7%, respectively [38•, 46]. The superior sensitivity of Babesia-specific PCR can be helpful in establishing a diagnosis, especially in settings where smears may be negative due to very low or intermittent parasitemias [44, 47]. The utility of Babesia PCR has been suggested in cases such as screening blood products to prevent transfusion-associated babesiosis or in immunocompromised patients who have negative smears but still have ongoing symptoms [48, 49]. Significantly elevated positive IgM titers may be suggestive of acute infection, but serology is often most helpful when acute and convalescent titers are obtained, as otherwise acute titers alone cannot distinguish new infection from old [38•]. First-line therapy is a combination of azithromycin and atovaquone, with a typical duration lasting 7–10 days, although if a highly immunocompromised host (i.e., rituximab along with splenectomy, HIV/AIDS) may be extended for 6 weeks or longer [38•, 44]. Exchange transfusion can also be considered for patients with parasitemia >10% or with evidence of end-organ damage [38•].
Borrelia miyamotoi
Borrelia miyamotoi is classified as a relapsing fever spirochete, and although B. miyamotoi is genetically distinct from Borrelia burgdorferi sensu lato, both are transmitted by the same Ixodes deer tick species [50, 51•]. First discovered in Japan in 1995 and later realized to be a major pathogen in 2011, B. miyamotoi is recognized as an emerging infectious disease, and one survey of US ticks found B. miyamotoi in 1.72% of I. scapularis tested [51•].
The typical clinical manifestations of B. miyamotoi disease (BMD) include fevers, chills, headaches, myalgia, and possibly recurrent, intermittent fever [51•]. A retrospective study performed by Marcos et al. involving a review of 9 PCR confirmed cases of BMD suggested that common laboratory findings may include leukopenia, thrombocytopenia, and transaminitis [50]. However, some patients, especially those with immunosuppression, may develop neurologic symptoms such as reduced cognition, gait disturbance, confusion, and meningoencephalitis [51•]. Despite this, a case of meningitis due to BMD confirmed by CSF 16s rRNA testing has been documented in an otherwise healthy woman [52•]. Case reports of meningitis due to BMD have largely shown CSF with mononuclear predominance, and some have even shown weakly positive, B. burgdorferi IgM in CSF [52•, 53•, 54•].
While the mechanism of BMD is not entirely known, a robust adaptive immune response with functional humoral immunity has been proposed to help clear the infection. Immunologic studies have demonstrated that dendritic cells phagocytose the bacteria during infection in response to inflammatory cytokines. Studies of other relapsing fever spirochetes have shown that functional humoral immunity is required to contain the infection, and it has been postulated that perhaps immunosuppression and loss of this vital process predisposes patients to developing severe manifestations such as meningoencephalitis [51•]. While further study is required to elucidate if a relationship between the two processes exist, three cases of BMD meningoencephalitis all involved patients who were on rituximab therapy, which possibly supports the notion that antibody-mediated immunity is necessary to help protect against severe B. miyamotoi infection [52•, 53•, 54•].
To the best of our knowledge, there is no official standardized method of diagnosing BMD, but many cases have been diagnosed via PCR or molecular-based testing [50, 51•, 52•, 53•, 54•]. B. miyamotoi can be detected by microscopic examination of blood smears with Wright–Giemsa stain or dark field microscopy, but sensitivity is low; detection is highly dependent on spirochete burden, and microscopy alone cannot differentiate between Borrelia species [51•]. PCR-based methods focus on detecting genes such as glpQ which is found in relapsing fever Borrelia but not in Lyme disease causing Borrelia [55]. Fourfold rise of acute and convalescent antibody titers can be useful in making a diagnosis, especially when GlpQ and variable large protein (Vlp)-15/16 antigens are used in serologic testing [51•, 55]. Caution should be taken with interpreting serologic testing for B. miyamotoi since studies have shown cross reaction with certain B. burgdorferi serologic tests such as C6 antigen-based antibody assays [28•, 55].
There are no formal guidelines for treating BMD, but a recent systematic review suggested that doxycycline appears to be the antibiotic of choice for BMD without neurological sequelae, but Ceftriaxone is favored in those with meningoencephalitis [56]. Little is known about long-term neurological symptoms of BMD, and further research is needed on this specific topic since B. miyamotoi may co-exist in Lyme endemic areas [12, 50].
