Abstract
Purpose of Review
Biofeedback is a promising technique that has been used as a treatment tool for different psychological disorders. In this regard, central (neurofeedback) and peripheral psychophysiological signals are presented as comprehensible stimuli with the aim of training specific processes. This review summarizes recent evidence about its use for the treatment of impulsivity-related processes in addictive disorders.
Recent Findings
Neurofeedback (NFB) protocols, based on electroencephalography (EEG) and functional magnetic resonance imaging (fMRI), have focused on substance use disorders. Biofeedback protocols using peripheral measures have been mainly based on heart rate variability and focused on behavioral addictions. EEG-NFB reported good results in the reduction of hyperarousal, impulsivity and risk taking in alcohol use disorder, and decreased rates of smoking and less craving in nicotine addiction. In fMRI-NFB, effective NFB performance has been related with better clinical outcomes in substance use disorders; however, its implication for treatment is still unclear. Heart rate variability biofeedback results are scarce, but some interventions have been recently designed aimed at treating behavioral addictions.
Summary
In addictive disorders, biofeedback interventions for impulsivity-related processes have shown promising results, although the literature is still scarce. Further research should aim at proving the effectiveness of biofeedback protocols as a treatment option for impulsivity in addictive disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
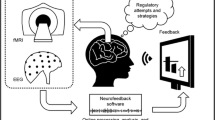
Biofeedback is a technique that provides real-time information of physiological activity and helps to improve self-perception and train specific regulatory strategies. Biofeedback uses sensors to measure central [neurofeedback (NFB)] or peripheral physiological activity and provide real-time feedback to the users in comprehensible stimuli (e.g., on a computer screen), so they can train self-regulation [1]. In other words, individuals can learn to modulate specific mental regulatory processes and thus improve behavioral, cognitive, or emotional self-regulation [2••, 3•, 4]. Biofeedback interventions have already been used to improve the symptomatology of anxiety disorders [5], mood disorders [6], obsessive–compulsive disorder [7], or attention deficit hyperactive disorder [8]. However, in most cases, the results are heterogeneous and the evidence of therapeutic efficacy is limited, considering the existing literature to date [9].
Biofeedback therapeutic approaches can be used to modulate specific central and peripheral responses that are thought to be useful in regulating impulsivity-related behaviors, and so, they are a promising tool for the treatment of addictive disorders [10•]. The most common techniques used in NFB interventions for addictive disorders are electroencephalography (EEG) and functional magnetic resonance imaging (fMRI). However, peripheral psychophysiological responses such as heart rate (HR) or heart rate variability (HRV) reflect the self-regulatory capacity and, therefore, have also been employed as a biomarker of top-down self-regulation [11]. Indeed, the higher an individual’s HRV, the better the performance in tasks requiring response inhibition [12].
Impulsivity is an important component of human behavior that has been broadly defined as a predisposition to act without premeditation or consideration of immediate or future consequences [13]. However, when there is a persistent impulsive pattern of performance extending into adulthood, impulsivity may trigger the development of several psychiatric disorders [14]. Because of its importance in research and clinical fields, the concept of impulsivity has been investigated from various perspectives and several definitions and measures have been proposed [15].
From a theoretical point of view, the impulsivity construct has been divided into three major domains: choice impulsivity, response impulsivity, and impulsive personality traits. Firstly, impulsive choices reflect a poor evaluation of future consequences and the inability to resist immediate rewards [16, 17]. Secondly, response impulsivity (or impulsive action) is the capacity to inhibit a prepotent motor response [18]. Finally, it has been proposed that trait impulsivity has multiple components. For instance, Lynam and colleagues identified five components (positive urgency, negative urgency, lack of premeditation, lack of perseverance, and sensation seeking), which are measured with a self-report inventory, the UPPS-P Impulsive Behavior Scale [19]. Similarly, Patton and colleagues proposed the Barratt Impulsivity Scale (BIS-11) [20] which fractionates impulsiveness into cognition, motor, and planning components. Together, these findings suggest impulsivity is a complex, multidimensional component of human behavior [13].
This narrative review aimed to compile recent literature on biofeedback interventions involving central and peripheral measures for impulsivity-related processes in addictive disorders, both substance use disorders (SUD) and behavioral addictions.
EEG Neurofeedback
Using electroencephalography neurofeedback (EEG-NFB), individuals can learn to modulate their own brain waves to improve behavioral, cognitive, and emotional self-regulation [10•]. Three different models have been proposed to explain how the regulation of EEG activity might be learned [21]. The first model is operant conditioning, which declares that the occurrence of a positively reinforced behavior will increase. Therefore, in NFB studies, correct or desired brain responses are positively reinforced by obtaining reward points, a smiling face, auditory feedback, etc. Secondly, it has also been proposed that self-regulation of EEG parameters is comparable to motor learning, such as riding a bicycle. The third model of how to regulate one’s own brain activity is the dual process theory, which describes learning as an interaction of feed-forward and feed-back processes [22].
In short, brain waves are patterns of electrical activity that can be recognized by their amplitudes (the power of the waves measured in microvolts) and frequencies (how fast the waves oscillate measured by the number of waves per second). Based on their frequency, brain waves are categorized into delta (less than 4 Hz), which are observed in the EEG signal when a person is asleep; theta (4–8 Hz), observed when the person is sleepy; alpha (8–13 Hz), usually seen when the person is awake but relaxed; beta (13–30 Hz), present when individuals are alert; and gamma (30–100 Hz), typically observed when a person is trying to solve a problem. Moreover, these frequency components have subsets, such as the sensorimotor rhythm (SMR) frequency bands (12–15 Hz), which are categorized by low beta waves, observed when the person is alert but physically relaxed [23].
That being said, in the past few decades, several EEG-NFB protocols targeting different brain signals have been developed, which can be divided into different categories [1]. The most commonly used training is the frequency training, which aims to change the power ratio of the EEG frequency bands [24]. Among the most well-studied and used frequency training protocols, are the EEG alpha/theta ratio training and the enhancement of the SMR frequency (12–15 Hz) [25]. Secondly, there is the coherence training, which focuses on the coherence or the degree of correlation between two or more brain regions. Specifically, this protocol aims to change the connectivity patterns among brain areas [26]. Distorted connectivity has been shown in various neurologic disorders compared to healthy controls [27]. Finally, several developments in low-frequency NFB have been introduced recently. Although they have their origins in traditional higher-frequency training, infra-low-frequency training and slow cortical potentials NFB in the range of 0.0001–0.01 Hz are two slightly different methodologies in a similar spectral regime [28].
