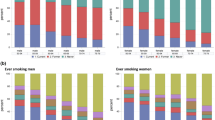
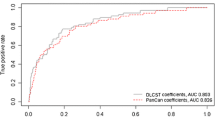
Abstract
Purpose of Review
Risk prediction models may be useful for facilitating effective and high-quality decision-making at critical steps in the lung cancer screening process. This review provides a current overview of published lung cancer risk prediction models and their applications to lung cancer screening and highlights both challenges and strategies for improving their predictive performance and use in clinical practice.
Recent Findings
Since the 2011 publication of the National Lung Screening Trial results, numerous prediction models have been proposed to estimate the probability of developing or dying from lung cancer or the probability that a pulmonary nodule is malignant. Respective models appear to exhibit high discriminatory accuracy in identifying individuals at highest risk of lung cancer or differentiating malignant from benign pulmonary nodules. However, validation and critical comparison of the performance of these models in independent populations are limited. Little is also known about the extent to which risk prediction models are being applied in clinical practice and influencing decision-making processes and outcomes related to lung cancer screening.
Summary
Current evidence is insufficient to determine which lung cancer risk prediction models are most clinically useful and how to best implement their use to optimize screening effectiveness and quality. To address these knowledge gaps, future research should be directed toward validating and enhancing existing risk prediction models for lung cancer and evaluating the application of model-based risk calculators and its corresponding impact on screening processes and outcomes.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. https://doi.org/10.1056/NEJMoa1102873.
•• Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–8. https://doi.org/10.7326/m13-2771. Describes the US Preventive Services Task Force recommendation to screen high-risk adults annually for lung cancer with low-dose computed tomography.
Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, MacMahon H, et al. Development of the American Association for Thoracic Surgery guidelines for low-dose computed tomography scans to screen for lung cancer in North America: recommendations of the American Association for Thoracic Surgery Task Force for Lung Cancer Screening and Surveillance. J Thorac Cardiovasc Surg. 2012;144(1):25–32. https://doi.org/10.1016/j.jtcvs.2012.05.059.
Wender R, Fontham ET, Barrera E Jr, Colditz GA, Church TR, Ettinger DS, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107–17. https://doi.org/10.3322/caac.21172.
Wood DE, Eapen GA, Ettinger DS, Hou L, Jackman D, Kazerooni E, et al. Lung cancer screening. J Natl Compr Cancer Netw. 2012;10(2):240–65.
Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418–29. https://doi.org/10.1001/jama.2012.5521.
Marcus PM, Pashayan N, Church TR, Doria-Rose VP, Gould MK, Hubbard RA, et al. Population-based precision cancer screening: a symposium on evidence, epidemiology, and next steps. Cancer Epidemiol Biomark Prev. 2016;25(11):1449–55. https://doi.org/10.1158/1055-9965.epi-16-0555.
Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ (Clin Res ed). 2009;338:b605. https://doi.org/10.1136/bmj.b605.
Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515–24.
Bleeker SE, Moll HA, Steyerberg EW, Donders AR, Derksen-Lubsen G, Grobbee DE, et al. External validation is necessary in prediction research: a clinical example. J Clin Epidemiol. 2003;56(9):826–32.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. https://doi.org/10.1148/radiology.143.1.7063747.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Dec Mak. 2006;26(6):565–74. https://doi.org/10.1177/0272989x06295361.
Tammemagi MC. Application of risk prediction models to lung cancer screening: a review. J Thorac Imaging. 2015;30(2):88–100. https://doi.org/10.1097/rti.0000000000000142.
Van Calster B, Vickers AJ. Calibration of risk prediction models: impact on decision-analytic performance. Med Dec Mak. 2015;35(2):162–9. https://doi.org/10.1177/0272989x14547233.
Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95(6):470–8.
Cassidy A, Myles JP, van Tongeren M, Page RD, Liloglou T, Duffy SW, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer. 2008;98(2):270–6. https://doi.org/10.1038/sj.bjc.6604158.
Etzel CJ, Kachroo S, Liu M, D'Amelio A, Dong Q, Cote ML, et al. Development and validation of a lung cancer risk prediction model for African-Americans. Cancer Prev Res (Philadelphia, Pa). 2008;1(4):255–65. https://doi.org/10.1158/1940–6207.capr-08-0082.
El-Zein RA, Lopez MS, D’Amelio AM Jr, Liu M, Munden RF, Christiani D, et al. The cytokinesis-blocked micronucleus assay as a strong predictor of lung cancer: extension of a lung cancer risk prediction model. Cancer Epidemiol Biomark Prev. 2014;23(11):2462–70. https://doi.org/10.1158/1055-9965.epi-14-0462.
