Abstract
Purpose of Review
Here, we review the use of cell sheet technology using different cell types and its potential for restoring the extracellular matrix microenvironment, perfusion, and immunomodulatory action on islets and beta cells.
Recent Findings
Cell sheets can be produced with different fabrication techniques ranging from the widely used temperature responsive system to the magnetic system. A variety of cells have been used to produce cell sheets including skin fibroblasts, smooth muscle cells, human umbilical vein endothelial cells, and mesenchymal stem cells.
Summary
CST would allow to recreate the ECM of islets which would provide cues to support islet survival and improvement of islet function. Depending on the used cell type, different additional supporting properties like immunoprotection or cues for better revascularization could be provided. Furthermore, CST offers the possibility to use other implantation sites than inside the liver. Further research should focus on cell sheet thickness and size to generate a potential translational therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes, also known as insulin-dependent diabetes, is an autoimmune disease that starts mostly during childhood and follows patients throughout their entire life. The main characteristic of this disease is a specific autoimmune reaction to the beta cells and their subsequent destruction [1]. Beta cells reside in pancreatic islets, which are spherical aggregates of a mixed population of cells that produce various blood glucose–controlling hormones. Beta cells can sense very accurately variations in blood glucose levels, and secrete an appropriate amount of insulin in response, to lower the glucose concentration and encompass ≈ 60% of the total amount of cells in human islets [2]. The counterpart of beta cells is the alpha cells which secrete glucagon which allow the release of glucose to increase blood glucose, by triggering its release from liver cells among others when needed. Without proper insulin secretion, blood glucose homeostasis cannot be reached which leads, when not treated with exogenous insulin therapy, to very severe complications resulting in the death of the affected person [3].
Successful clinical islet transplantation (CIT) was developed around 2 decades ago, after successful trials in 1990 showed that a minimally invasive procedure of perfusing donor islets obtained from a deceased donor via the portal vein into the liver’s vasculature was able to replenish immunocompromised islets and restore long-term normoglycemia using a tailored steroid-free immunosuppressive protocol [4]. CIT is currently reserved for adults with severe type 1 diabetes (T1D) suffering from hypoglycemia unawareness, labile blood glucose control, and long-term complications due the side effects of the accompanying immunosuppressive therapy [5, 6]. When successful, Patient treated with CIT, can become insulin-independent for at least 7 years, after which much better glucose control can be achieved, even if graft function declines over time. However successful CIT may be, restoration of blood glucose levels continues to be transient (~ 5–7 years) due to the loss of function of transplanted islets due a to a continuous complicated multifactorial reaction against the allogeneic cells implanted in the liver [4].
Donor islets used for CIT are exposed to several stress factors, starting at the moment a pancreas is retrieved from a donor, which continues during the isolation procedure using enzymatic digestion and mechanical disruption to separate the endocrine cells from the surrounding acinar tissue to an immediate blood-mediated inflammatory response, hypoxia, and long-term auto- and alloimmune reactions after transplantation [7]. [8]. Almost 60% of the transplanted donor islets are lost immediately after transplantation. This is mainly caused by an instant blood-mediated inflammatory reaction to the allogeneic cells placed directly in the bloodstream [9]. In addition, enzymes used during isolation will destroy part of the islet extracellular matrix (ECM) [10•], while also the intra islet microvasculature is removed [11,12,13], resulting in poor engraftment [14], and consequently a loss of function due to a significant loss of transplanted cells in the first 2 weeks [10•, 14].
In recent years, a vast number of alternative methods to transplant islets and beta cells have been proposed to create a more suitable environment for islets and beta cells to improve their survival and function [15]. However, these extrahepatic islet transplantation strategies typically rely on the use of synthetic biomaterials as a carrier for beta cells in the form of so-called micro or macroencapsulation delivery devices [16]. Even though biomaterials are deemed biocompatible, they will always elicit a foreign body reaction after implantation leading to inflammation and the formation of a fibrotic capsule (scar tissue) around the implant [17]. This creates a suboptimal situation from the start that will eventually hinder revascularization, and consequently impair the proper exchange of oxygen and nutrients, leading to cell death and loss of the endocrine cells. The consensus in the field is that a delivery device strategy is needed to have control over confinement of the allogeneic cells, or stem cell–derived beta cells, providing a possibility to retrieve, protect, and replace the transplanted cells, while another reason is to be able to place them in the most optimal location stimulating revascularization.
A less explored alternative in beta cell replacement therapy is based on cell sheet technology (CST) that can be applied to create a modular tissue–engineered structure composed of different cell layers combined into a patch [18]. This technology can potentially be used to support long-term survival of fully biological grafts created from different cell types, to for example stimulate vascularization [19], or support immunomodulation [20]. An additional benefit is that cell sheets can also be used to create a specific extracellular matrix microenvironment depending on the cells used [21, 22].
However, its high potential, CST requires significant expertise and experience before one can consistently and reproducibly apply this in the field of beta cell replacement therapy. Until now, CST has not been widely used for beta cell delivery strategies. Here, we report on the available literature and focus on two main categories: the cells used to create cell sheets and the techniques applied to create and harvest cell sheets for potential clinical use.