Currently, EEG-NFB remains the primary and most often used NFB approach, mainly because they are low-cost, non-invasive, and easy-to-use methods. However, the majority of findings in SUD are mixed and are limited by the small number of studies [3•, 29•]. Whereas some studies have reported that EEG-NFB can improve clinical outcomes and prolong treatment retention, other authors claim no significant effect of this intervention on addiction [29•]. However, in spite of the heterogeneous findings, EEG-NFB is now considered a promising tool for modulating brain activity related to cognitive and emotional impairment in those patients [3•]. Indeed, since 2002, EEG-NFB has been listed as a probable efficacious treatment for SUD in the guidelines for the evaluation of the clinical efficacy of psychophysiological interventions [30].
In alcohol use disorder (AUD), the majority of the studies using EEG-NFB adopted the Peniston and Kulkosky [31] protocol, which consists of 15–30-minute sessions of an alpha-theta brainwave training program [2••]. In this pioneering study, the authors compared a control group without alcohol use disorder (AUD), a traditionally treated group of people with AUD, and a group of people with AUD receiving brainwave training (BWT) and observed that the latter showed significant increases in percentages of EEG record in alpha and theta rhythms and increased alpha rhythm amplitudes. Moreover, the authors found that, after training, patients with AUD showed a sharp reduction in depressive symptoms. Follow-up data also indicated sustained prevention of relapse in those who received the training [31]. Recently, Lackner and colleagues [32] also used the Peniston and Kulkosky [31] protocol to analyze the efficacy of NFB with a new visual paradigm in a cohort of patients with AUD treated in an Austrian therapeutic community center. To measure training effects, participants were randomly allocated to 2 groups: an experimental group and a control group. After 12 sessions of visual NFB training, patients with AUD improved control of their brain activity, suggesting the effectiveness of visual short-term NFB. Although many studies have used the alpha-theta protocol, this EEG brainwave program has not escaped criticism and some questions have been raised regarding the methodology used in the Peniston studies [33]. A few years later, this protocol was expanded by Scott and colleagues [34] who combined the alpha-theta training with beta (16–21 Hz) or SMR (12–15 Hz) training. The aim of this study was to assess whether an EEG-NFB protocol could improve outcome measures for the inpatient population with mixed SUD. To do so, the patients were randomly assigned to the EEG-NFB or control group. Overall, results showed that the combined program aims to improve response inhibition and is supposed to be more suitable for patients with an addiction to mixed substances. Ko and Park [35] assessed whether EEG-NFB may help to normalize abnormally high beta and low alpha waves in patients with AUD, by dividing them into two groups: an experimental group with NFB training and a control group that did not receive NFB training. These two groups showed no significant difference in terms of lowering brain hyperarousal. On the other hand, the hyperarousal state was maintained in the experimental group but worsened in the control group. Finally, the experimental group showed a significant increase in alcohol abstinence self-efficacy and self-regulation. Bonfiglio and colleagues [36] evaluated the use of EEG-NFB training for risk taking that is strongly related to impulsivity in a sample that included patients with AUD and patients addicted to cocaine. The training involved the use of a brain computer interface that allows the user to monitor concentration and relaxation levels. Then, a subject can be trained to voluntarily produce a concentration condition. The comparison between the experimental and control groups showed significant efficacy in reducing risk taking in subjects with SUD.
The first attempts to use EEG-NFB interventions on smoking cessation date back to the eighties and used occipital alpha (8–12 Hz) modulation protocols [37, 38]. Despite both studies reporting promising results, they had very low power of evidence and cannot provide enough scientific validation of the effectiveness of this type of treatment [39•]. A recent study [40] attempted to address these shortcomings. Patients with nicotine addiction were randomized into an experimental arm, performing real NFB, and a control arm, performing sham NFB (yoked arm). Overall, the authors reported decreased rates of smoking in the participants that were randomized to perform the real NFB in comparison to participants in the yoked NFB. Moreover, individuals in the real feedback group showed a significant reduction in cigarette cravings and a successful deactivation of the altered EEG pattern. Finally, it was observed that those participants who were more successful in the task also showed a higher decrease in craving scores. In a more recent study by the same group, the authors used the novel cognition-guided NFB brain–computer interface paradigm based on a cue-reactivity model to resolve the shortcomings of traditional NFB [41]. It has been reported that cue-reactivity leads to impulsive behavior in drug-seeking behavior as well as relapse; however, cue-reactivity has multiple EEG features including both time (e.g., P300, slow positive wave) and frequency-domain (e.g., alpha oscillation). The authors claim that, compared with the single signal EEG, this novel NFB process, which involves both an offline classifier construction and real-time NFB training, can better enhance sensitivity [41]. The participants were people with nicotine dependence. Overall, the authors observed that during NFB training, the participants’ smoking cue-reactivity patterns were significantly reduced. The authors also suggested that this novel protocol should be used with other NFB systems for the development of other brain-computer interface algorithms and paradigms for addiction.
