Abstract
Purpose of Review
Research in deceased organ donor management offers an opportunity to increase the quantity and quality of organs available for transplantation. This article aims to appraise the current literature with a focus on reviewing deceased donor intervention trials.
Recent Findings
Aggressive critical care management after determination of brain death resulting in meeting of a donor management goal bundle has consistently demonstrated an association with significantly more organs transplanted per donor as well as improved graft outcomes. Although there is a dearth of experience with randomized donor intervention studies, dopamine and targeted mild therapeutic hypothermia have been found to significantly reduce delayed graft function in kidney recipients.
Summary
Progress in understanding the ethical, legal, regulatory, policy, and organizational elements of organ donor research has provided a mechanism that allows for the endorsement of potentially impactful donor management studies. Ongoing trials should incorporate methods to ensure safety to all organs donated from donors enrolled in interventional trials.
Similar content being viewed by others
Introduction
Solid organ transplantation is a life-altering intervention for those suffering from end-stage organ disease and provides a significant survival benefit to patients. Unfortunately, despite substantial progress in the field, a pervasive shortage of donor organs continues to lead to excess deaths on transplant waiting lists internationally. As such, efforts to improve the quantity and quality of organs available for transplantation have focused on addressing this imbalance through a multifaceted approach. Strategies have included raising awareness of organ donation and improving donor designation rates; increasing the proportion of living donors, expanded criteria donors, and donors after circulatory determination of death (DCDs); developing protocols to successfully use organs from donors who are at risk of infection or have known infection (e.g., human immune deficiency virus or hepatitis C); optimizing organ allocation; developing ex vivo perfusion devices to store, assess, and repair procured organs; and reducing the number of discarded organs through improved deceased donor management and intervention.
The following review article highlights progress in deceased organ donor management and intervention trials. Specifically, a brief summary of developments in critical care management is provided followed by a more detailed review of completed, ongoing, and future donor intervention studies which hold promise to provide more lifesaving organs for transplantation.
Critical Care of The Potential Organ Donor
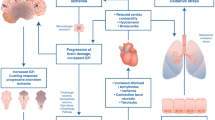
Providing appropriate critical care to patients with devastating brain injuries serves to increase the chance of neurologic recovery as well as simultaneously preserve the option for organ donation in those who regress to brain death. A timeline of potential care of the patient with neurologic injury, depicting the phases of donor management, is presented in Fig. 1. In this construct, the donor hospital phase of care refers to the care provided from the time referral for imminent brain death is made to the organ procurement organization (OPO) through to the time authorization is obtained for organ donation. The latter phase of management, the OPO phase, represents the critical care management after authorization for donation is obtained and extends to organ recovery.
Timeline and phases of management of the potential organ donor. From Patel MS, De La Cruz S, Sally MB, Groat T, Malinoski DJ. Active Donor Management During the Hospital Phase of Care Is Associated with More Organs Transplanted per Donor. J Am Coll Surg. 2017;225 [4]:525–31.Used with permission from Elsevier
Following brain death, donor physiology changes as a result of loss of the neurohormonal axis, development of diabetes insipidus, and alteration in normal homeostasis [1]. Management of donors after determination of brain death is thus crucial in order to maintain adequate end-organ perfusion and ensure optimal recovery of potentially lifesaving organs. Continuous assessment of critical care endpoints in the donor is imperative to help guide intensive care unit (ICU) management and improve the quality of potentially transplantable organs [2••, 3,4,5,6,7, 8•].