Cells Used for Cell Sheets
In literature, different applications of cell sheets have been described, including single-cell sheets, multilayer cell sheets, and cell sheets combined with scaffolds (Table 1). Single-cell sheets have been predominantly used for correcting corneal, skin, periodontal, and urinary bladder disorders, whereas multilayered cell sheets have been described for the regeneration of the cardiac, liver, vascular, and respiratory smooth muscle and other complex tissues [23].
A combination of cell sheets and scaffolds has been predominantly reported for the reconstruction of bone and cartilage defects due to the load-bearing characteristics of the tissue. Regardless, the different cell types used in creating such cell sheets can be broadly classified into three types: (a) non-mesenchymal stem cells, (b) mesenchymal stem cells (MSCs), and (c) cell sheets derived from other stem cells including embryonic and induced pluripotent stem cells (iPSCs).
Non-Mesenchymal Stem Cells
The non-mesenchymal stem cells widely reported in literature for making cell sheets are epithelial cells, skin fibroblasts, human umbilical vein endothelial cells, cardiomyocytes, smooth muscle cells, and periodontal ligament cells. The most commonly used epithelial cells are the limbal and oral mucosal epithelial cells, which are used for the reconstruction of corneal epithelial cell layers and corneal repair [24, 25]. Clinical trial outcomes with corneal cell sheets have been remarkable with restoration in corneal transparency and marked improvement in postoperative visual acuity with no reported complications or inflammation [25, 26].
Stratified epithelial cell sheet grafts, derived from oral mucosal epithelial cells, have been successfully used in reconstructing the esophageal luminal surface, to prevent stenosis and inflammation [27,28,29]. Bioengineered dermal fibroblast cell sheets have been primarily studied in the context of lung disorders as a novel lung sealant to repair pulmonary air leakage and in the closure of pleural defects [30,31,32].
A combination of dermal fibroblast cells and human umbilical vein endothelial (HUVEC) cell sheets has been explored in the reconstruction of the vascular network, to form tubular structures resembling a native primitive microvasculature [33]. CST has been shown to have great potential in periodontal tissue regeneration, while preclinical studies with periodontal ligament cell sheet transplantation resulted in new bone and ligament formation with well-oriented collagen fibers [34,35,36].
Transplantation of layered myocardial cell sheets to the site of infarcted myocardium resulted in homogeneous tissue integration resembling native cardiac tissue, prolonged survival, enhanced angiogenesis, and improved cardiac performance in an ischemic animal model [37,38,39]. In liver tissue, engineering layers of hepatocytes combined with a fibroblast growth factor improved vascularization, resulting in a highly vascularized liver cell sheet resembling liver tissue [18, 40]. Combinations of smooth muscle and urothelial cells have resulted in the development of a bladder cell sheet to repair and improve bladder function though this is still in a nascent state [41].
Mesenchymal Stem Cells
MSCs have been used in the generation of cell sheets for various applications. MSCs have certain advantages: they are multipotent in nature, have immunomodulatory properties, can produce extracellular matrix, relatively easy scalable in cell culture, and can be derived and applied autologously, thereby making them a promising candidate for a wide range of different tissue engineering and regenerative medicine applications.
Bone marrow–derived MSCs have been widely used in the generation of MSC-based cell sheets, especially for bone regeneration. Multiple layers of MSCs stacked on top of each other can be used to create large surface area-to-volume ratio patches of living cells and used to efficiently deliver a relative large number of cells at high density compared to constructs based on a combination of MSCs with biomaterial-based scaffolds [42, 43]. Bone marrow–derived MSC-based cell sheets have been mainly used in bone regeneration studies due to their positive role in enhancing vascularization, and their potential contribution to bone formation.
The challenges associated with vascularization of large tissue construct using conventional biomaterial-based scaffolds can be overcome by CST, as thin layers of cell sheets support the formation of capillaries and aid in vascularization of for example tissue-engineered bone grafts. Studies have demonstrated the potential of human MSCs engineered into multilayered cell sheets to differentiate into the osteochondral lineage, subsequently maturing into fully mineralized compact bone, and aid in repairing both segmental and long bone defects [44,45,46]. In addition, these cell sheets have been shown to be effective in inducing bone union at the fracture site [47, 48].
In other skeletal repair studies, similar MSC-based cells sheets have been shown to provide the necessary ECM environment required for cartilage regeneration which can possibly lead to improved cartilage repair when used in osteoarthritic patients [21, 22]. Alternatively, MSCs derived from adipose tissue (ADMSCs) have been mainly used in cardiac tissue repair and regeneration. Implantation of ADMSC cell sheets into the myocardial infarction site resulted in engraftment of cells, differentiation into cardiomyocytes and cardiomyoblast-like cells, improved cardiac function, and long-term survival [49, 50].