The aforementioned protocol combining SMR training with theta-alpha protocol has also been used to treat methamphetamine [42] or opioid [43] addiction. In both studies, patients were randomly assigned to either an experimental group that received NFB training in addition to their usual medication, or to a control group that received only their usual medication. Although both studies found decreased severity of addiction and reductions in craving compared to treatment as usual (in that case, pharmacotherapy), neither of them included a follow-up assessment. Recently, Faridi and colleagues [44] investigated the effectiveness of EEG-NFB in individuals with opioid addiction. Specifically, the authors aimed to compare the effectiveness of LORETA (Low-Resolution Brain Electromagnetic Tomography) Z Score NFB and cognitive rehabilitation therapy with Methadone Maintenance Treatment (MMT) in reducing cravings in patients with opioid addiction. To do so, patients were randomly divided into three groups: a LORETA Z score NFB group, a cognitive rehabilitation intervention group or a control group. Indeed, results obtained do support the effectiveness of LORETA Z Score NFB and cognitive rehabilitation in reducing opioid craving, improving attentional bias toward craving cues, and the quality of life among patients. Finally, EEG-NFB intervention for cocaine addiction has also been investigated [45]. In patients with cocaine dependence, a combination of NFB and motivation treatment lowered EEG reactivity to drug-related images. Interestingly, in a very recent work by Corominas-Roso and colleagues [10•], the authors conducted a single-blind sham-controlled NFB protocol on individuals in prison with a past cocaine and heroin addiction diagnosis (now abstinent). They investigated the benefits of infra-low-frequency EEG-NFB on the modulation of impulsivity, which was assessed with the BIS-11 and the commission-errors of the continuous performance test [46]. After the EEG-NFB training, clinical symptoms, such as depressive symptoms, anxiety, impulsivity, and attention deficits, had improved and the benefits were higher than in the control group. The authors suggested that infra-low-frequency EEG-NFB was better than placebo in the modulation of impulsivity [10•].
fMRI Neurofeedback
In 1995, Cox and colleagues [47] reported the first experiment using real-time fMRI. The possibility of providing hemodynamic changes in the brain in real time opened a new framework for designing interventions using this technique [8]. fMRI-NFB transforms the brain activity of one specific brain region into comprehensible stimuli that act as feedback. It requires a complex setup, and neural data have to be processed online; however, fMRI-NFB has high spatial resolution and can provide direct information about the activity of subcortical structures (e.g., reward circuit), which is useful for addictive-related processes, such as craving [48•].
Scientific evidence about fMRI-NFB for the treatment of AUD is scarce [2••]. In a study with patients with AUD, the objective was to downregulate the salience response associated with alcohol-related stimuli and upregulate the salience in front of positive goals by mental imagery [49]. Regions of interest (ROIs) were defined for each participant, considering the brain clusters that were more activated in front of alcohol or goal-related pictures than when viewing neutral stimuli. The NFB was displayed by the size of the image. Results showed no differences in clinical outcomes between the group that received NFB in addition to the standard treatment for AUD and the group that only received the standard treatment. Regarding NFB performance, most patients in the NFB group had decreased activation in front of alcohol-related stimuli without significantly increased activation in front of positive goals. Besides, there was an association between task performance and less alcohol consumption and lower craving within the NFB group. Increased craving in front of alcohol cues is associated with impaired response inhibition, a characteristic feature of impulsivity [50]. Furthermore, Karch and colleagues [51•] reported the results of a double-blind fMRI-NFB design in patients diagnosed with AUD. The objective of the NFB task was to modulate the neural activity associated with craving in front of alcohol-related cues; thus, the selected ROIs were the anterior cingulate cortex or insula, associated with craving [52], or the dorsolateral prefrontal cortex, associated with cognitive control [53, 54]. The NFB was presented by a graphical thermometer of BOLD activity. The active group showed more decreased activity in the ACC, claustrum, and caudate nucleus than the sham group, suggesting effective top-down modulation of the automatic response to high salient stimuli during NFB. Clinical outcomes were similar for both groups; however, within the active group, those that did not relapse at three months showed more of a decrease in neural activity. Moreover, new protocols can also be found in the literature, although experimental data have not yet been published. Weiss and colleagues [55] proposed a randomized control trial in patients with AUD to evaluate if mindfulness training could improve the efficacy of fMRI-NFB for controlling the cue-reactivity to alcohol-related stimuli in the ventral striatum. Also, Gerchen and colleagues [56] presented a randomized control trial with a single-blind design in patients with AUD, based on the increased reward system activity and the diminished cognitive control in front of alcohol-related stimuli, which are characteristic features of people with AUD.
Most fMRI-NFB studies in persons with nicotine addiction were aimed at modulating the craving response [39•]. Craving is a key factor for nicotine consumption and relapse [57], and it is related to the impulsivity dimension of urgency [58]. To our knowledge, three studies reported data on fMRI-NFB in persons with nicotine addiction [51•, 59, 60]. Karch and colleagues [51•] aimed to assess if, in those patients who remained abstinent after three months, NFB performance was different from the ones who had relapsed. They analyzed the data of an NFB group and a sham group. The paradigm was formed by blocks where they were exposed to either tobacco-related pictures or neutral pictures while receiving NFB via a graphical thermometer. The real NFB group received the signal from brain areas associated with craving (anterior cingulate cortex, insula, or dorsolateral prefrontal cortex), whereas the sham group received the NFB of other areas such as the parietal cortex. After three months, the relapse rates showed no difference between real NFB and sham conditions, but within the real NFB group, the ability to reduce the neural response during the first session was a good predictor of treatment success. Moreover, these results are supported by another study that analyzed the data from the same group of patients to evaluate the correlation between the activity of the default mode network and smoking cessation [59]. This study reported no differences between the real NFB and sham conditions in terms of smoking relapses. However, it showed that a broader default mode network connectivity and a lower negative coupling with the salience network were associated with higher relapse rates in patients that underwent a smoking cessation program that included an fMRI-NFB intervention. These patterns would be associated with the cognitive end-emotional control of withdrawal in people with nicotine dependence. Another study by Rana and colleagues [60] aimed at reducing craving by the downregulation of the bilateral insula response in front of tobacco-related cues. Four participants were selected by their scores in nicotine dependence and number of cigarette consumption. They achieved to downregulate insula activity with NFB in the presence of tobacco-related pictures and a reduction in craving and cigarette consumption that continued at the 6-month follow-up. The insula plays a central role in craving processes, and its downregulation may have helped to reduce the salience of tobacco-related stimuli. However, this study had several limitations, as the sample was small and also lacked a control group or sham condition to compare.