In order to aid the management of donors after brain death (DBDs), the Donation and Transplantation Community of Practice (DTCP) recommends that OPOs use a checklist of critical care endpoints, also known as donor management goals (DMGs), during the OPO phase of management. Specifically, a DMG bundle (Table 1) is a set of variables which capture the hemodynamic, acid-base, respiratory, renal, and endocrine status of a donor, along with corresponding target values which reflect normal physiology. During the OPO phase of care, the United Network Organ Sharing (UNOS) clinical pathway or other regional practices may be used [9]. Catastrophic brain injury guidelines (CBIGs) or other established protocols can be used by critical care physicians and providers to help guide management of potential organ donors during the donor hospital phase of care [10]. Many hospital CBIGs incorporate the same critical care endpoints included in the DMG bundle, as they merely reflect good critical care practices that would benefit any patient in the ICU. Indeed, prior studies have assessed the impact of meeting a DMG bundle (defined as achieving any seven of nine critical care endpoints) during both the hospital and OPO phases of care. Results have consistently demonstrated an association with significantly more organs transplanted per donor (OTPD) as well as improved graft outcomes [3, 4, 11]. Most recently, a prospective observational study of DBDs from ten OPOs across three United Network Organ Sharing (UNOS) regions strengthened these findings by noting that in donors not meeting the DMG bundle at referral, critical care practices leading to the bundle being met by the time of authorization for donation led to a twofold increase in achieving ≥ 4 OTPD [8•]. This study is important in demonstrating that active critical care management during the donor hospital phase of care can substantially impact the number of organs available for transplant.
The importance of providing care to patients with devastating injuries has been highlighted by consensus statements from the Neurocritical Care Society [12••] as well as from a consortium of the Society of Critical Care Medicine (SCCM), the American College of Chest Physicians (ACCP), and the Association of Organ Procurement Organizations (AOPO) [2••]. Detailed recommendations are provided in these guidelines and are beyond the scope of this review.
Donor Intervention Trials
Deceased organ donor intervention research is considered to have tremendous potential to increase the quantity and quality of organs available for transplantation [13, 14, 15••]. However, this type of research has faced substantial challenges due to logistical complexity, diversity of stakeholders, and a lack of regulatory guidance. This has been extensively discussed in the literature [13, 16,17,18]. To this end, in October 2017, the National Academies of Sciences, Engineering, and Medicine (NASEM) released a seminal report focused on the ethical, legal, regulatory, policy, and organizational elements of organ donor research. The report clearly emphasizes the need for further coordination and design of donor intervention studies [19••].
A review of current clinical trials in deceased donor intervention is thus timely as it highlights work that has been completed and that which is both ongoing and planned. ClinicalTrials.gov, which is run by the United States National Library of Medicine (NLM) at the National Institutes of Health (NIH), is the largest clinical trials database with registrations from 204 countries. This database was queried for all studies involving solid organ transplantation, deceased donor intervention trials. Interventions were defined as those related to procedures (e.g., ischemic preconditioning), drug administration, or other management change (e.g., use of checklists for donor management). Trials evaluating ex vivo perfusion of procured organs were excluded, as were studies which were terminated.
As of June 2018, there are 26 trials registered in the ClinicalTrials.gov database meeting the aforementioned criteria, with the earliest completed in March 2007 (Table 2). Of these, 14 are completed, 5 are actively recruiting, 2 are registered but not yet recruiting, and 4 have an unknown status. The most common type of intervention involved drug delivery to the deceased donor. Interestingly, only 10 trials (38%) had full-length publications indexed to the study by their ClinicalTrails.gov National Clinical Trial (NCT) number. Notable donor intervention efforts, findings, and ongoing trials are discussed below.