Conclusions
This review highlights some of the latest updates and advancements in the recognition, diagnosis, and management of nervous system sequelae involving the major pathogens transmitted by I. scapularis. While further study is required to elucidate the pathophysiologic mechanisms of each illness, clinicians should maintain a high index of suspicion when dealing with patients from the appropriate endemic areas so that an appropriate broad evaluation can be performed given that several of I. scapularis-borne diseases can have serious neurologic syndromes/consequences.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Rochlin I, Toledo A. Emerging tick-borne pathogens of public health importance: a mini-review. J Med Microbiol. 2020;69(6):781–91. https://doi.org/10.1099/jmm.0.001206.
Rodino KG, Theel ES, Pritt BS. Tick-borne diseases in the United States. Clin Chem. 2020;66(4):537–48. https://doi.org/10.1093/clinchem/hvaa040.
Tran T, Prusinski MA, White JL, Falco RC, Vinci V, Gall WK, et al. Spatio-temporal variation in environmental features predicts the distribution and abundance of Ixodes scapularis. Int J Parasitol. 2021;51(4):311–20. https://doi.org/10.1016/j.ijpara.2020.10.002.
Dunaj J, Moniuszko-Malinowska A, Swiecicka I, Andersson M, Czupryna P, Rutkowski K, et al. Tick-borne infections and co-infections in patients with non-specific symptoms in Poland. Adv Med Sci. 2018;63(1):167–72. https://doi.org/10.1016/j.advms.2017.09.004.
Rauer S, Kastenbauer S, Fingerle V, Hunfeld KP, Huppertz HI, Dersch R. Lyme neuroborreliosis. Dtsch Arztebl Int. 2018;115(45):751–6. https://doi.org/10.3238/arztebl.2018.0751.
Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet. 2008;371(9627):1861–71. https://doi.org/10.1016/S0140-6736(08)60800-4.
Bazer DA, Orwitz M, Koroneos N, Syritsyna O, Wirkowski E. Powassan encephalitis: a case report from New York, USA. Case Rep Neurol Med. 2022;2022:8630349. https://doi.org/10.1155/2022/8630349.
Basu S, Chakravarty A. Neurological manifestations of scrub typhus. Curr Neurol Neurosci Rep. 2022;22(8):491–8. https://doi.org/10.1007/s11910-022-01215-5.
Sekeyova Z, Danchenko M, Filipcik P, Fournier PE. Rickettsial infections of the central nervous system. PLoS Negl Trop Dis. 2019;13(8):e0007469. https://doi.org/10.1371/journal.pntd.0007469.
Fill MA, Compton ML, McDonald EC, Moncayo AC, Dunn JR, Schaffner W, et al. Novel clinical and pathologic findings in a heartland virus-associated death. Clin Infect Dis. 2017;64(4):510–2. https://doi.org/10.1093/cid/ciw766.
• Ford L, Tufts DM. Lyme Neuroborreliosis: Mechanisms of B. burgdorferi infection of the nervous system. Brain Sci. 2021;11(6) https://doi.org/10.3390/brainsci11060789.Highlights the current understanding of the relevant pathophysiology of neuroborreliosis and also associated neurologic syndromes associated with Lyme disease.
Eisen RJ, Eisen L. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 2018;34(4):295–309. https://doi.org/10.1016/j.pt.2017.12.006.
Oschmann P, Dorndorf W, Hornig C, Schafer C, Wellensiek HJ, Pflughaupt KW. Stages and syndromes of neuroborreliosis. J Neurol. 1998;245(5):262–72. https://doi.org/10.1007/s004150050216.
Strle F, Henningsson AJ, Strle K. Diagnostic utility of CXCL13 in lyme neuroborreliosis. Clin Infect Dis. 2021;72(10):1727–9. https://doi.org/10.1093/cid/ciaa337.
Halperin JJ. Neuroborreliosis. J Neurol. 2017;264(6):1292–7. https://doi.org/10.1007/s00415-016-8346-2.