To date, only one study reported results of an fMRI-NFB paradigm in patients with cocaine addiction. Addiction to cocaine is associated with an enhanced reward response toward drug-related stimuli, a diminished response toward non-drug-related potential rewards and impaired executive control [61,62,63], leading to elevated impulsive drug use. Kirschner and colleagues [64] aimed to upregulate the reward response to non-drug-related stimuli in a mental imagery paradigm with fMRI-NFB. The areas selected for the NFB training were the substantia nigra and the ventral tegmental area of the midbrain, characterized by their dopaminergic neurons [65]. Both patients and healthy controls were able to self-regulate reward circuity areas, but this ability was hampered in those patients with more obsessive–compulsive thoughts and more cocaine consumption. However, the impulsivity traits assessed with the BIS-11 [20] were not associated with brain activity during the task, and the ability to self-regulate reward response did not improve after the NFB training.
HRV Biofeedback
HRV, defined by the variations of intervals between heartbeats, is associated with the regulatory function of the autonomous nervous system according to environmental demands [66]. In addictive disorders, the lower HRV described in SUD has allowed researchers to hypothesize that the reward circuit modulates the autonomous nervous system [67•]. However, there are fewer studies evaluating this biofeedback technique to regulate impulsive tendencies in SUD than in behavioral addictions.
In gambling disorder (GD), biofeedback techniques are usually integrated into gamification, using video games as complementary tools for mental health treatments (i.e., serious games). Relaxation techniques (e.g., breathing training) are incorporated and enhanced by the management of physiological variables (e.g., HRV) to handle stressful situations and achieve better emotion regulation. The biofeedback sensors connect emotional reactions to the media display. The patient learns how to better manage their emotional reactions by interacting with the application, being visually rewarded when they do the training properly and consolidating their learning via breathing techniques. An increase in HRV would translate to better well-being and emotional control, being able to better regulate negative affect, having more flexible emotional responses, and using more adaptive emotional regulatory strategies. Furthermore, a reduction in impulsive behaviors and arousal as well as the enhancement of self-control have been reported [68, 69]. The relationship between emotion regulation and impulsivity traits has been previously described [70, 71], and whether changes in impulsivity levels could derive from greater emotional control associated with biofeedback techniques should be further studied.
One recent intervention for GD using HRV biofeedback is the serious game e-Estesia, a complementary tool for traditional cognitive behavioral therapy (CBT) intervention designed to reduce arousal, improve emotion regulation, and increase well-being [72,73,74]. e-Estesia is displayed on a tablet connected to a thoracic biosensor registering HR and HRV data and providing biofeedback in a gamified environment. Interestingly, the usability of e-Estesia has been supported by patients diagnosed with GD [72]. Patients diagnosed with GD enrolled in the e-Estesia intervention had significantly less relapses and better indicators of treatment compliance than patients only treated with CBT. No significant reductions in self-report impulsivity levels (measured by the UPPS-P Impulsive Behavior Scale [19]) nor in emotion dysregulation were achieved after using e-Estesia [73]. However, in the group treated with the serious game, a reduction in emotional reappraisal (considered as a maladaptive emotional coping strategy) was observed [73]. These studies mentioned a precursor serious game with biofeedback sensors called Playmancer, applied to patients with GD, which also trained users in relaxation, self-awareness, and self-regulation [69, 75]. In the context of e-Estesia, a study regarding biofeedback interventions in other impulsivity-related behaviors has been published based on a patient with Parkinson’s Disease and hypersexuality [74]. He was treated with 20 sessions of CBT, followed by 15 sessions of e-Estesia. After the combined treatment, the patient scored lower in emotion dysregulation as well as in the lack of perseverance, positive urgency and negative urgency scales of the UPPS-P [19]. Likewise, the frequency and severity of the relapses decreased. However, the clinical complexity of this case limited the possibility of attributing the improvement to the intervention with the serious game [74]. Furthermore, Giordano and colleagues [76] described another serious game treatment protocol (Alter Game) aimed at preventing gambling relapse, which employed biofeedback techniques measuring HR, HRV, skin conductance, and temperature to assess psychophysiological arousal in the face of gambling cues. In this vein, psychophysiological arousal is considered as an indirect measure of craving which, in turn, has been related to impulsivity in GD [77, 78]. The authors suggested a pre-post experimental design in adults with GD, comparing a group treated with traditional CBT versus a group treated with CBT plus Alter Game, with a one-month follow-up. Among the psychometric assessments, they proposed to evaluate impulsivity with the BIS-11 [20]. Although promising, no results have been reported so far.
Studies related to biofeedback and gaming have been mostly performed in the adolescent population. Poskotinova and colleagues [79] evaluated the effectiveness of short-term HVR biofeedback training to increase the total power of the HRV spectrum in 20 adolescents between 15 and 16 years old. They distinguished between adolescents at minimal risk or at significant risk of internet addiction. While the total power increased pre-post training in the first group, there were no significant differences in the second group. Moreover, the total power after the training was significantly higher in the first group, and a negative correlation was observed between the risk of internet addiction and total power in the total sample. The authors concluded that a significant risk of internet addiction developing in puberty may be associated with a decreased autonomous nervous system reactivity during the training. Then, the risk of developing internet addiction in adolescents may be accompanied by a decrease in the ability to self-regulate. Besides, withdrawal symptoms associated with excessive internet use had the greatest influence in reducing the HRV biofeedback efficiency during short-term training. Similarly, Demin and Poskotinova [80] compared three groups of adolescents between 16 and 17 years old at minimal risk of internet addiction, at high risk of internet addiction, and those with a diagnosis of internet addiction. Apart from HRV, they added EEG measures at baseline and during the short-term HRV biofeedback training period to identify the most sensitive brain regions to HRV biofeedback. In all groups, HRV biofeedback training increased HRV. While an increase in EEG activity during HRV biofeedback might suggest a higher emotional control, the group with internet addiction showed the least reactivity of emotiogenic areas during the training. A recent systematic review and meta-analysis included 12 studies regarding HRV at baseline and after stimuli exposition in individuals with problematic use of the internet [67•]. The authors reported that these individuals had significant differences in parasympathetic activity in resting state compared with healthy subjects, those with problematic internet use having lower baseline HRV. However, there were no significant intergroup differences regarding HRV reactivity. Some of the limitations described were related to the heterogeneity between studies in the types of population (e.g., people with problematic use of the internet and people with internet addiction), as well as in the measures of physiological variables (e.g., HRV indices), or the lack of adjustment for potential confounders that affect these physiological variables, among others. It is worth mentioning that works exploring biofeedback and gaming referred to physiological variables that could be related to impulsivity. In contrast to the aforementioned studies in GD, they did not include a direct evaluation of impulsivity, such as specific psychometric assessments.