Ischemic Preconditioning of the Donor
Ischemic preconditioning, a technique first described in 1986, involves inducing short intervals of ischemia either directly to or remotely from a target organ, as a means of providing protection during a subsequent ischemic insult such as that which occurs during ischemia-reperfusion of a transplanted graft [50, 51]. Initial clinical studies of ischemic preconditioning in transplantation were performed in deceased donor liver transplants and involved hepatic ischemic preconditioning by hilar clamping for 10 min followed by release of the clamp prior to procurement of the liver from the donor [20]. Results from this trial did not show a difference in patient or graft survival, but did show evidence of an increase in reperfusion injury [20]. Subsequent systematic review and meta-analyses of ten studies including 593 patients (286 donors with ischemic preconditioning and 307 controls) have demonstrated a decrease in 1-year mortality, but did not reach statistical significance (OR 0.54, CI 0.28–1.04, p = 0.06) [50]. There was, however, a significantly lower postoperative day 3 aspartate aminotransferase (AST) level, resulting in the conclusion that any confirmation of benefit in the liver transplant recipient would require an adequately powered prospective randomized controlled trial [50]. A trial evaluating the effect of remote ischemic preconditioning in neurologic death organ donors (RIPNODs) has also been performed; it subjected donors to 4 cycles of 5 min of pneumatic tourniquet induced ischemia on the mid-thigh 6 h prior to as well as directly before organ recovery (ClinicalTrials.gov Identifier: NCT01515072). Results have been published in abstract form only and suggest that remote ischemic preconditioning does not increase the number of organs procured or transplanted but may lead to improvement in kidney survival [21]. Lastly, efforts investigating ischemic preconditioning of the recipient have also been a focus of study, but are beyond the scope of this review which focuses on deceased donor management.
Dopamine
Low-dose dopamine infusion (4 μg/kg per minute) administered to donors after determination of brain death has been shown to improve outcomes of transplanted organs in kidney and heart recipients [22, 23••, 24, 25••, 26, 27]. Initial results of a randomized controlled trial in 2009 by Schnuelle et al. demonstrated a significantly lower dialysis requirement during the first week post-transplant [25]. Further, a post hoc analysis of 93 heart transplants from multiorgan donors of this original trial demonstrated improved 3-year graft survival for organs recovered from donors receiving pretreatment with dopamine (87% vs. 67.8%, p = 0.03), which persisted on adjusted analysis (HR 0.33, CI 0.12–0.89, p = 0.03) [24]. Mechanistically, it is thought that the observed protective effect is due to dopamine’s ability to mitigate cellular injury by scavenging reactive oxygen species that accumulate and lead to cell death under cold storage conditions rather than its circulatory effects [27, 28]. Most recently, long-term follow-up results of the 487 renal transplant patients receiving grafts in the dopamine donor pretreatment trial failed to demonstrated a significant graft survival advantage on intention-to-treat analysis [23••]. This was thought to be secondary to the duration of continuous infusion being too brief in a substantial number of donors [23••]. This observation was supported by further analysis of the data, in that the investigators noted a nonlinear exposure-response relationship suggesting that benefit was noted for infusion times of around 7 h [23••]. Although dopamine infusion in the donor was noted to be relatively safe, further prospective studies are needed to confirm a recipient or graft survival benefit [23••, 27].
Ventilatory Strategies
Optimization of ventilator management has been investigated as a means to improve lung function and increase the number of lungs eligible for transplantation. In 2010, results of the Protective Ventilatory Strategy in Potential Lung Donors Study were published [29]. In this report, the effects of a protective ventilator strategy with tidal volumes of 6–8 mL/kg of predicted body weight (vs. 10–12 mL/kg), positive end-expiratory pressure (PEEP) of 8–10 cm H2O (vs. 3–5 cm H2O), apnea tests performed using continuous positive airway pressure (vs. disconnection from the ventilator), and maintenance of a closed (vs. open) circuit for airway suction were evaluated [29]. In the 59 of 118 donors with a protective ventilatory strategy, eligibility for lung donation was significantly higher (95% vs. 54%, p < 0.001) and significantly more lungs were procured (54% vs. 27%, p = 0.004) [29]. This study was performed as a European clinical trial during the 6-h interval between brain death exams; given that patients are not considered to be organ donors until after a final determination of brain death and receipt of authorization for donation in the USA as well as other differences in the logistics of donor management and organ recovery in Europe, it is unclear if these findings are translatable in the USA. Thus, the Goal of Open Lung Ventilation in Donors (GOLD) trial has been recently registered (ClinicalTrials.gov Identifier: NCT03439995) with an estimated start date in the USA of June 2018.