Hippisley-Cox J, Coupland C. Development and validation of risk prediction algorithms to estimate future risk of common cancers in men and women: prospective cohort study. BMJ Open. 2015;5(3):e007825. https://doi.org/10.1136/bmjopen-2015-007825.
Hoggart C, Brennan P, Tjonneland A, Vogel U, Overvad K, Ostergaard JN, et al. A risk model for lung cancer incidence. Cancer Prev Res (Philadelphia, Pa). 2012;5(6):834–46. https://doi.org/10.1158/1940–6207.capr-11-0237.
Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;315(21):2300–11. https://doi.org/10.1001/jama.2016.6255.
Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245–54. https://doi.org/10.1056/NEJMoa1301851.
Li H, Yang L, Zhao X, Wang J, Qian J, Chen H, et al. Prediction of lung cancer risk in a Chinese population using a multifactorial genetic model. BMC Med Genet. 2012;13:118. https://doi.org/10.1186/1471-2350-13-118.
Marcus MW, Chen Y, Raji OY, Duffy SW, Field JK. LLPi: Liverpool Lung Project risk prediction model for lung cancer incidence. Cancer Prev Res (Philadelphia, Pa). 2015;8(6):570–5. https://doi.org/10.1158/1940–6207.capr-14-0438.
Marcus MW, Raji OY, Duffy SW, Young RP, Hopkins RJ, Field JK. Incorporating epistasis interaction of genetic susceptibility single nucleotide polymorphisms in a lung cancer risk prediction model. Int J Oncol. 2016;49(1):361–70. https://doi.org/10.3892/ijo.2016.3499.
Muller DC, Johansson M, Brennan P. Lung cancer risk prediction model incorporating lung function: development and validation in the UK Biobank Prospective Cohort Study. J Clin Oncol. 2017;Jco2016692467 https://doi.org/10.1200/jco.2016.69.2467.
Park S, Nam BH, Yang HR, Lee JA, Lim H, Han JT, et al. Individualized risk prediction model for lung cancer in Korean men. PLoS One. 2013;8(2):e54823. https://doi.org/10.1371/journal.pone.0054823.
Raji OY, Agbaje OF, Duffy SW, Cassidy A, Field JK. Incorporation of a genetic factor into an epidemiologic model for prediction of individual risk of lung cancer: the Liverpool Lung Project. Cancer Prev Res (Philadelphia, Pa). 2010;3(5):664–9. https://doi.org/10.1158/1940–6207.capr-09-0141.
Sin DD, Tammemagi CM, Lam S, Barnett MJ, Duan X, Tam A, et al. Pro-surfactant protein B as a biomarker for lung cancer prediction. J Clin Oncol. 2013;31(36):4536–43. https://doi.org/10.1200/jco.2013.50.6105.
Spitz MR, Hong WK, Amos CI, Wu X, Schabath MB, Dong Q, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst. 2007;99(9):715–26. https://doi.org/10.1093/jnci/djk153.
Spitz MR, Etzel CJ, Dong Q, Amos CI, Wei Q, Wu X, et al. An expanded risk prediction model for lung cancer. Cancer Prev Res (Philadelphia, Pa). 2008;1(4):250–4. https://doi.org/10.1158/1940–6207.capr-08-0060.
Spitz MR, Amos CI, Land S, Wu X, Dong Q, Wenzlaff AS, et al. Role of selected genetic variants in lung cancer risk in African Americans. J Thorac Oncol. 2013;8(4):391–7. https://doi.org/10.1097/JTO.0b013e318283da29.
Tammemagi MC, Lam SC, McWilliams AM, Sin DD. Incremental value of pulmonary function and sputum DNA image cytometry in lung cancer risk prediction. Cancer Prev Res (Philadelphia, Pa). 2011;4(4):552–61. https://doi.org/10.1158/1940–6207.capr-10-0183.
Tammemagi CM, Pinsky PF, Caporaso NE, Kvale PA, Hocking WG, Church TR, et al. Lung cancer risk prediction: prostate, lung, colorectal and ovarian cancer screening trial models and validation. J Natl Cancer Inst. 2011;103(13):1058–68. https://doi.org/10.1093/jnci/djr173.
Tammemagi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–36. https://doi.org/10.1056/NEJMoa1211776.
Tammemagi MC, Church TR, Hocking WG, Silvestri GA, Kvale PA, Riley TL, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med. 2014;11(12):e1001764. https://doi.org/10.1371/journal.pmed.1001764.