Embryonic and Induced pluripotent Stem Cells
Apart from non-MSC and MSCs, other stem cells have also been reported in the creation of cell sheets such as muscle-derived stem cells, cardiac stem cells, anterior cruciate ligament stem cells, and tendon-derived stem cells for various tissue repair purposes. Of all the stem cells, embryonic stem cells (ESCs) have more multipurpose applicability due to their pluripotent differentiation potential. Cells sheets created from ESC-derived cardiomyocytes have been shown to beat spontaneously and synchronously in vitro and, hence, could be developed into a functional bioengineered myocardium [51]. Similarly, in corneal tissue engineering, transplantation of ESC-derived corneal epithelial cells facilitated the reconstruction of the ocular surface in rabbits in 75% of the treated animals without the use of a biomaterial based scaffold [52].
Despite the vast potential of ESC, there are few disadvantages related to ethical issues, immune rejection, and potential teratoma formation, which might hinder the widespread clinical application of ESC-derived cell sheets. These disadvantages can be overcome by using iPSCs, which can be derived from the patient’s own cells, thereby overcoming immune rejection while having a similar pluripotent capacity as ESCs do to differentiate into various cell lineages, thereby leading potentially to the development of personalized cell therapy.
In cardiovascular disease, the transplantation of iPSC-derived cardiomyocyte cell sheets for the treatment of ischemic cardiomyopathy in a large preclinical porcine model resulted in improved cardiac function and, most notably, without any teratoma formation [53]. Differentiated iPSC sheets have been studied for the generation of vascular grafts, and iPSC-derived receptor tyrosine kinase Flk-1 ( +) cell sheets have been shown to repair hind limb ischemia, by increasing vascular endothelial growth factor (VEGF) expression leading to enhanced vascularization, increased capillary density, and increased blood flow in a murine model [54]. Despite the many advantages of iPSC derived cell sheets, limited induced differentiation potential capacity depending on the iPSC line derived from a patient is still a significant drawback.
Cell Sheet Technology — a Possible New Technology Platform for the Treatment of Type 1 Diabetes
The mechanisms leading to islet graft failure after CIT are mainly caused by the isolation procedure (pre-transplantation) and poor engraftment and immune responses which (post-transplantation) induce significant cell stress leading ultimately to suboptimal β-cell survival and function [55]. These factors include instant blood-mediated inflammatory reaction (IBMIR), proinflammatory cytokines, hypoxia, and nutrient deprivation, and deleterious effects of the toxic immunosuppressive drugs used to prevent rejection. As a result, in most cases, two or more pancreata are required per patient to achieve full insulin independence.
To avoid the IBMIR, various extrahepatic transplantation sites such as the peritoneal cavity, omentum, subcutaneous site, kidney capsule, and intramuscular are being explored with limited success depending on their location [56]. Among these sites, the subcutaneous space remains attractive due to its accessibility and the relative minimally invasive surgical procedures required to transplant islets [56]. To improve graft survival, prevascularization strategies [57, 58] with or without the help scaffolds [59, 60] seem to be required to further promote islet engraftment. CST can help overcome these limitations and is currently being explored as a potential strategy for islet transplantation at various extrahepatic sites.
Some examples of CST in the field of beta cell replacement have been published before, and in these reports, the sheets are generally composed of dispersed islets combined with supporting cells that aid in the engraftment of the transplanted islets at an extrahepatic site.
Early studies on the use of cell sheet technology for beta cells were published by Saito et al. and Shimizu et al. who used temperature responsive poly-N-isopropylacrylamide (PIPAAm)-coated cell culture dishes to make cell sheets composed of dispersed islets [61, 62]. In those studies, the islets were dispersed into single cells using trypsin–EDTA and then plated onto temperature-responsive PIPAAm plates coated with laminin. The cells were cultured for a couple of days until they became confluent, and the islet cell sheets were detached from the plate by lowering the culture temperature to 20 ºC. The islet cell sheet created in this way showed good viability [61]. Histology revealed that the islet cell sheets contained both insulin and glucagon-positive cells. Subsequent subcutaneous implantation of the islet cell sheets into diabetic rodents showed good engraftment, normalized blood sugar levels, and demonstrated quick glucose clearance during intraperitoneal glucose tolerance tests [62]. The topographical arrangement of alpha and beta cells within the islet cell sheet after explanation resembled primary rodent islets with beta cells located in the core surrounded by alpha cells at the periphery [63].
The same group showed in follow up studies that the addition of fibroblast or MSCs could improve some of the abovementioned outcomes [64,65,66]. MSCs are known to have protective effects on islets and can improve their engraftment by secreting various angiogenic factors such as VEGF, HGF, and TGF-β1 and immunomodulatory cytokines such as IL-6 and IL-10 [67,68,69]. The islets and MSCs were cocultured into sheets by seeding islets onto a confluent MSC layer (46). The viability and insulin secretory stimulation index of islets in these sheets were found to be significantly higher compared to the islet alone controls. Furthermore, VEGF, HGF, and TGFβ1 levels were significantly higher in the coculture group compared to islets alone [64].