With regard to pharmacologic strategies, the Beta-agonists for Oxygenation in Lung Donors (BOLD) trial deserves mention. In this randomized, placebo-controlled trial assessing the effect of aerosolized albuterol on donors, both oxygenation and lung utilization did not improve [30]. Rather, the use of albuterol was associated with increased tachycardia, thus suggesting that this drug should not be used in donor management to aid in the resolution of pulmonary edema or increase lung utilization [30].
N-Acetylcysteine
The role of N-acetylcysteine (NAC), as it is known to regenerate glutathione and scavenges free oxygen radicals, has been studied in DBDs as a means of potentially improving graft survival for liver transplant recipients and reducing delayed graft function (DGF) in kidney transplant recipients [31, 32]. In the former of these studies, a prospective randomized clinical trial assessing the systemic and portal infusion of NAC prior to liver procurement was assessed. On adjusted analyses, NAC infusion in the donor was noted to benefit 3-month (HR 1.65, CI 1.01–2.93, p = 0.04) and 12-month (HR 1.73, CI 1.14–2.76, p ≤ 0.01) graft survival. In the latter of these studies, an attempt to address the notion that contrast media is often utilized to confirm the diagnosis of brain death through acquisition of cerebral or computed tomography scan angiography, and that deceased donors are at higher risk of contrast-induced acute kidney injury, was made. In a randomized, open-label, single-center clinical trial, DBDs were randomized in the treatment group to receive 600 mg of intravenous NAC 1 h before and 2 h after angiography used to confirm brain death [31]. The primary endpoint was DGF in the recipient, defined as the need for at least one dialysis session within the first week of transplantation or a serum creatinine level noted to be greater than 200 μmol/L at day 7 after transplantation [31]. There was no statistically significant difference in DGF rates noted in recipients of donor grafts that were pretreated with NAC and thus no benefit was noted for administration of this drug at the evaluated dose [31].
Therapeutic Hypothermia
The clinical impact of DGF on renal transplant graft and patient survival as well as resource utilization is well described in the literature [33,34,35,36,37,38,39,40], and reduction of DGF remains a critical goal for the global transplant community. Therapeutic hypothermia of the deceased donor has been studied as an intervention to improve renal function in the transplant recipient [15••]. In a recent clinical trial, 394 brain-dead donors with documented research authorization received either targeted mild hypothermia (34–35 °C) or normothermia (36.5–37.5 °C). Randomization was stratified by standard versus expanded criteria donor status (SCD vs. ECD), organ procurement organization, and whether or not the donor had received therapeutic hypothermia to treat their primary neurologic insult prior to death. ECDs were defined using standard clinical definitions [15••]. Targeted temperatures were achieved using forced air systems or external cooling devices and followed a protocol adopted across all donor hospitals by OPO coordinators. This trial was terminated early for efficacy as targeted mild hypothermia was found to significantly reduce DGF (OR 0.62, CI 0.43–0.92, p = 0.02) after half of the maximum planned enrollment was reached [15••]. Importantly, in the trial, hypothermia was found to not impact organ utilization rates compared with normothermia [15••].
While the benefit of hypothermia in reducing the incidence of DGF was noted in the overall study population, the effect of therapeutic hypothermia was most evident in higher risk donors [15••]. A follow-up report demonstrating the impact of the hypothermia protocol on graft survival of the targeted (kidney) and non-targeted (extra-renal) organs is expected to be published in the near future. Lastly, as the completed trial was in kidneys not undergoing pulsatile perfusion after organ removal from the donor, a comparison of targeted hypothermia and pulsatile perfusion is currently being investigated in a follow-up national clinical trial (ClinicalTrials.gov Identifier: NCT02525510) which is actively recruiting.