Wang X, Ma K, Cui J, Chen X, Jin L, Li W. An individual risk prediction model for lung cancer based on a study in a Chinese population. Tumori. 2015;101(1):16–23. https://doi.org/10.5301/tj.5000205.
Wilson DO, Weissfeld J. A simple model for predicting lung cancer occurrence in a lung cancer screening program: the Pittsburgh Predictor. Lung Cancer (Amsterdam, Netherlands). 2015;89(1):31–7. https://doi.org/10.1016/j.lungcan.2015.03.021.
Wu X, Wen CP, Ye Y, Tsai M, Wen C, Roth JA, et al. Personalized risk assessment in never, light, and heavy smokers in a prospective cohort in Taiwan. Sci Rep. 2016;6:36482. https://doi.org/10.1038/srep36482.
Young RP, Hopkins RJ, Hay BA, Epton MJ, Mills GD, Black PN, et al. A gene-based risk score for lung cancer susceptibility in smokers and ex-smokers. Postgrad Med J. 2009;85(1008):515–24. https://doi.org/10.1136/pgmj.2008.077107.
Young RP, Hopkins RJ, Hay BA, Epton MJ, Mills GD, Black PN, et al. Lung cancer susceptibility model based on age, family history and genetic variants. PLoS One. 2009;4(4):e5302. https://doi.org/10.1371/journal.pone.0005302.
Cronin KA, Gail MH, Zou Z, Bach PB, Virtamo J, Albanes D. Validation of a model of lung cancer risk prediction among smokers. J Natl Cancer Inst. 2006;98(9):637–40. https://doi.org/10.1093/jnci/djj163.
D’Amelio AM Jr, Cassidy A, Asomaning K, Raji OY, Duffy SW, Field JK, et al. Comparison of discriminatory power and accuracy of three lung cancer risk models. Br J Cancer. 2010;103(3):423–9. https://doi.org/10.1038/sj.bjc.6605759.
Raji OY, Duffy SW, Agbaje OF, Baker SG, Christiani DC, Cassidy A, et al. Predictive accuracy of the Liverpool Lung Project risk model for stratifying patients for computed tomography screening for lung cancer: a case-control and cohort validation study. Ann Intern Med. 2012;157(4):242–50. https://doi.org/10.7326/0003-4819-157-4-201208210-00004.
Li K, Husing A, Sookthai D, Bergmann M, Boeing H, Becker N, et al. Selecting high-risk individuals for lung cancer screening: a prospective evaluation of existing risk models and eligibility criteria in the German EPIC cohort. Cancer Prev Res (Philadelphia, Pa). 2015;8(9):777–85. https://doi.org/10.1158/1940–6207.capr-14-0424.
Ten Haaf K, Jeon J, Tammemagi MC, Han SS, Kong CY, Plevritis SK, et al. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med. 2017;14(4):e1002277. https://doi.org/10.1371/journal.pmed.1002277.
Weber M, Yap S, Goldsbury D, Manners D, Tammemagi M, Marshall H, et al. Identifying high risk individuals for targeted lung cancer screening: independent validation of the PLCOM2012 risk prediction tool. Int J Cancer. 2017; https://doi.org/10.1002/ijc.30673.
Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–e120S. https://doi.org/10.1378/chest.12-2351.
Dong J, Sun N, Li J, Liu Z, Zhang B, Chen Z, et al. Development and validation of clinical diagnostic models for the probability of malignancy in solitary pulmonary nodules. Thorac Cancer. 2014;5(2):162–8. https://doi.org/10.1111/1759-7714.12077.
Deppen SA, Blume JD, Aldrich MC, Fletcher SA, Massion PP, Walker RC, et al. Predicting lung cancer prior to surgical resection in patients with lung nodules. J Thorac Oncol. 2014;9(10):1477–84. https://doi.org/10.1097/jto.0000000000000287.
Gould MK, Ananth L, Barnett PG. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131(2):383–8. https://doi.org/10.1378/chest.06-1261.
Herder GJ, van Tinteren H, Golding RP, Kostense PJ, Comans EF, Smit EF, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest. 2005;128(4):2490–6. https://doi.org/10.1378/chest.128.4.2490.
Jin C, Cao J, Cai Y, Wang L, Liu K, Shen W, et al. A nomogram for predicting the risk of invasive pulmonary adenocarcinoma for patients with solitary peripheral subsolid nodules. J Thorac Cardiovasc Surg. 2017;153(2):462–9.e1. https://doi.org/10.1016/j.jtcvs.2016.10.019.
