Abstract
Purpose of Review
This work aims to provide an up-to-date review of the preclinical and clinical scientific literature on the therapeutic value of kratom to better understand the underlying mechanisms related to its use and inform future therapeutic applications.
Recent Findings
A growing number of studies, mainly of cross-sectional nature, describe the widespread use of kratom by individuals to self-treat pain, psychiatric symptoms, and substance use disorders (SUD) outside a controlled clinical setting. Preclinical evidence suggests kratom is effective as an analgesic agent and might decrease the self-administration of other drugs. A randomized controlled trial has further supported kratom’s therapeutic value as an analgesic. Investigations in nonclinical samples of long-term kratom users also indicate its therapeutic benefit in managing SUD symptoms (e.g., craving) and long-term or acute symptoms (e.g., withdrawal) for alcohol, opioids, and other illicit drugs. However, episodes of kratom-related intoxications have also been reported, often due to the adulteration and the contamination of kratom products mainly sold online or mixed toxicities when consumed outside clinical and traditional settings.
Summary
Evidence on the clinical implications of kratom use is still limited and uncertain, with kratom research constantly evolving. Therefore, further randomized trials are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical Background
Over the past decade, kratom (Mitragyna speciosa Korth.) has widely spread from its Eastern native regions (Malaysia, Thailand, and Indonesia) to the West, especially in Europe and the United States (US) [1, 2••]. It is mainly used by white adults (ages 30–50 on average) who are well-educated and employed at least part-time (e.g., [3, 4••]). Estimates of kratom users largely differ, but millions of people have been using this plant in the US where it is federally legal in all the constituent states apart from Alabama, Arkansas, Indiana, Vermont, Wisconsin, and Rhode Island [1, 2••]. Kratom is sold in various formulations (i.e., capsules, resin, tinctures, powder) in headshops and on the internet [1, 2••]. Recent evidence has shown that kratom products are becoming quite popular also in darknet markets [5]. Kratom is used recreationally as a plant-based substance by some users. Its effects are described as psychostimulant in small dosages of up to 5 g of plant material and similar to opioids at higher doses of approximately 5 to 15 g [1, 6]. However, its native use in South Asia has been associated with the nonmedical self-treatment of several conditions (i.e., substance use disorder (SUD) symptoms, such as opioid and other drugs’ withdrawal, pain, and improving energy) described even in the West, as well as hypertension, stomach ailments, diarrhea, infections, and diabetes, among others [1,7,8].
Preclinical Data
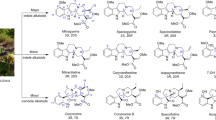
From a pharmacological perspective, mitragynine (MG) is the main lipophilic alkaloid present in kratom. Its psychoactive metabolite 7-hydroxy-mitragynine (7-HMG) is widely studied in preclinical models for its analgesic properties, while few studies have been conducted on the other alkaloids present in the plant. Another alkaloid, speciociliatine, has also been recently studied [9••]. MG possesses a poly-pharmacological profile and a primary opiate receptor activity, with preliminary evidence suggesting the possibility of mu-opioid receptor (MOR) activity without β-arrestin-2 recruitment in the signaling pathway [10•]. Recent evidence has shown that high doses (between 20 and 400 mg/kg) of oral MG do not exert respiratory depression compared to oxycodone in animal models [11]. However, information related to the safety profile of MG is still quite limited. Furthermore, in February 2018, the Food and Drug Administration (FDA) expressed concerns about the abuse potential of MG and 7-HMG contained in unregulated kratom-related products, highlighting that no medical indication has been approved for such herbal supplements [12]. Similar concerns have emerged in Europe, where kratom remains illegal in various countries (e.g., Sweden, Poland, Romania, and Denmark, among others) [13].
With the increasing scientific interest in the balance between kratom’s risks and potential therapeutic use, the present paper aims to provide an up-to-date review of the preclinical and clinical scientific literature on this topic to understand the underlying mechanism of kratom use and inform future therapeutic applications. The key objectives of our search were to (a) evaluate evidence suggesting kratom’s therapeutic value from a clinical perspective, including both anecdotal information and (pre)clinical evidence; (b) identify the clinical issues/health hazards linked to kratom use; and (c) understand if any information is available on the clinical consequences of kratom use in naïve users.