Protocolized Care of Donors
As noted above, in retrospective and prospective observational studies, standardized care of the potential organ donor using a checklist of DMGs has been associated with an increase in the number and quality of organs available for transplantation [2••, 3,4,5,6,7, 8•]. The Monitoring Organ Donors to Increase Transplantation Results (MOnIToR) study was designed as a randomized controlled, multicenter trial to compare use of a protocol-guided fluid therapy algorithm which focused on cardiac index, mean arterial pressure, and pulse pressure variation versus a non-protocolized standard of care [41, 42]. There was no significant difference in the number of OTPD in the protocolized care group (3.39 vs. 3.29, p = 0.56) [42]. Most recently, a cluster-randomized clinical trial is recruiting donors across 60 Brazilian intensive care units (ICUs) to further assess the impact of standardized care by randomizing the management of potential organ donors to an evidence-based checklist arm and comparing outcomes to those managed according to usual care (ClinicalTrials.gov Identifier: NCT03179020). The study is estimated to complete in December 2019.
Future Considerations
As avenues to increase the supply and quality of deceased organs available for transplant are explored, and donor intervention research continues to gain traction with endorsement of the NASEM, new challenges will arise that require attention [19••]. For instance, it will be vital to establish systems to ensure that donor-based interventions do not have a negative effect on donor physiology or non-target organs, as an intervention delivered to the donor has the potential to impact multiple recipients. In order for ongoing trials to ensure safety of the donor, changes in critical care parameters from the time of authorization for donation, through the time of a donor intervention, and up to organ recovery in any prospective donor intervention trial should be recorded and studied. Organized data registries, such as the UNOS Donor Management Goals (DMG) Registry Web Portal (https://nationaldmg.org), provide the necessary framework that can enable multicenter data collection of relevant physiologic endpoints.
Further, with increasing experience in designing donor intervention studies, a clear delineation of metrics used to gauge outcomes and standards of reporting will need to be formalized to assure meaningful interpretation of results. For example, the appropriate time frame for assessment of impact delivered to the deceased donor has been variable. It can be argued that survival through 1 year should be used, as this metric is standardly reported through the Scientific Registry of Transplant Recipients to assess program performance. However, intuitively, more distant time points make drawing causal inference of a donor intervention increasingly confounded by other clinical events [43]. As such, several of the donor-based trials reported in this review have chosen kidney recipient DGF as their primary outcome measure as have numerous pharmaceutical transplant recipient intervention trials. Establishing a standard, either broadly applicable or individualized for a particular study, will require continued discussion and expert consensus, but remains imperative as donor research becomes more formalized. Lastly, in addition to traditional outcomes, additional considerations such as the impact of a donor intervention on the distribution of organs and waitlist mortality are metrics that should be considered in determining the overall utility of adopting specific interventions.
Conclusions
Deceased donor intervention provides an opportunity to ameliorate graft injury and rescue organs that may otherwise be discarded as well as improve post-transplant function in those which would otherwise be transplanted. Although significant progress has been made in donor management over the past few years, there remains a dearth of experience with conducting meaningful donor intervention trials. Now that discussion of logistical, ethical, and regulatory challenges pertaining to donor intervention studies has begun, it is likely that an increasing number of trials will be underway and additional practical considerations as well as limitations will surface. Given the promise that deceased donor management holds for narrowing the supply and demand gap of organs available for transplantation, maintaining persistence in continuing to advance this evolving field of research remains imperative.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
McKeown DW, Bonser RS, Kellum JA. Management of the heartbeating brain-dead organ donor. Br J Anaesth. 2012;108(Suppl 1):i96–107.
•• Kotloff RM, Blosser S, Fulda GJ, Malinoski D, Ahya VN, Angel L, et al. Management of the potential organ donor in the ICU: Society of Critical Care Medicine/American College of Chest Physicians/Association of Organ Procurement Organizations Consensus Statement. Crit Care Med. 2015;43(6):1291–325. Consensus statement providing practical recommendations to aid critical care practitioners in the management of potential organ donors.