Li Y, Chen KZ, Wang J. Development and validation of a clinical prediction model to estimate the probability of malignancy in solitary pulmonary nodules in Chinese people. Clin Lung Cancer. 2011;12(5):313–9. https://doi.org/10.1016/j.cllc.2011.06.005.
McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369(10):910–9. https://doi.org/10.1056/NEJMoa1214726.
Mehta HJ, Ravenel JG, Shaftman SR, Tanner NT, Paoletti L, Taylor KK, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464–72. https://doi.org/10.1378/chest.13-0708.
Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849–55.
Yang L, Zhang Q, Bai L, Li T-Y, He C, Ma Q-L, et al. Assessment of the cancer risk factors of solitary pulmonary nodules. Oncotarget. 2017;8(17):29318–27.
Yonemori K, Tateishi U, Uno H, Yonemori Y, Tsuta K, Takeuchi M, et al. Development and validation of diagnostic prediction model for solitary pulmonary nodules. Respirology (Carlton, Vic). 2007;12(6):856–62. https://doi.org/10.1111/j.1440-1843.2007.01158.x.
Zhang M, Zhuo N, Guo Z, Zhang X, Liang W, Zhao S, et al. Establishment of a mathematic model for predicting malignancy in solitary pulmonary nodules. J Thorac Dis. 2015;7(10):1833–41. https://doi.org/10.3978/j.issn.2072-1439.2015.10.56.
Zheng B, Zhou X, Chen J, Zheng W, Duan Q, Chen C. A modified model for preoperatively predicting malignancy of solitary pulmonary nodules: an Asia cohort study. Ann Thorac Surg. 2015;100(1):288–94. https://doi.org/10.1016/j.athoracsur.2015.03.071.
Pinsky PF, Gierada DS, Black W, Munden R, Nath H, Aberle D, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162(7):485–91. https://doi.org/10.7326/M14-2086.
American College of Radiology. Lung-RADS version 1.0 assessment categories Release Date: April 28, 2014. https://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/LungRADS/AssessmentCategories.pdf. Last accessed 09/12/2017.
van Riel SJ, Ciompi F, Jacobs C, Winkler Wille MM, Scholten ET, Naqibullah M, et al. Malignancy risk estimation of screen-detected nodules at baseline CT: comparison of the PanCan model, Lung-RADS and NCCN guidelines. Eur Radiol. 2017; https://doi.org/10.1007/s00330-017-4767-2.
Isbell JM, Deppen S, Putnam JB Jr, Nesbitt JC, Lambright ES, Dawes A, et al. Existing general population models inaccurately predict lung cancer risk in patients referred for surgical evaluation. Ann Thorac Surg. 2011;91(1):227–233; discussion 33. https://doi.org/10.1016/j.athoracsur.2010.08.054.
Melo CB, Perfeito JA, Daud DF, Costa Junior Ada S, Santoro IL, Leao LE. Analysis and validation of probabilistic models for predicting malignancy in solitary pulmonary nodules in a population in Brazil. J Bras Pneumol. 2012;38(5):559–65.
Schultz EM, Sanders GD, Trotter PR, Patz EF Jr, Silvestri GA, Owens DK, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335–41. https://doi.org/10.1136/thx.2007.084731.
Shinohara S, Hanagiri T, Takenaka M, Chikaishi Y, Oka S, Shimokawa H, et al. Evaluation of undiagnosed solitary lung nodules according to the probability of malignancy in the American College of Chest Physicians (ACCP) evidence-based clinical practice guidelines. Radiol Oncol. 2014;48(1):50–5. https://doi.org/10.2478/raon-2013-0064.
Xiao F, Liu D, Guo Y, Shi B, Song Z, Tian Y, et al. Novel and convenient method to evaluate the character of solitary pulmonary nodule-comparison of three mathematical prediction models and further stratification of risk factors. PLoS One. 2013;8(10):e78271. https://doi.org/10.1371/journal.pone.0078271.
White CS, Dharaiya E, Campbell E, Boroczky L. The Vancouver Lung Cancer Risk Prediction Model: assessment by using a subset of the National Lung Screening Trial Cohort. Radiology. 2016;152627 https://doi.org/10.1148/radiol.2016152627.
Winkler Wille MM, van Riel SJ, Saghir Z, Dirksen A, Pedersen JH, Jacobs C, et al. Predictive accuracy of the PanCan lung cancer risk prediction model—external validation based on CT from the Danish Lung Cancer Screening Trial. Eur Radiol. 2015;25(10):3093–9. https://doi.org/10.1007/s00330-015-3689-0.