Furthermore, one recent study reported a biofeedback protocol aimed at reducing craving and negative affect in patients with SUD by the use of an app for breath pace training [81]. This technique aims at optimizing HRV by synchronizing breath with heart rhythm. Participants were women with SUD who were receiving outpatient addiction treatment. They were assigned to either the group who did cardiovascular resonance breathing (6 breaths per minute) or sham (14 breaths per minute). In participants who used the app frequently, those who were assigned to the sham condition experienced the typical elevated levels of craving during the intervention, but those who did cardiovascular resonance breathing did not experience these increases. These results supported the use of cardiovascular breath training as a method for reducing elevated craving episodes during outpatient treatment.
Combined EEG-Skin Temperature Biofeedback
Besides the interventions with only central or peripheral measures of biofeedback, the combination of both measures could also be an interesting approach to benefit from the features of each method. In that sense, Pandria and colleagues carried out an intervention with combined peripheral biofeedback from skin temperature and EEG-NFB for smoking cessation in people with nicotine addiction [82]. Both biofeedback interventions were performed separately and showed changes across sessions. The intervention yielded positive results in both objective and self-reported measures of smoking, but with the limitation of no control or sham groups.
Conclusions
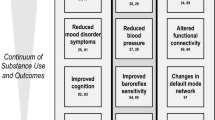
This review compiles the most recent evidence about biofeedback paradigms as complementary interventions aimed at reducing impulsivity-related features of addictive disorders. In the last years, NFB protocols have been aimed at SUD, mainly alcohol and nicotine addiction, particularly craving and the stimuli salience. EEG-NFB has been related to better clinical outcomes in AUD, as well as a reduction of hyperarousal and an effective reduction of impulsivity and risk taking. In nicotine addiction, recent evidence about EEG-NFB reports decreased rates of smoking, decreased cue-reactivity toward smoke-related stimuli and less craving, with more reduction associated with better NFB performance. Also, for other drugs, EEG-NFB have been associated with an improved attentional bias toward craving cues as well as improvements in clinical symptomatology, including impulsivity and attention deficits. According to fMRI-NFB studies in AUD, patients who achieve more effective downregulation in front of alcohol-related stimuli tend to present better treatment outcomes, although the clinical improvement due to fMRI-NFB training is still not adequately demonstrated. In nicotine addiction, results support the idea of NFB performance as a predictor of treatment outcomes, as those patients who have better performance from the initial sessions and thus, better cognitive control over craving, would present better treatment outcomes. However, available studies do not provide evidence about the possible beneficial effect of fMRI-NFB training for the treatment of this disorder. In cocaine addiction, impaired reward modulation using fMRI-NFB is related to more severe symptomatology; however, its transference effect toward treatment is still not confirmed. Besides, most recent biofeedback protocols using peripheral measures are based on HRV and have been mainly focused on behavioral addictions, such as GD or gaming disorder. Studies exploring biofeedback to modulate impulsive tendencies in behavioral addictions are scarce and mostly based on measures of physiological variables such as HRV. Further research regarding biofeedback techniques different from HRV is needed, such as interventions specifically focused on impulsivity and studies with a bigger sample size and longer follow-up periods.
In summary, biofeedback interventions for impulsivity-related processes in addictive disorders have shown promising results; however, the literature is still scarce, and some findings are limited by methodological issues like a short follow-up period or lacking a comparison group. Future designs should include a control and/or sham group to validate the effectiveness of these interventions and evaluate the impact of biofeedback interventions over the long term.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Orndorff-Plunkett F, Singh F, Aragón O, Pineda J. Assessing the effectiveness of neurofeedback training in the context of clinical and social neuroscience. Brain Sci. 2017;7:95.
•• Dave F, Tripathi R. The efficacy of neurofeedback for alcohol use disorders – a systematic review. World J Biol Psychiatry. 2022;1–12. A recent review that offers a broad view of the effectiveness of non-pharmacological neurofeedback (NF) interventions in patients with alcohol use disorders (AUDs). Although more research is required, the authors conclude that neuromodulation approaches are promising for the treatment of AUD.
• Dousset CC, Kajosch H, Ingels AA, Schroder E, Kornreich C, Campanella S, et al. Preventing relapse in alcohol disorder with EEG-neurofeedback as a neuromodulation technique: a review and new insights regarding its application. Addict Behav England. 2020;106:106391. An important review of the application of EEG-NF protocols for AUD. It supports the application of EEG-NF as an add-on tool in order to enhance the cognitive abilities required to maintain abstinence, especially inhibition and attentional skills.
Ferreri F, Bourla A, Mouchabac S, Karila L. e-Addictology: an overview of new technologies for assessing and intervening in addictive behaviors. Front Psych. 2018;9:51.
Tolin DF, Davies CD, Moskow DM, Hofmann SG. Biofeedback and neurofeedback for anxiety disorders: a quantitative and qualitative systematic review. Adv Exp Med Biol. 2020;1305:265–289.
Patil AU, Lin C, Lee S-H, Huang H-W, Wu S-C, Madathil D, et al. Review of EEG-based neurofeedback as a therapeutic intervention to treat depression. Psychiatry Res Neuroimaging Elsevier BV. 2023;329:111591.
Ferreira S, Pêgo JM, Morgado P. The efficacy of biofeedback approaches for obsessive-compulsive and related disorders: a systematic review and meta-analysis. Psychiatry Res Elsevier Ireland Ltd. 2019;272:237–45.
Taschereau-Dumouchel V, Cushing CA, Lau H. Real-time functional MRI in the treatment of mental health disorders. Annu Rev Clin Psychol. 2022;18:125–54.
Schoenberg PLA, David AS. Biofeedback for psychiatric disorders: a systematic review. Appl Psychophysiol Biofeedback. 2014;39:109–35.