Malinoski DJ, Patel MS, Ahmed O, Daly MC, Mooney S, Graybill CO, et al. The impact of meeting donor management goals on the development of delayed graft function in kidney transplant recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. 2013;13(4):993–1000.
Malinoski DJ, Patel MS, Daly MC, Oley-Graybill C, Salim A. The impact of meeting donor management goals on the number of organs transplanted per donor: results from the United Network for Organ Sharing Region 5 prospective donor management goals study. Crit Care Med. 2012;40(10):2773–80.
Malinoski DJ, Daly MC, Patel MS, Oley-Graybill C, Foster CE 3rd, Salim A. Achieving donor management goals before deceased donor procurement is associated with more organs transplanted per donor. J Trauma. 2011;71(4):990–5. discussion 6
Franklin GA, Santos AP, Smith JW, Galbraith S, Harbrecht BG, Garrison RN. Optimization of donor management goals yields increased organ use. Am Surg. 2010;76(6):587–94.
Westphal GA. A simple bedside approach to therapeutic goals achievement during the management of deceased organ donors--an adapted version of the “VIP” approach. Clin Transpl. 2016;30(2):138–44.
• Patel MS, De La Cruz S, Sally MB, Groat T, Malinoski DJ. Active donor management during the hospital phase of care is associated with more organs transplanted per donor. J Am Coll Surg. 2017;225(4):525–31. Prospective observational study demonstrating a substantial increase in organs transplanted per donor with active critical care of the potential organ donor.
Critical Pathway for the Organ Donor [Available from: https://www.unos.org/wp-content/uploads/unos/Critical_Pathway.pdf.
Malinoski DJ, Patel MS, Lush S, Willis ML, Navarro S, Schulman D, et al. Impact of compliance with the American College of Surgeons trauma center verification requirements on organ donation-related outcomes. J Am Coll Surg. 2012;215(2):186–92.
Malinoski DJ, Daly MC, Patel MS, Oley-Graybill C, Foster CE 3rd, Salim A. Achieving donor management goals before deceased donor procurement is associated with more organs transplanted per donor. J Trauma. 2011;71(4):990–5. discussion 6
•• Souter MJ, Blissitt PA, Blosser S, Bonomo J, Greer D, Jichici D, et al. Recommendations for the critical care management of devastating brain injury: prognostication, psychosocial, and ethical management : a position statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care. 2015;23(1):4–13. Position statement to help guide management during the first 72 h after devastating brain injury.
Abt PL, Marsh CL, Dunn TB, Hewitt WR, Rodrigue JR, Ham JM, et al. Challenges to research and innovation to optimize deceased donor organ quality and quantity. Am J Transplant. 2013;13(6):1400–4.
Feng S. Donor intervention and organ preservation: where is the science and what are the obstacles? Am J Transplant. 2010;10(5):1155–62.
•• Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M, et al. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med. 2015;373(5):405–14. Randomized controlled trial demonstrating the impact of therapeutic hypotermia in the decased organ donor on signficantly decreasing delayed kidney graft function in recipients.
Rodrigue JR, Feng S, Johansson AC, Glazier AK, Abt PL. Deceased donor intervention research: a survey of transplant surgeons, organ procurement professionals, and institutional review board members. Am J Transplant. 2016;16(1):278–86.
Glazier AK, Heffernan KG, Rodrigue JR. A framework for conducting deceased donor research in the United States. Transplantation. 2015;99(11):2252–7.
Mone T, Heldens J, Niemann CU. Deceased organ donor research: the last research frontier? Liver Transpl. 2013;19(2):118–21.
•• National Academies of Sciences E, Medicine. Opportunities for organ donor intervention research: saving lives by improving the quality and quantity of organs for transplantation. In: Childress JF, Domnitz S, Liverman CT, editors. . Washington, DC: The National Academies Press; 2017. 220 p. Report discussing the ethical, legal, regulatory, policy, and organizational elements of organ donor research, and endorsing the need for further coordination and design of donor intervention studies.