Subsequent subcutaneous implantation of these cocultured sheets in diabetic mice resulted in better cell survival, engraftment, and normalization of blood sugar levels compared to those transplanted with islets alone [64]. The improved efficiency may be attributed to enhanced vascularization seen in the coculture group compared to the islet alone group. A similar benefit was seen when ADMSCs were used to create similar islet cell sheets, however with a higher viability, recovery rate, and increased IL-6 secretion compared to bone marrow–derived MSCs [65]. Fibroblasts, as an alternative to MSCs, can be used to achieve a similar outcome by promoting angiogenesis due to their VEGF and FGF secretion thereby promoting islet survival and function [66].
The subcutaneous site is easily accessible for cell sheet implantation. However, this transplant site is hampered by poor vascularization, a limited blood flow and low oxygen tension, and generally more immunogenic, requiring a larger islet sheet mass to normalize blood sugar levels in diabetic animals than other sites explored for islet cell sheet implantation. Fujita et al. compared the liver surface site to the subcutaneous space for islet cell sheet transplantation and found the liver surface site more optimal as it needed less islet cell sheet mass to reach normoglycemia [73]. They did however disperse intact primary islets to create the cell sheets, which might have limitations for their clinical application since this method is well known to lead to a loss of endocrine cells and completely disrupts the beta cell’s native microenvironment. Maintaining the native spheroid structure is crucial for the proper physiological function of primary islets [74, 75].
In a recent study by Lee et al., they used an adipose-derived stem cell (ADSC) sheet as a supporting cell layer to deliver intact islets and compared various transplantation sites such as the liver surface, peritoneal wall, and the subcutaneous space [76]. Transplantation of this islets-ADSC sheet exhibited better glucose control at all transplantation sites liver, peritoneal wall, and the subcutaneous space compared to the islet alone group. Among the various transplantation sites tested, the liver surface and the peritoneal wall appeared to give the best outcomes. The authors found better glycemic control, body weight increase, and improved glucose clearance during intraperitoneal glucose tolerance tests (IPGTTs) compared to the subcutaneous space.
Based on the reports described above, transplantation of bioengineered islet cell sheets seems to be a great alternative for biomaterial-based beta cell delivery devices by creating a biological scaffold-free implant for beta cell replacement. Furthermore, CST can be used with insulin-producing cells derived from other sources, as shown recently with transdifferentiated liver cells [77••]. In this study, IPSCs were created from human hepatocytes after which these were differentiated into insulin-producing cells; the cells were then seeded onto thermo-responsive dishes to generate insulin-producing cell sheets which showed improved insulin expression compared to those cultured on conventional dishes. Subsequently, the sheets demonstrated better engraftment, improved glucose control, and lower hepatotoxicity after implantation compared to cells grown on conventional cell culture plastic.
Techniques Used to Create Cell Sheets
Temperature Responsive Systems
Cell sheets can be generated in different ways. One of the widely used systems are temperature-responsive cell culture dishes. Here, culture surfaces are coated with a temperature-responsive polymer which changes its properties from hydrophobic to hydrophilic with a change of temperature releasing the cell sheet [78]. An often-used polymer is a poly(N-isopropyl acrylamide) (PIPAAm). The surface of the culture is coated with PIPAAm. The cells are cultured on it until confluent. The dish is cooled down to 32 °C or lower, allowing the PIPAAm to reach a reversible lower critical solution temperature (LCST) phase transition from an insoluble dehydrated state to a soluble hydrated state, thereby releasing an intact cell sheet [79].
Since temperature-responsive systems are widely used and relatively easy to work with, most in vivo studies and some clinical trials have been done with this system. In vivo studies include using cell sheets made from autologous epithelial cells for corneal epithelial reconstruction in rabbits [80] or MSC-based cell sheets to improve heart failure in rats [80]. The usage of MSCs to treat heart problems has been translated into clinic use. A phase one clinical trial using autologous MSCs to treat cardiomyopathy has successfully been done [81]. Another clinical application was the transplantation of autologous mucosal epithelial cell sheets to repair the esophageal wall after endoscopic submucosal dissection [82].
Electro Responsive Systems
Here, cells are released through an electrical trigger. Different surfaces (e.g., glass, cell culture insert, or a photocurable resin) can be gold coated and modified with a so-called self-assembling alkanethiol monolayer, to which cell-adhesive RGD peptides can be adsorbed [83,84,85]. Detachment of cell sheets from these surfaces is achieved by applying \(-\) 1.0 V for 10 min, which has been described to work faster than thermo-responsive surfaces.
Electro-responsive systems are versatile, comprising different cell culture surfaces, allowing for the creation of varying cell sheet sizes, with different forms and thicknesses [83]. Electro-responsive systems based on gold-coated cell culture inserts enable the production of cell sheets from fibroblasts with ~ 50 µm thickness. Subsequent stacking of these cell sheets allows for better handling. However, the limitation is that if these stacked cell sheets become too thick, a necrotic core starts to develop, a problem which can be overcome by providing a better diffusion oxygen and nutrients through the cell sheet by generating a continuous medium flow around the stacked sheets. This method allows for the creation of multi-layered cell sheets containing up to 5 sheets, which can easily be handled during subcutaneous transplantation [84]. Thick cell sheets from neonatal skin fibroblasts were created this way and then subcutaneously implanted in nude mice. One and two months after transplantation, no inflammation and tumor formation at the transplantation site was observed, while the transplanted cells sheets seemed to be well engrafted.