• Corominas-Roso M, Ibern I, Capdevila M, Ramon R, Roncero C, Ramos-Quiroga JA. Benefits of EEG-neurofeedback on the modulation of impulsivity in a sample of cocaine and heroin long-term abstinent inmates: a pilot study. Int J Offender Ther Comp Criminol. 2020;64:1275–98. A very recent study using a single-blind sham-controlled NFB protocol to assess the effects of NFB on impulsivity in 10 cocaine and 10 heroin long-term abstinent addicts. Despite limitations, results suggest that NFB is better than placebo in improving impulsivity and clinical symptoms of anxiety and depression in patients.
Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev. 2017;74:233–55.
Schumann A, Köhler S, Brotte L, Bär K-J. Effect of an eight-week smartphone-guided HRV-biofeedback intervention on autonomic function and impulsivity in healthy controls. Physiol Meas. 2019;40:064001.
Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75:63–75.
Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014;1327:1–26.
MacKillop J, Weafer J, C Gray J, Oshri A, Palmer A, de Wit H. The latent structure of impulsivity: impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacology (Berl). 2016;233:3361–70.
Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–92.
Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, Murphy JG. The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol. 2014;10:641–77.
Fillmore MT, Weafer J. Behavioral inhibition and addiction. In Mackillop J, de Wit H, editors. The Wiley‐Blackwell Handbook of Addiction Psychopharmacology. Oxford, UK: Wiley-Blackwell; 2013; 135–64.
Lynam DR, Smith GT, Whiteside SP, Cyders MA. The UPPS-P: assessing five personality pathways to impulsive behavior. West Lafayette: Purdue Univ; 2006. p. 10.
Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–74.
Hammer EM, Halder S, Blankertz B, Sannelli C, Dickhaus T, Kleih S, et al. Psychological predictors of SMR-BCI performance. Biol Psychol. 2012;89:80–6.
Kober SE, Witte M, Ninaus M, Neuper C, Wood G. Learning to modulate one’s own brain activity: the effect of spontaneous mental strategies. Front Hum Neurosci. 2013;7:695.
Marzbani H, Marateb H, Mansourian M. Methodological note: neurofeedback: a comprehensive review on system design, methodology and clinical applications. Basic Clin Neurosci J. 2016;7:143–158.
Noachtar S, Binnie C, Ebersole J, Mauguière F, Sakamoto A, Westmoreland B. A glossary of terms most commonly used by clinical electroencephalographers and proposal for the report form for the EEG findings. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:21–41.
Weber E, Köberl A, Frank S, Doppelmayr M. Predicting successful learning of SMR neurofeedback in healthy participants: methodological considerations. Appl Psychophysiol Biofeedback. 2011;36:37–45.
Bowyer SM. Coherence a measure of the brain networks: past and present. Neuropsychiatr Electrophysiol. 2016;2:1.
Omejc N, Rojc B, Battaglini PP, Marusic U. Review of the therapeutic neurofeedback method using electroencephalography: EEG Neurofeedback. Bosn J Basic Med Sci. 2019;19:213–220.
Bazzana F, Finzi S, Di Fini G, Veglia F. Infra-low frequency neurofeedback: a systematic mixed studies review. Front Hum Neurosci. 2022;16:920659.
• Gabrielsen KB, Clausen T, Haugland SH, Hollup SA, Vederhus J-K. Infralow neurofeedback in the treatment of substance use disorders: a randomized controlled trial. J Psychiatry \ Neurosci. 2022;47:E222-9. The article applies a recently developed subtype of traditional, frequency-based neurofeedback that targets cerebral rhythmic activity below 0.5 Hz. As there is still a research gap regarding the effectivity of IFL-NF, the authors conducted a randomized trial and showed that ILF-NF offered limited additional benefit when combined with treatment-as-usual.
La Vaque T, Hammond DC. Template for developing guidelines for the evaluation of the clinical efficacy of psychophysiological interventions. Appl Psychophysiol Biofeedback Germany. 2002;27:273–81.
Peniston EG, Kulkosky PJ. alpha-theta Brainwave Training and beta-Endorphin Levels in Alcoholics. Alcohol Clin Exp Res. 1989;13:271–9.
Lackner N, Unterrainer HF, Skliris D, Wood G, Wallner-Liebmann SJ, Neuper C, et al. The effectiveness of visual short-time neurofeedback on brain activity and clinical characteristics in alcohol use disorders. Clin EEG Neurosci. 2016;47:188–95.
Trudeau DL. The treatment of addictive disorders by brain wave biofeedback: a review and suggestions for future research. Clin Electroencephalogr. 2000;31:13–22.
Scott WC, Kaiser D, Othmer S, Sideroff SI. Effects of an EEG biofeedback protocol on a mixed substance abusing population. Am J Drug Alcohol Abuse. 2005;31:455–69.
Ko S, Park W. Effects of quantitative electroencephalography based neurofeedback training on autonomous regulations in patients with alcohol use disorder. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:136–44.
Bonfiglio NS, Parodi D, Rollo D, Renati R, Pessa E, Penna MP. Use of training with BCI (brain computer interface) in the management of impulsivity. 2020 IEEE Int Symp Med Meas Appl. 2020. p. 1–5.
Griffith EE, Crossman E. Biofeedback: a possible substitute for smoking, experiment I. Addict Behav. 1983;8:277–85.
Szalai JP, Allon R, Doyle J, Zamel N. EEG alpha reactivity and self-regulation correlates of smoking and smoking deprivation. Psychosom Med. 1986;48:67–72.
• Pandria N, Athanasiou A, Konstantara L, Karagianni M, Bamidis PD. Advances in biofeedback and neurofeedback studies on smoking. Neuroimage-Clin Elsevier. 2020;28:102397. Review of studies about biofeedback and neurofeedback training on smokers.
Bu J, Young KD, Hong W, Ma R, Song H, Wang Y, et al. Effect of deactivation of activity patterns related to smoking cue reactivity on nicotine addiction. Brain. 2019;142:1827–41.
Bu J, Liu C, Gou H, Gan H, Cheng Y, Liu M, et al. A novel cognition-guided neurofeedback BCI dataset on nicotine addiction. Front Neurosci. 2021;15:647844.
Rostami R, Dehghani-Arani F. Neurofeedback training as a new method in treatment of crystal methamphetamine dependent patients: a preliminary study. Appl Psychophysiol Biofeedback. 2015;40:151–61.