Koneru B, Shareef A, Dikdan G, Desai K, Klein KM, Peng B, et al. The ischemic preconditioning paradox in deceased donor liver transplantation-evidence from a prospective randomized single blind clinical trial. Am J Transplant. 2007;7(12):2788–96.
Bongu A, Washburn WK, Oliver JB, Davidow AL, Nespral J, Pentakota SR, et al. Remote ischemic preconditioning in neurological death organ donors: the RIPNOD Prospective Randomized Clinical Trial. J Am Coll Surg. 2017;225(4):e171.
Schnuelle P, Mundt HM, Druschler F, Schmitt WH, Yard BA, Kramer BK, et al. Impact of spontaneous donor hypothermia on graft outcomes after kidney transplantation. Am J Transplant. 2018;18(3):704–14.
•• Schnuelle P, Schmitt WH, Weiss C, Habicht A, Renders L, Zeier M, et al. Effects of dopamine donor pretreatment on graft survival after kidney transplantation: a randomized trial. Clin J Am Soc Nephrol. 2017;12(3):493–501. Five-year follow-up of renal transplant patients participating in dopamine donor pretreatment clinical trial showing no signficant graft survival advanatage on intention-to-treat analysis but noting an exposure-response relationship which may provide advanatage to a subset.
Benck U, Hoeger S, Brinkkoetter PT, Gottmann U, Doenmez D, Boesebeck D, et al. Effects of donor pre-treatment with dopamine on survival after heart transplantation: a cohort study of heart transplant recipients nested in a randomized controlled multicenter trial. J Am Coll Cardiol. 2011;58(17):1768–77.
Schnuelle P, Gottmann U, Hoeger S, Boesebeck D, Lauchart W, Weiss C, et al. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA. 2009;302(10):1067–75.
Benck U, Gottmann U, Hoeger S, Lammert A, Rose D, Boesebeck D, et al. Donor desmopressin is associated with superior graft survival after kidney transplantation. Transplantation. 2011;92(11):1252–8.
Schnuelle P, Benck U, Yard BA. Dopamine in transplantation: written off or comeback with novel indication? Clin Transpl. 2018:e13292.
Brinkkoetter PT, Song H, Losel R, Schnetzke U, Gottmann U, Feng Y, et al. Hypothermic injury: the mitochondrial calcium, ATP and ROS love-hate triangle out of balance. Cell Physiol Biochem. 2008;22(1–4):195–204.
Mascia L, Pasero D, Slutsky AS, Arguis MJ, Berardino M, Grasso S, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304(23):2620–7.
Ware LB, Landeck M, Koyama T, Zhao Z, Singer J, Kern R, et al. A randomized trial of the effects of nebulized albuterol on pulmonary edema in brain-dead organ donors. Am J Transplant. 2014;14(3):621–8.
Orban JC, Quintard H, Cassuto E, Jambou P, Samat-Long C, Ichai C. Effect of N-acetylcysteine pretreatment of deceased organ donors on renal allograft function: a randomized controlled trial. Transplantation. 2015;99(4):746–53.
D’Amico F, Vitale A, Piovan D, Bertacco A, Ramirez Morales R, Chiara Frigo A, et al. Use of N-acetylcysteine during liver procurement: a prospective randomized controlled study. Liver Transpl. 2013;19(2):135–44.
Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24(3):1039–47.
Butala NM, Reese PP, Doshi MD, Parikh CR. Is delayed graft function causally associated with long-term outcomes after kidney transplantation? Instrumental variable analysis. Transplantation. 2013;95(8):1008–14.
McLaren AJ, Jassem W, Gray DW, Fuggle SV, Welsh KI, Morris PJ. Delayed graft function: risk factors and the relative effects of early function and acute rejection on long-term survival in cadaveric renal transplantation. Clin Transpl. 1999;13(3):266–72.
Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21(1):153–61.