• Theel ES, Aguero-Rosenfeld ME, Pritt B, Adem PV, Wormser GP. Limitations and confusing aspects of diagnostic testing for neurologic lyme disease in the United States. J Clin Microbiol. 2019;57(1) https://doi.org/10.1128/JCM.01406-18. Reviews the current advantages and limitations of current modalities of diagnosing neuroborreliosis.
Khan F, Allehebi Z, Shabi Y, Davis I, LeBlanc J, Lindsay R, et al. Modified two-tiered testing enzyme immunoassay algorithm for serologic diagnosis of Lyme disease. Open Forum. Infect Dis Ther. 2022;9(7):ofac272. https://doi.org/10.1093/ofid/ofac272.
Rauer S, Kastenbauer S, Hofmann H, Fingerle V, Huppertz HI, Hunfeld KP, et al. Guidelines for diagnosis and treatment in neurology - Lyme neuroborreliosis. Ger. Med Sci. 2020;18:Doc03. https://doi.org/10.3205/000279.
Leeflang MM, Ang CW, Berkhout J, Bijlmer HA, Van Bortel W, Brandenburg AH, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140. https://doi.org/10.1186/s12879-016-1468-4.
Avery RA, Frank G, Eppes SC. Diagnostic utility of Borrelia burgdorferi cerebrospinal fluid polymerase chain reaction in children with Lyme meningitis. Pediatr Infect Dis J. 2005;24(8):705–8. https://doi.org/10.1097/01.inf.0000172903.14077.4c.
Pedersen RR, Kragh KN, Fritz BG, Orbaek M, Ostrup Jensen P, Lebech AM, et al. A novel Borrelia-specific real-time PCR assay is not suitable for diagnosing Lyme neuroborreliosis. Ticks Tick Borne Dis. 2022;13(5):101971. https://doi.org/10.1016/j.ttbdis.2022.101971.
Marques AR. Lyme neuroborreliosis. Continuum (Minneap Minn). 2015;21(6):1729–44. https://doi.org/10.1212/CON.0000000000000252.
De Koning J, Bosma RB, Hoogkamp-Korstanje JA. Demonstration of spirochaetes in patients with Lyme disease with a modified silver stain. J Med Microbiol. 1987;23(3):261–7. https://doi.org/10.1099/00222615-23-3-261.
• Lantos PM, Rumbaugh J, Bockenstedt LK, Falck-Ytter YT, Aguero-Rosenfeld ME, Auwaerter PG, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme disease. Clin Infect Dis. 2021;72(1):1–8. https://doi.org/10.1093/cid/ciab049. Publication of the current IDSA guideline which reviews current recommended treatments of Lyme disease.
Chung MK, Caboni M, Strandwitz P, D'Onofrio A, Lewis K, Patel CJ. Systematic comparisons between Lyme disease and post-treatment Lyme disease syndrome in the U.S. with administrative claims data. EBioMedicine. 2023;90:104524. https://doi.org/10.1016/j.ebiom.2023.104524.
Piantadosi A, Solomon IH. Powassan virus encephalitis. Infect Dis Clin N Am. 2022;36(3):671–88. https://doi.org/10.1016/j.idc.2022.03.003.
• Hermance ME, Thangamani S. Powassan virus: an emerging arbovirus of public health concern in North America. Vector Borne Zoonotic Dis. 2017;17(7):453–62. https://doi.org/10.1089/vbz.2017.2110. Thorough review of current knowledge regarding the Powassan virus, including postulated mechanisms driving neurologic manifestations of infection with the Powassan virus.
• Della-Giustina D, Duke C, Goldflam K. Underrecognized tickborne illnesses: Borrelia miyamotoi and Powassan virus. Wilderness Environ Med. 2021;32(2):240–6. https://doi.org/10.1016/j.wem.2021.01.005. Thorough review of current knowledge regarding the Powassan virus including the range of clinical manifestations.
Pastula DM, Smith DE, Beckham JD, Tyler KL. Four emerging arboviral diseases in North America: Jamestown Canyon, Powassan, chikungunya, and Zika virus diseases. J Neuro-Oncol. 2016;22(3):257–60. https://doi.org/10.1007/s13365-016-0428-5.