Even more interesting is the possibility to culture more complex 3D-structured cell sheets. Different designed molds can be gold coated and modified for cell attachment, and cells can then be electrically released to create a three-dimensional shaped tissue construct.
One can create anatomically correct molds from a photocurable resin using micro stereolithography, gold coat these, and use them for cell culture to create a three-dimensional shape. The cells can be detached by first encapsulating the mold with a collagen hydrogel prior to releasing the cells to ensure the shape is maintained. The idea behind these types of cell sheets would be to apply them to repair the intestinal wall since they can be made to fit its anatomical features in a precise way [85]. The advantage of these three dimensionally shaped cell sheets is their use for repair of more complex structures and to regenerate more complex organs.
Photoresponsive Systems
Different approaches have been used to release cell sheets from photoresponsive cell culture surfaces. One material combination is the usage of poly(3,4-ethylene dioxythiophene) (PEDOT) substrate and collagen type I. A near-infrared (NIR) laser can be used to heat up the collagen on top of the PEDOT, which then leads to cell sheet detachment. The advantage of this method is the tunability of the release mechanism. The NIR laser allows for a fast detachment (5 min) and a high spatial control over the release process [86, 87]. The spatial control makes it possible to harvest small monolayer cell sheets (between 2.3 and 4.7 mm) [86] and larger cell sheets with a surface area of 7.7 cm2 or detach a patterned cell sheet from a confluent cell layer, and harvest multiple cell sheets simultaneously with one light source increasing the production efficiency [87]. This method was used by Na et al., to grow cell sheets from fibroblasts and ADSCs, but interestingly, it turned out that using this method, it was more challenging to detach ADSC-based sheets. These results suggest that adjustments might be needed to optimize the photoresponsive technique to suit different cell types [87]. The possibility to grow large cell sheets and control the patterning cell sheets might be advantageous since it allows for the creation of complex organ-like structures in a flexible way.
Another approach is the usage of nanodot titanium dioxide (TiO2) films and cold LED UV light to detach cells and cells sheets from cell culture substrates. In a recent study, the authors used a relatively low dose of UV light to detach cell sheets and found that cell viability was not negatively affected, and no influence their function nor on cell–cell interactions was observed. Cell sheets harvested with this method were able to reattach again and could be used for further cell culture [88]. Furthermore, it was proven that no residual material remained attached to the harvested cell sheet, which could happen when using the other detachment methods previously described making it more difficult to apply these in a clinical setting. A similar method was used to culture a more complex cell sheet made from different cell types. In this case, HUVECs and hMSCs were co-cultured then detached and stacked into a multilayered cell sheet to form a thicker pre-vascularized cell sheet [89]. This approach is promising since one of biggest hurdles in complex tissue engineering is the vascularization of large tissue constructs leading often to implant failure [90].
Dispase Treatment
Dispase treatment has been used for quite a while to harvest cell sheets. Green et al. [91] used the dispase to detach epithelial cell sheets without reducing cell viability. Even though dispase treatment can be effective, it seems not as efficient as other techniques like temperature-responsive systems. Cell sheets harvested from temperature-responsive dishes have better structure compared to dispase harvested cell sheets which facilitates easy harvesting and handling during in vivo implantation [92].
Further extensive washing of dispase harvested cell sheets is needed if they are used in clinical applications. If not all dispase is removed, the enzyme could negatively influence the wound healing process [93].
Since enzymatic treatment with dispase has some drawbacks, researchers have looked into other possible enzymatic treatments. Another option for enzymatic cell sheet harvesting could be the usage of collagenase. In a direct comparison, collagenase cell sheet harvesting has had better results on some occasions than in the dispase treatment. Cell sheets detached with collagenase were easier to handle, with better overall sheet integrity and better ex vivo attachment. Collagenase might also be easier to translate for clinical use since it has been approved for a clinical use in specific interventions [94].
Magnetic Systems
In general, coated magnetic nanoparticles are introduced into the cell, and those “magnetized cells” are cultured on top of magnets to generate and release cell sheets. Gonçalves et al. [95] used coated iron oxide nanoparticles to culture cell sheets that had a tendon-like rich matrix, which displayed good mechanoelastic properties. The cells were not negatively affected by the uptake of nanoparticles.
In another experiment, magnetite (Fe3o4) nanoparticles coated with N-(a-trimethylammonioacetyl-)didodecyl-d-glutamate chloride were phagocytosed by keratinocytes which were then cultured on an ultralow attachment plate with a magnet underneath until confluence was reached. Removal of the magnet allowed the cell sheet to detach and be lifted using another different magnet allowing for easy transfer. The use of magnetite nanoparticles did not lead to cell toxicity, and pilot studies showed they had no adverse effects in vivo [93].