Zhao H, Marshall HM, Yang IA, Bowman RV, Ayres J, Crossin J, et al. Screen-detected subsolid pulmonary nodules: long-term follow-up and application of the PanCan lung cancer risk prediction model. Br J Radiol. 2016;89(1060):20160016. https://doi.org/10.1259/bjr.20160016.
Al-Ameri A, Malhotra P, Thygesen H, Plant PK, Vaidyanathan S, Karthik S, et al. Risk of malignancy in pulmonary nodules: a validation study of four prediction models. Lung cancer (Amsterdam, Netherlands). 2015;89(1):27–30. https://doi.org/10.1016/j.lungcan.2015.03.018.
Talwar A, Rahman NM, Kadir T, Pickup LC, Gleeson F. A retrospective validation study of three models to estimate the probability of malignancy in patients with small pulmonary nodules from a tertiary oncology follow-up centre. Clin Radiol. 2017;72(2):177.e1–8. https://doi.org/10.1016/j.crad.2016.09.014.
National Cancer Institute, Division of Cancer Epidemiology & Genetics, Lung Cancer Risk Models for Screening (R package: lcrisks). http://dceg.cancer.gov/tools/risk-assessment/lcrisks. Last accessed 09/12/2017.
National Cancer Institute, Division of Cancer Epidemiology & Genetics, R package: lcmodels. http://dceg.cancer.gov/tools/risk-assessment/lcmodels. Last accessed 09/12/2017.
• Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. https://doi.org/10.7326/m14-0697. Presents guidelines for the systematic and transparent reporting of studies designed to develop, validate, or update a prediction model.
Simonson MA, Wills AG, Keller MC, McQueen MB. Recent methods for polygenic analysis of genome-wide data implicate an important effect of common variants on cardiovascular disease risk. BMC Med Genet. 2011;12:146. https://doi.org/10.1186/1471-2350-12-146.
Qian DC, Han Y, Byun J, Shin HR, Hung RJ, McLaughlin JR, et al. A novel pathway-based approach improves lung cancer risk prediction using germline genetic variations. Cancer Epidemiol Biomark Prev. 2016;25(8):1208–15. https://doi.org/10.1158/1055-9965.epi-15-1318.
Khurana R, Wolf R, Berney S, Caldito G, Hayat S, Berney SM. Risk of development of lung cancer is increased in patients with rheumatoid arthritis: a large case control study in US veterans. J Rheumatol. 2008;35(9):1704–8.
Stocks T, Van Hemelrijck M, Manjer J, Bjorge T, Ulmer H, Hallmans G, et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension (Dallas, Tex: 1979). 2012;59(4):802–10. https://doi.org/10.1161/hypertensionaha.111.189258.
Carter BW, Godoy MC, Erasmus JJ. Predicting malignant nodules from screening CTs. J Thorac Oncol. 2016;11(12):2045–7. https://doi.org/10.1016/j.jtho.2016.09.117.
Memorial Sloan Ketting Cancer Center. Lung Cancer Screening Decision Tool. http://nomograms.mskcc.org/Lung/Screening.aspx. Last accessed 09/12/2017.
Lung Cancer CT Screening. http://www.shouldiscreen.com/. Last accessed 09/12/2017.
National Cancer Institute, Division of Cancer Epidemiology & Genetics, Risk-based NLST Outcomes Tool (RNOT). http://analysistools.nci.nih.gov/lungCancerScreening/. Last accessed 09/12/2017.
• NCCN Clinical Practice Guidelines in Oncology—Lung Cancer Screening Version 2.2018. Represents the first clinical practice guidelines for lung cancer screening in the USA to endorse LDCT screening based on model-based predicted lung cancer risk.
Funding
This work was supported in part by a career development award to Dr. Sakoda (K07 CA188142).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Lori C. Sakoda, Louise M. Henderson, Karen J. Wernli, and Hormuzd A. Katki each declare no potential conflicts of interest.
Tanner J. Caverly reports grants from Genentech Corporate Giving Scientific Project Support Program outside the submitted work.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Cancer Epidemiology
Rights and permissions
About this article
Cite this article
Sakoda, L.C., Henderson, L.M., Caverly, T.J. et al. Applying Risk Prediction Models to Optimize Lung Cancer Screening: Current Knowledge, Challenges, and Future Directions. Curr Epidemiol Rep 4, 307–320 (2017). https://doi.org/10.1007/s40471-017-0126-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40471-017-0126-8