Dehghani-Arani F, Rostami R, Nadali H. Neurofeedback training for opiate addiction: improvement of mental health and craving. Appl Psychophysiol Biofeedback. 2013;38:133–41.
Faridi A, Taremian F, Thatcher RW, Dadashi M, Moloodi R. Comparison of LORETA Z score neurofeedback and cognitive rehabilitation in terms of their effectiveness in reducing craving in opioid addicts. Basic Clin Neurosci J. 2022;13:81–96.
Horrell T, El-Baz A, Baruth J, Tasman A, Sokhadze G, Stewart C, et al. Neurofeedback effects on evoked and induced EEG gamma band reactivity to drug-related cues in cocaine addiction. J Neurother. 2010;14:195–216.
Conners CK. Conners’ continuous performance test (CPTII) [The technical guide and software manual]. Multi Heal Syst. 2002.
Cox RW, Jesmanowicz A, Hyde JS. Real-time functional magnetic resonance imaging. Magn Reson Med. 1995;33:230–6.
• Martz ME, Hart T, Heitzeg MM, Peltier SJ. Neuromodulation of brain activation associated with addiction: a review of real-time fMRI neurofeedback studies. NeuroImage Clin Elsevier. 2020;27:102350. Review of real-time fMRI studies associated with addictive processes, including studies that used neurofeedback interventions to reduce craving in individuals with substance use.
Subramanian L, Skottnik L, Cox WM, Luhrs M, McNamara R, Hood K, et al. Neurofeedback training versus treatment-as-usual for alcohol dependence: results of an early-phase randomized controlled trial and neuroimaging correlates. Eur Addict Res. 2021;27:381–94.
Papachristou H, Nederkoorn C, Havermans R, Van Der Horst M, Jansen A. Can’t stop the craving: the effect of impulsivity on cue-elicited craving for alcohol in heavy and light social drinkers. Psychopharmacology. 2012;219:511–8.
• Karch S, Krause D, Lehnert K, Konrad J, Haller D, Rauchmann B-S, et al. Functional and clinical outcomes of FMRI-based neurofeedback training in patients with alcohol dependence: a pilot study. Eur Arch Psychiatry Clin Neurosci. 2022;272:557–69. Double-blind, placebo-controlled real-time fMRI study with follow-up that provided evidence of modulation of addiction-related brain activity in individuals with alcohol dependence.
Verdejo-García A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56:48–62.
Jansen JM, Daams JG, Koeter MWJ, Veltman DJ, van den Brink W, Goudriaan AE. Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev. 2013;37:2472–80.
Almeida-Antunes N, Vasconcelos M, Crego A, Rodrigues R, Sampaio A, López-Caneda E. Forgetting alcohol: a double-blind, randomized controlled trial investigating memory inhibition training in young binge drinkers. Front Neurosci. 2022;16:1–13.
Weiss F, Aslan A, Zhang J, Gerchen MF, Kiefer F, Kirsch P. Using mind control to modify cue-reactivity in AUD: the impact of mindfulness-based relapse prevention on real-time fMRI neurofeedback to modify cue-reactivity in alcohol use disorder: a randomized controlled trial. BMC Psychiatry. 2020;20:309.
Gerchen MF, Rentsch A, Kirsch M, Kiefer F, Kirsch P. Shifts in the functional topography of frontal cortex-striatum connectivity in alcohol use disorder. Addict Biol. 2019;24:1245–53.
Vafaie N, Kober H. Association of drug cues and craving with drug use and relapse: a systematic review and meta-analysis. JAMA Psychiat. 2022;79:641–50.
Billieux J, Van der Linden M, Ceschi G. Which dimensions of impulsivity are related to cigarette craving? Addict Behav. 2007;32:1189–99.
Paolini M, Keeser D, Rauchmann B-S, Gschwendtner S, Jeanty H, Reckenfelderbaeumer A, et al. Correlations between the DMN and the smoking cessation outcome of a real-time fMRI neurofeedback supported exploratory therapy approach: descriptive statistics on tobacco-dependent patients. Clin EEG Neurosci. 2022;53:287–96.
Rana M, Ruiz S, Sanchez Corzo A, Muehleck A, Eck S, Salinas C, et al. Use of real-time functional magnetic resonance imaging-based neurofeedback to downregulate insular cortex in nicotine-addicted smokers. Jove-J Vis Exp. 2020;160:e59441.
Goldstein RZ, D P, Alia-klein N, Tomasi D, Zhang L, Cottone LA, et al. Is decreased prefrontal cortical sensitivity to monetary and self-control in cocaine addiction ? Psychiatry Interpers Biol Process. 2007;164:43–51.
Costumero V, Bustamante JC, Rosell-Negre P, Fuentes P, Llopis JJ, Ávila C, et al. Reduced activity in functional networks during reward processing is modulated by abstinence in cocaine addicts. Addict Biol. 2017;22:479–89.
Hobkirk AL, Bell RP, Utevsky AV, Huettel S, Meade CS. Reward and executive control network resting-state functional connectivity is associated with impulsivity during reward-based decision making for cocaine users. Drug Alcohol Depend. 2019;194:32–9.
Kirschner M, Sladky R, Haugg A, Stampfli P, Jehli E, Hodel M, et al. Self-regulation of the dopaminergic reward circuit in cocaine users with mental imagery and neurofeedback. EBioMedicine. 2018;37:489–98.
Gantz SC, Ford CP, Morikawa H, Williams JT. The evolving understanding of dopamine neurons in the substantia nigra and ventral tegmental area. Annu Rev Physiol. 2018;80:219–41.
Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258.
• Cheng Y-C, Huang Y-C, Huang W-L. Can heart rate variability be viewed as a biomarker of problematic internet use? A systematic review and meta-analysis. Appl Psychophysiol Biofeedback. 2023;48:1–10. A recent systematic review and meta-analysis using PubMed, Medline, EMBASE, and PsycINFO; 106 studies were included for qualitative synthesis, and 12 studies were included in the quantitative analysis.
Tárrega S, Castro-Carreras L, Fernández-Aranda F, Granero R, Giner-Bartolomé C, Aymamí N, et al. A serious videogame as an additional therapy tool for training emotional regulation and impulsivity control in severe gambling disorder. Front Psychol. 2015;6:1–12.