Conclusion and Next Steps
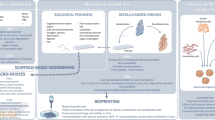
CITs have unmet clinical needs that can potentially be solved using cell sheet technology. CST can be used to recreate islet extracellular matrix (ECM), which is removed or damaged during the islet isolation process using support cells such as HUVECs and MSCs. Recreating islets, ECM will provide structural components and biochemical cues to support cell survival and improve beta cell function. Additionally, CST can be used to support islet revascularization and to provide immunoprotective properties, supporting cell survival after transplantation. MSCs can be best used as a supporting cell for the creation of cell sheets, and as a tool to deliver intact islets, serving as reliable base layer. Additionally, MSCs can be harvested from the patient’s own bone marrow. Furthermore, MSC-based sheets can potentially improve the therapeutic efficacy of islets, as MSCs are known to secrete islet protective factors and enhance islet function and can to a limited extend induce neovascularization of the transplanted islets. Considering the transplant site, the liver surface and peritoneal wall seems to be the best cell sheet implantation location compared to the subcutaneous space and can potentially be used by applying minimal invasive surgical techniques in future clinical applications. Moreover, it is essential to optimize cell sheet thickness and size for superior handling and translation into clinical practice to compromise between cell survival and function and surgical implementation (Fig. 1).
Schematic overview of cell sheet technology applied to beta cell replacement therapy. (1) Different supporting cell types are cultured into a confluent layer. (2) Cells sheet are harvested and collected, (3) different cell sheets are stacked while beta cells or islets are placed in-between, (4) the living cell sheet–based implant is then implanted, after which the various support cells can mitigate inflammatory responses and stimulate vascularization of the newly created endocrine tissue
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82.
Cabrera O, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103(7):2334–9.
Diabetes C, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–30.
Shapiro AMJ, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8.
Othonos N, Choudhary P. Who should be considered for islet transplantation alone? Curr Diab Rep. 2017;17(4):23.
Buitinga M, et al. Micro-fabricated scaffolds lead to efficient remission of diabetes in mice. Biomaterials. 2017;135:10–22.
Olsson R, et al. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes. 2011;60(9):2350–3.
Shapiro AM, et al. The portal immunosuppressive storm: relevance to islet transplantation? Ther Drug Monit. 2005;27(1):35–7.
• Townsend SE, Gannon M. Extracellular matrix-associated factors play critical roles in regulating pancreatic beta-cell proliferation and survival. Endocrinology. 2019;160(8):1885–94. (This review summarise the importance of the ECM and microenvironment of the islets of Langerhans and its influence on the survival of islets of Langerhans.)
Linn T, et al. Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB J. 2003;17(8):881–3.
Brissova M, et al. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53(5):1318–25.
Nyqvist D, et al. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54(8):2287–93.
Pepper AR, et al. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clin Dev Immunol. 2013;2013:352315.
Farney AC, Sutherland DE, Opara EC. Evolution of islet transplantation for the last 30 years. Pancreas. 2016;45(1):8–20.
Vantyghem MC, et al. Advances in beta-cell replacement therapy for the treatment of type 1 diabetes. Lancet. 2019;394(10205):1274–85.
Vaithilingam V, Bal S, Tuch BE. Encapsulated islet transplantation: where do we stand? Rev Diabet Stud. 2017;14(1):51–78.
Matsuura K, et al. Cell sheet approach for tissue engineering and regenerative medicine. J Control Release. 2014;190:228–39.
Moschouris K, Firoozi N, Kang Y. The application of cell sheet engineering in the vascularization of tissue regeneration. Regen Med. 2016;11(6):559–70.
Li H, et al. Immunomodulatory functions of mesenchymal stem cells in tissue engineering. Stem Cells Int. 2019;2019:9671206–9671206.
Qi YY, Yan WQ. Mesenchymal stem cell sheet encapsulated cartilage debris provides great potential for cartilage defects repair in osteoarthritis. Med Hypotheses. 2012;79(3):420–1.
Qi YY, et al. Cartilage repair using mesenchymal stem cell (MSC) sheet and MSCs-loaded bilayer PLGA scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1424–33.
Kobayashi J, et al. Cell sheet tissue engineering: cell sheet preparation, harvesting/manipulation, and transplantation. J Biomed Mater Res, Part A. 2019;107(5):955–67.
Nishida K, et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77(3):379–85.
Nishida K, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351(12):1187–96.
Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343(2):86–93.
Ohki T, et al. Application of regenerative medical technology using tissue-engineered cell sheets for endoscopic submucosal dissection of esophageal neoplasms. Dig Endosc. 2015;27(2):182–8.
Takagi R, et al. Cell sheet technology for regeneration of esophageal mucosa. World J Gastroenterol. 2012;18(37):5145–50.
Ohki T, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143(3):582-U56.