Fatmi SS, Zehra R, Carpenter DO. Powassan virus-a new reemerging tick-borne disease. Front Public Health. 2017;5:342. https://doi.org/10.3389/fpubh.2017.00342.
Conde JN, Sanchez-Vicente S, Saladino N, Gorbunova EE, Schutt WR, Mladinich MC, et al. Powassan viruses spread cell to cell during direct isolation from ixodes ticks and persistently infect human brain endothelial cells and pericytes. J Virol. 2022;96(1):e0168221. https://doi.org/10.1128/JVI.01682-21.
• Dumic I, Jevtic D, Veselinovic M, Nordstrom CW, Jovanovic M, Mogulla V, et al. Human granulocytic anaplasmosis-a systematic review of published cases. Microorganisms. 2022;10(7) https://doi.org/10.3390/microorganisms10071433. Thorough review of current knowledge regarding anaplasmosis.
Eldaour Y, Hariri R, Yassin M. Severe Anaplasmosis presenting as possible CVA: case report and 3-year Anaplasma infection diagnosis data is based on PCR testing and serology. IDCases. 2021;24:e01073. https://doi.org/10.1016/j.idcr.2021.e01073.
Kim SW, Kim CM, Kim DM, Yun NR. Manifestation of anaplasmosis as cerebral infarction: a case report. BMC Infect Dis. 2018;18(1):409. https://doi.org/10.1186/s12879-018-3321-4.
• Cosiquien RJS, Stojiljkovic N, Nordstrom CW, Amadi E, Lutwick L, Dumic I. Anaplasma phagocytophilum encephalitis: a case report and literature review of neurologic manifestations of anaplasmosis. Infect Dis Rep. 2023;15(4):354–9. https://doi.org/10.3390/idr15040035. Reviews current understanding of documented neurologic manifestations of anaplasmosis, as well as postulated mechanisms driving these processes.
LeDonne MJ, Ahmed SA, Keeney SM, Nadworny H. Trigeminal neuralgia as the principal manifestation of anaplasmosis: a case report. Cureus. 2022;14(1):e21668. https://doi.org/10.7759/cureus.21668.
Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and Babesiosis: a review. JAMA. 2016;315(16):1767–77. https://doi.org/10.1001/jama.2016.2884.
• Krause PJ. Human babesiosis. Int J Parasitol. 2019;49(2):165–74. https://doi.org/10.1016/j.ijpara.2018.11.007. Thorough review of current knowledge regarding babesiosis, including relevant clinical manifestations, diagnostics, and treatment modalities.
Venigalla T, Adekayode C, Doreswamy S, Al-Sudani H, Sekhar S. Atypical presentation of Babesiosis with neurological manifestations as well as hematological manifestations. Cureus. 2022;14(7):e26811. https://doi.org/10.7759/cureus.26811.
• Usmani-Brown S, Halperin JJ, Krause PJ. Neurological manifestations of human babesiosis. Handb Clin Neurol. 2013;114:199–203. https://doi.org/10.1016/B978-0-444-53490-3.00014-5. Provides information regarding the pathophysiology of cerebral malaria and some similarities seen in neurological manifestations of babesiosis.
• Locke S, O'Bryan J, Zubair AS, Rethana M, Moffarah AS, Krause PJ, et al. Neurologic complications of babesiosis, United States, 2011-2021. Emerg Infect Dis. 2023;29(6):1127–35. https://doi.org/10.3201/eid2906.221890. Provides information regarding the pathophysiology of cerebral malaria and some similarities seen in neurological manifestations of babesiosis.
• Trivedi S, Chakravarty A. Neurological complications of malaria. Curr Neurol Neurosci Rep. 2022;22(8):499–513. https://doi.org/10.1007/s11910-022-01214-6. Provides information regarding the pathophysiology of cerebral malaria and some similarities seen in neurological manifestations of babesiosis.
• Djokic V, Rocha SC, Parveen N. Lessons learned for pathogenesis, immunology, and disease of erythrocytic parasites: Plasmodium and Babesia. Front Cell Infect Microbiol. 2021;11:685239. https://doi.org/10.3389/fcimb.2021.685239. Provides information regarding the pathophysiology of cerebral malaria and some similarities seen in neurological manifestations of babesiosis.