Jiménez-Murcia S, Fernández-Aranda F, Kalapanidas E, Konstantas D, Ganchev T, Kocsis O, et al. Playmancer project: a serious videogame as an additional therapy tool for eating and impulse control disorders. Stud Health Technol Inform. 2009;144:163–6.
Rogier G, BeomonteZobel S, Velotti P. Pathological personality facets and emotion (dys)regulation in gambling disorder. Scand J Psychol. 2020;61:262–70.
Vintró-Alcaraz C, Mestre-Bach G, Granero R, Gómez-Peña M, Moragas L, Fernández-Aranda F, et al. Do emotion regulation and impulsivity differ according to gambling preferences in clinical samples of gamblers? Addict Behav. 2022;126:107176.
Mena-Moreno T, Fernández-Aranda F, Granero R, Munguía L, Steward T, López-González H, et al. A serious game to improve emotion regulation in treatment-seeking individuals with gambling disorder: a usability study. Front Psychol. 2021;12:621953.
Mena-Moreno T, Munguía L, Granero R, Lucas I, Fernández-Aranda F, Gómez-Peña M, et al. e-Estesia: a serious game for reducing arousal, improving emotional regulation and increasing wellbeing in individuals with gambling disorder. J Clin Med. 2022;11:6798.
Mena-Moreno T, Munguía L, Granero R, Lucas I, Sánchez-Gómez A, Cámara A, et al. Cognitive behavioral therapy plus a serious game as a complementary tool for a patient with Parkinson disease and impulse control disorder: case report. JMIR Serious Games. 2022;10:e33858.
Fernández-Aranda F, Jiménez-Murcia S, Santamaría JJ, Gunnard K, Soto A, Kalapanidas E, et al. Video games as a complementary therapy tool in mental disorders: PlayMancer, a European multicentre study. J Ment Heal. 2012;21:364–74.
Giordano R, Donati MA, Zamboni L, Fusina F, Primi C, Lugoboni F. Alter Game: a study protocol on a virtual “serious game” for relapse prevention in patients with gambling disorder. Front Psychiatry. 2022;13:854088.
Albein-Urios N, Pilatti A, Lozano Ó, Martínez-González JM, Verdejo-García A. The value of impulsivity to define subgroups of addicted individuals differing in personality dysfunction, craving, psychosocial adjustment, and wellbeing: a latent class analysis. Arch Clin Neuropsychol. 2014;29:38–46.
Miedl SF, Büchel C, Peters J. Cue-induced craving increases impulsivity via changes in striatal value signals in problem gamblers. J Neurosci. 2014;34:4750–5.
Poskotinova LV, Krivonogova OV, Zaborsky OS. Effectiveness of short-term heart rate variability biofeedback training and the risk of internet addiction in adolescents 15–16 years of age. Int J Biomed. 2020;10:153–6.
Demin D, Poskotinova L. Neurophysiologic reactions during heart rate variability biofeedback session in adolescents with different risk of internet addiction. Int J Environ Res Public Health. 2022;19:2759.
Price JL, Bates ME, Morgano J, Todaro S, Uhouse SG, Vaschillo E, et al. Effects of arousal modulation via resonance breathing on craving and affect in women with substance use disorder. Addict Behav Elsevier Ltd. 2022;127:107207.
Pandria N, Athanasiou A, Styliadis C, Terzopoulos N, Mitsopoulos K, Paraskevopoulos E, et al. Does combined training of biofeedback and neurofeedback affect smoking status, behavior, and longitudinal brain plasticity? Front Behav Neurosci. 2023;17:1–26.
Acknowledgements
CERCA Programme/Generalitat de Catalunya gave institutional support.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by a grant from the Ministerio de Ciencia e Innovación (PDI2021-124887OB-I00), the Delegación del Gobierno para el Plan Nacional sobre Drogas (2021I031), Instituto de Salud Carlos III (ISCIII) (PI20/00132), co-funded by FEDER funds/European Regional Development Fund (ERDF), a way to build Europe. CIBEROBN is an initiative of ISCIII. Additional funding was received by AGAUR-Generalitat de Catalunya (2021-SGR-00824) and European Union’s Horizon 2020 research and innovation program under Grant agreement no. 847879 (PRIME/H2020, Prevention and Remediation of Insulin Multimorbidity in Europe) and eprObes (Preventing lifetime obesity by early risk-factor identification, prognosis and intervention) (Ref 101080219-2). IL is supported by the Ministerio de Ciencia e Innovación (MCIN), Agencia Estatal de Investigación (AEI) (DOI:10.13039/501100011033), and by the European Union “NextGenerationEU/Plan de Recuperación, Transformación y Resiliencia (PRTR)” (Juan de la Cierva-Formación program, FJC2021-046494-I). IB is supported by Instituto de Salud Carlos III through the grant CM21/00172 2022–2023 (co-funded by European Social Fund-ESF investing in your future). MR is supported by a FI grant from the Catalan Agency for the Management of Grants for University – AGAUR (2020 FISDU 00579). The funders had no role in the study design, data collection and interpretation, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Concept – I.L., N.S.-M., I.B., F.F.-A., S.J.-M.; design – I.L., N.S.-M., I.B., F.F.-A., S.J.-M.; supervision – F.F.-A., S.J.-M.; funding – F.F.-A., S.J.-M.; literature review – I.L., N.S.-M.; writing – I.L., N.S.-M., I.B., M.R.; critical review – F.F.-A., S.J.-M.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Fernández-Aranda and Dr. Jiménez-Murcia received consultancy honoraria from Novo Nordisk, and Dr. Fernández-Aranda received editorial honoraria as EIC from Wiley.
Human and Animal Rights and Informed Consent
Not applicable as no human or animal subjects were involved in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ignacio Lucas and Neus Solé-Morata shared first authorship.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lucas, I., Solé-Morata, N., Baenas, I. et al. Biofeedback Interventions for Impulsivity-related Processes in Addictive Disorders. Curr Addict Rep 10, 543–552 (2023). https://doi.org/10.1007/s40429-023-00499-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40429-023-00499-y