Fonseca I, Teixeira L, Malheiro J, Martins LS, Dias L, Castro Henriques A, et al. The effect of delayed graft function on graft and patient survival in kidney transplantation: an approach using competing events analysis. Transpl Int. 2015;28(6):738–50.
Gill J, Dong J, Rose C, Gill JS. The risk of allograft failure and the survival benefit of kidney transplantation are complicated by delayed graft function. Kidney Int. 2016;89(6):1331–6.
Lee J, Song SH, Lee JY, Kim DG, Lee JG, Kim BS, et al. The recovery status from delayed graft function can predict long-term outcome after deceased donor kidney transplantation. Sci Rep. 2017;7(1):13725.
Troppmann C, Gillingham KJ, Benedetti E, Almond PS, Gruessner RW, Najarian JS, et al. Delayed graft function, acute rejection, and outcome after cadaver renal transplantation. The multivariate analysis. Transplantation. 1995;59(7):962–8.
Al-Khafaji A, Murugan R, Wahed AS, Lebovitz DJ, Souter MJ, Kellum JA, et al. Monitoring Organ Donors to Improve Transplantation Results (MOnIToR) trial methodology. Crit Care Resusc. 2013;15(3):234–40.
Al-Khafaji A, Elder M, Lebovitz DJ, Murugan R, Souter M, Stuart S, et al. Protocolized fluid therapy in brain-dead donors: the multicenter randomized MOnIToR trial. Intensive Care Med. 2015;41(3):418–26.
Niemann CU, Broglio K, Malinoski D. Comments on “Impact of spontaneous donor hypothermia on graft outcomes after kidney transplantation”. Am J Transplant. 2018;18(3):763.
Koneru B, Fisher A, He Y, Klein KM, Skurnick J, Wilson DJ, et al. Ischemic preconditioning in deceased donor liver transplantation: a prospective randomized clinical trial of safety and efficacy. Liver Transpl. 2005;11(2):196–202.
Raza A, Dikdan G, Desai KK, Shareef A, Fernandes H, Aris V, et al. Global gene expression profiles of ischemic preconditioning in deceased donor liver transplantation. Liver Transpl. 2010;16(5):588–99.
Desai KK, Dikdan GS, Shareef A, Koneru B. Ischemic preconditioning of the liver: a few perspectives from the bench to bedside translation. Liver Transpl. 2008;14(11):1569–77.
Sharpe MD, van Rassel B, Haddara W. Oral and intravenous thyroxine (T4) achieve comparable serum levels for hormonal resuscitation protocol in organ donors: a randomized double-blinded study. Can J Anaesth. 2013;60(10):998–1002.
Ware LB, Koyama T, Billheimer D, Landeck M, Johnson E, Brady S, et al. Advancing donor management research: design and implementation of a large, randomized, placebo-controlled trial. Ann Intensive Care. 2011;1(1):20.
Orban JC, Fontaine E, Cassuto E, Baumstarck K, Leone M, Constantin JM, et al. Effects of cyclosporine A pretreatment of deceased organ donors on kidney graft function (Cis-A-rein): study protocol for a randomized controlled trial. Trials. 2018;19(1):231.
Robertson FP, Magill LJ, Wright GP, Fuller B, Davidson BR. A systematic review and meta-analysis of donor ischaemic preconditioning in liver transplantation. Transpl Int. 2016;29(11):1147–54.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36.
Funding
This work was supported in part by the Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Madhukar Patel, Mitchell Sally, and Claus Niemann declare no conflict of interest. Darren Malinoski reports grants from the Laura and John Arnold Foundation, during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Anesthesia and Critical Care in Transplantation
Rights and permissions
About this article
Cite this article
Patel, M.S., Sally, M., Niemann, C.U. et al. State of the Science in Deceased Organ Donor Management. Curr Transpl Rep 5, 273–281 (2018). https://doi.org/10.1007/s40472-018-0207-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-018-0207-8