Bloch EM, Kumar S, Krause PJ. Persistence of Babesia microti infection in humans. Pathogens. 2019;8(3) https://doi.org/10.3390/pathogens8030102.
Parija SC, Kp D, Venugopal H. Diagnosis and management of human babesiosis. Trop Parasitol. 2015;5(2):88–93. https://doi.org/10.4103/2229-5070.162489.
Wang G, Wormser GP, Zhuge J, Villafuerte P, Ip D, Zeren C, et al. Utilization of a real-time PCR assay for diagnosis of Babesia microti infection in clinical practice. Ticks Tick Borne Dis. 2015;6(3):376–82. https://doi.org/10.1016/j.ttbdis.2015.03.001.
Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366(25):2397–407. https://doi.org/10.1056/NEJMra1202018.
Akoolo L, Schlachter S, Khan R, Alter L, Rojtman AD, Gedroic K, et al. A novel quantitative PCR detects Babesia infection in patients not identified by currently available non-nucleic acid amplification tests. BMC Microbiol. 2017;17(1):16. https://doi.org/10.1186/s12866-017-0929-2.
Krause PJ, Auwaerter PG, Bannuru RR, Branda JA, Falck-Ytter YT, Lantos PM, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA): 2020 guideline on diagnosis and management of babesiosis. Clin Infect Dis. 2021;72(2):185–9. https://doi.org/10.1093/cid/ciab050.
Marcos LA, Smith K, Reardon K, Weinbaum F, Spitzer ED. Presence of Borrelia miyamotoi infection in a highly endemic area of Lyme disease. Ann Clin Microbiol Antimicrob. 2020;19(1):22. https://doi.org/10.1186/s12941-020-00364-0.
• Cleveland DW, Anderson CC, Brissette CA. Borrelia miyamotoi: a comprehensive review. Pathogens. 2023;12:2. https://doi.org/10.3390/pathogens12020267. Thorough review of current knowledge regarding Borrelia miyamotoi disease.
• Henningsson AJ, Asgeirsson H, Hammas B, Karlsson E, Parke A, Hoornstra D, et al. Two cases of Borrelia miyamotoi meningitis, Sweden, 2018. Emerg Infect Dis. 2019;25(10):1965–8. https://doi.org/10.3201/eid2510.190416. Case reports of meningoencephalitis during Borrelia miyamotoi disease, also theorizes mechanism regarding these neurologic syndromes.
• Boden K, Lobenstein S, Hermann B, Margos G, Fingerle V. Borrelia miyamotoi-Associated neuroborreliosis in immunocompromised person. Emerg Infect Dis. 2016;22(9):1617–20. https://doi.org/10.3201/eid2209.152034. Case reports of meningoencephalitis during Borrelia miyamotoi disease, also theorizes mechanism regarding these neurologic syndromes.
• Schwartz T, Hoornstra D, Oie E, Hovius J, Quarsten H. Case report: first case of Borrelia miyamotoi meningitis in an immunocompromised patient in Norway. IDCases. 2023;33:e01867. https://doi.org/10.1016/j.idcr.2023.e01867. Case reports of meningoencephalitis during Borrelia miyamotoi disease, also theorizes mechanism regarding these neurologic syndromes.
Burde J, Bloch EM, Kelly JR, Krause PJ. Human Borrelia miyamotoi infection in North America. Pathogens. 2023;12(4) https://doi.org/10.3390/pathogens12040553.
Hoornstra D, Azagi T, van Eck JA, Wagemakers A, Koetsveld J, Spijker R, et al. Prevalence and clinical manifestation of Borrelia miyamotoi in Ixodes ticks and humans in the northern hemisphere: a systematic review and meta-analysis. Lancet Microbe. 2022;3(10):e772–e86. https://doi.org/10.1016/S2666-5247(22)00157-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on CNS Infections in Tropical and Subtropical Settings
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lum, M., Syritsyna, O., Spitzer, E.D. et al. Neurologic Manifestations of Tick-Borne Diseases Transmitted by Deer Ticks (Ixodes scapularis) in the USA. Curr Trop Med Rep 10, 213–221 (2023). https://doi.org/10.1007/s40475-023-00302-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40475-023-00302-y