Fabian T, Federico JA, Ponn RB. Fibrin glue in pulmonary resection: a prospective, randomized, blinded study. Ann Thorac Surg. 2003;75(5):1587–92.
Kanzaki M, et al. Dynamic sealing of lung air leaks by the transplantation of tissue engineered cell sheets. Biomaterials. 2007;28(29):4294–302.
Kanzaki M, et al. Functional closure of visceral pleural defects by autologous tissue engineered cell sheets. Eur J Cardiothorac Surg. 2008;34(4):864–9.
Asakawa N, et al. Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering. Biomaterials. 2010;31(14):3903–9.
Flores MG, et al. Cementum-periodontal ligament complex regeneration using the cell sheet technique. J Periodontal Res. 2008;43(3):364–71.
Akizuki T, et al. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J Periodontal Res. 2005;40(3):245–51.
Iwata T, et al. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009;30(14):2716–23.
Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. Faseb J. 2006;20(1):708–10. https://doi.org/10.1096/fj.05-4715fje.
Shimizu T, et al. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24(13):2309–16.
Miyagawa S, et al. Tissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: Their integration with recipient myocardium. Transplantation. 2005;80(11):1586–95.
Kidambi S, et al. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci USA. 2009;106(37):15714–9.
Staack A, et al. Molecular, cellular and developmental biology of urothelium as a basis of bladder regeneration. Differentiation. 2005;73(4):121–33.
Dumas A, et al. Bone grafts cultured with bone marrow stromal cells for the repair of critical bone defects: an experimental study in mice. J Biomed Mater Res A. 2009;90(4):1218–29.
Yang J, et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26(33):6415–22.
Gao Z, et al. Vitalisation of tubular coral scaffolds with cell sheets for regeneration of long bones: a preliminary study in nude mice. Br J Oral Maxillofac Surg. 2009;47(2):116–22.
Zhou Y, et al. Combined marrow stromal cell-sheet techniques and high-strength biodegradable composite scaffolds for engineered functional bone grafts. Biomaterials. 2007;28(5):814–24.
Zou XH, et al. A novel strategy incorporated the power of mesenchymal stem cells to allografts for segmental bone tissue engineering. Cell Transplant. 2009;18(4):433–41.
Nakamura A, et al. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone. 2010;46(2):418–24.
Ma D, et al. Reconstruction of rabbit critical-size calvarial defects using autologous bone marrow stromal cell sheets. Ann Plast Surg. 2010;65(2):259–65.
Miyahara Y, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12(4):459–65.
Okura H, et al. Cardiomyoblast-like cells differentiated from human adipose tissue-derived mesenchymal stem cells improve left ventricular dysfunction and survival in a rat myocardial infarction model. Tissue Eng Part C-Methods. 2010;16(3):417–25.
Matsuura K, et al. Creation of mouse embryonic stem cell-derived cardiac cell sheets. Biomaterials. 2011;32(30):7355–62.
Zhang W, et al. Rapidly constructed scaffold-free embryonic stem cell sheets for ocular surface reconstruction. Scanning. 2014;36(3):286–92.
Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126(11):S29–37. https://doi.org/10.1161/CIRCULATIONAHA.111.084343.
Kito T et al. iPS cell sheets created by a novel magnetite tissue engineering method for reparative angiogenesis. Sci Rep. 2013;3.
Anazawa T, et al. Current state and future evolution of pancreatic islet transplantation. Ann Gastroenterol Surg. 2019;3(1):34–42.
Addison P, Fatakhova K, Rodriguez Rilo HL. Considerations for an alternative site of islet cell transplantation. J Diabetes Sci Technol. 2020;14(2):338–44. https://doi.org/10.1177/1932296819868495.
Pepper AR, et al. Long-term function and optimization of mouse and human islet transplantation in the subcutaneous device-less site. Islets. 2016;8(6):186–94.
Pepper AR, et al. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol. 2015;33(5):518–23.
Dufour JM, et al. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue Eng. 2005;11(9–10):1323–31.
Blomeier H, et al. Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82(4):452–9.
Shimizu H, et al. Bioengineering of a functional sheet of islet cells for the treatment of diabetes mellitus. Biomaterials. 2009;30(30):5943–9.
Saito T, et al. Reversal of diabetes by the creation of neo-islet tissues into a subcutaneous site using islet cell sheets. Transplantation. 2011;92(11):1231–6.
Shimizu H, et al. Topographical Arrangement of alpha- and beta-cells within neo-islet tissues engineered by islet cell sheet transplantation in mice. Transpl Proc. 2013;45(5):1881–4.
Hirabaru M, et al. A method for performing islet transplantation using tissue-engineered sheets of islets and mesenchymal stem cells. Tissue Eng Part C-Methods. 2015;21(12):1205–15.
Imamura H et al. An engineered cell sheet composed of human islets and human fibroblast, bone marrow-derived mesenchymal stem cells, or adipose-derived mesenchymal stem cells: An in vitro comparison study. Islets. 2018;10(3).
Matsushima H, et al. Human fibroblast sheet promotes human pancreatic islet survival and function in vitro. Cell Transplant. 2016;25(8):1525–37.
Silva GV, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–6.
Boumaza I, et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun. 2009;32(1):33–42.
Vaithilingam V, Evans MDM, Lewy DM et al. Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted. Sci Rep. 2017;7. https://doi.org/10.1038/s41598-017-10359-1.
Jalili RB, et al. Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal. J Cell Physiol. 2011;226(7):1813–9.
Liu DZ, et al. The effect of fibroblast activation on vascularization in transplanted pancreatic islets. J Surg Res. 2013;183(1):450–6.
Perez-Basterrechea M et al. Cooperation by fibroblasts and bone marrow-mesenchymal stem cells to improve pancreatic rat-to-mouse islet xenotransplantation. Plos One, 2013;8(8).
Fujita I, et al. The liver surface as a favorable site for islet cell sheet transplantation in type 1 diabetes model mice. Regen Ther. 2018;8:65–72.
Hopcroft DW, Mason DR, Scott RS. Structure-function relationships in pancreatic islets: support for intraislet modulation of insulin secretion. Endocrinology. 1985;117(5):2073–80.
Lammert E, Thorn P. The role of the islet niche on beta cell structure and function. J Mol Biol. 2020;432(5):1407–18.
Lee YN et al. Evaluation of multi-layered pancreatic islets and adipose-derived stem cell sheets transplanted on various sites for diabetes treatment. Cells. 2020;9(9).
•• Lee YN et al. Improvement of the therapeutic capacity of insulin-producing cells trans-differentiated from human liver cells using engineered cell sheet. Stem Cell Res Ther. 2021;12(1). (This study shows promising results to generate beta cells from an alternative source. Further, CST is used to improve maturity and function of the generated beta cells.)
Lu Y, et al. Recent advances in cell sheet technology for bone and cartilage regeneration: from preparation to application. Int J Oral Sci. 2019;11(2):17–17.
Imashiro C, Shimizu T. Fundamental technologies and recent advances of cell-sheet-based tissue engineering. Int J Mol Sci. 2021;22(1):425.
Narita T, et al. The use of scaffold-free cell sheet technique to refine mesenchymal stromal cell-based therapy for heart failure. Mole Ther : the journal of the American Society of Gene Therapy. 2013;21(4):860–7.
Miyagawa S, et al. Phase I clinical trial of autologous stem cell-sheet transplantation therapy for treating cardiomyopathy. J Am Heart Assoc. 2017;6(4):e003918.
Yamaguchi N, et al. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep. 2017;7(1):17460–17460.
Inaba R, et al. Electrochemical desorption of self-assembled monolayers for engineering cellular tissues. Biomaterials. 2009;30(21):3573–9.
Enomoto J, et al. Engineering thick cell sheets by electrochemical desorption of oligopeptides on membrane substrates. Regen Ther. 2016;3:24–31.
Kobayashi Y, et al. Tailored cell sheet engineering using microstereolithography and electrochemical cell transfer. Sci Rep. 2019;9(1):10415–10415.
Kim JD, et al. Photothermally induced local dissociation of collagens for harvesting of cell sheets. Angew Chem Int Ed. 2015;54(20):5869–73.
Na J, et al. Harvesting of living cell sheets by the dynamic generation of diffractive photothermal pattern on PEDOT. Adv Func Mater. 2017;27(10):1604260.
Hong Y, et al. Light-induced cell detachment for cell sheet technology. Biomaterials. 2013;34(1):11–8.
Zhou Y, et al. Engineering prevascularized composite cell sheet by light-induced cell sheet technology. RSC Adv. 2017;7(52):32468–77.
Christoffersson G, et al. Clinical and experimental pancreatic islet transplantation to striated muscle: establishment of a vascular system similar to that in native islets. Diabetes. 2010;59(10):2569–78.
Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979;76(11):5665–8.
Osada A, et al. Harvesting epithelial keratinocyte sheets from temperature-responsive dishes preserves basement membrane proteins and improves cell survival in a skin defect model. J Tissue Eng Regen Med. 2017;11(9):2516–24.
Ito A, et al. Construction and harvest of multilayered keratinocyte sheets using magnetite nanoparticles and magnetic force. Tissue Eng. 2004;10(5–6):873–80.
Rovere MR, et al. Preserving basement membranes during detachment of cultivated oral mucosal epithelial cell sheets for the treatment of total bilateral limbal stem cell deficiency. Cell Transplant. 2018;27(2):264–74.
Goncalves AI, Rodrigues MT, Gomes ME. Tissue-engineered magnetic cell sheet patches for advanced strategies in tendon regeneration. Acta Biomater. 2017;63:110–22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cellular Transplants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hermanns, C., da Silva Filho, O.P., Vaithilingam, V. et al. The Potential of Cell Sheet Technology for Beta Cell Replacement Therapy. Curr Transpl Rep 9, 199–208 (2022). https://doi.org/10.1007/s40472-022-00371-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-022-00371-4