Methods
An exploratory literature search was performed between May 2022 and January 2023 in PubMed, Medline, Google Scholar, ScienceDirect, and Cochrane. “Kratom”, “mitragynine”, and “Mitragyna speciosa” were used as keywords to carry out the databases’ searches. We excluded all the duplicates and commentaries. Studies considered recent as published within the past 5 years were considered for analysis. Particular attention was given to all the (pre)clinical studies also if published outside this timeframe as limited clinical evidence is available.
Narrative Review Results
Evidence Suggesting Kratom’s Therapeutic Value from a Clinical Perspective
Anecdotal Information
There is an ongoing debate in the scientific community to understand the complex profile of kratom and its alkaloids regarding safety, health issues, and potential medical benefits. Anecdotal data, mainly from its native countries, suggest it has therapeutic value as an aid in several domains. Data from qualitative reports, e.g., social media analyses [14, 15] and large-scale surveys (e.g., [16••, 17••]), all document its widespread reported use in nonmedical settings for treating pain such as that related to the coronavirus disease 2019 (COVID-19) [18], cancer, multiple sclerosis, neuropathy and trigeminal neuralgia, fibromyalgia, headache, rheumatoid arthritis, and other inflammatory conditions (i.e., lupus, osteoarthritis, inflammatory bowel disease, complex regional pain syndrome, Ehlers-Danlos syndrome) [2••, 19]. Kratom use has also been reported as a substitute in nonmedical settings for dangerous drugs (i.e., methamphetamine, benzodiazepines, or heroin) or to mitigate the withdrawal from opioids, alcohol, and other illicit substances [20••, 21], especially among opioid poly-drug users [22]. Other underlying motivations for kratom intake include the improvement of sexual performance [23], coping strategies for negative emotional states [24], ameliorating psychiatric symptoms (i.e., anxiety, depression, insomnia, attention deficit hyperactivity disorder (ADHD), and post-traumatic stress disorder (PTSD) [e.g., 4••, 16••], and the enhancement of sociability, energy, and focus [14]. A recent case even reported its use in post-COVID insomnia [25].
Preclinical Evidence
Evidence emerging from the initial preclinical findings supports the anecdotal reports that kratom can be beneficial in treating pain and mental health–related conditions [26••]. In vitro and/or in vivo studies have shown that kratom and its alkaloids exert analgesic/antinociceptive effects as suggested by the increased latency of an antinociceptive response in the hot plate or tail-flick tests or in the model of inflammatory pain induced by acetic acid (27, 28••). Anti-allodynic efficacy in neuropathic pain has also been described [29••, 30•). Moreover, some preclinical models have suggested kratom’s antidepressant, anxiolytic, stress-mitigating [31••, 32•, 33] and antipsychotic effects [34•]. It has also emerged that kratom and its alkaloids exert gastroprotective, anti-inflammatory [35••, 36], antibacterial [37], antioxidant, antimutagen, and anticancer actions [38]. A recent preclinical study showed, for instance, that MG and speciociliatine acted as chemo-sensitizers for cisplatin and inhibited cell proliferation of nasopharyngeal carcinoma, malignant cancer [9••]. In another current study, MG has been shown to inhibit the enzyme acetylcholinesterase (AChE) involved in Alzheimer’s disease [39••]. It might also exert a lipolytic effect [40••] and antidiabetic action by inhibiting other biological enzymes (α-glucosidase, pancreatic lipase) when combined with the diabetes-2 drug (α-glucosidase inhibitor) acarbose [41••]. Preclinical evidence has also suggested its potential in treating (i) alcohol use disorder, alcohol withdrawal, and alcohol-seeking behavior, with a large therapeutic window (e.g., [42, 43••]); (ii) dependence and withdrawal from opioids (e.g., [28••, 44••]) without anxiogenic symptoms [45] and by improving simultaneously cognitive performance [46••]; and (iii) craving and addiction from methamphetamine [47] (for an overview of preclinical evidence and anecdotal data providing some evidence suggesting kratom’s therapeutic potential, see also Table 1).
In light of the anecdotal and preclinical findings, little data is available on kratom’s therapeutic potential.
Evidence from Clinical Studies
The discussion on the clinical implications of kratom use has been further enriched by the findings derived from some observational studies carried out in a traditional context (Malaysia or Thailand) among long-term/daily kratom users drinking daily several glasses of kratom juice/tea (between 2 and 6) [48, 49, 50, 51••]. In these cases, the estimated daily dose of MG ranges between 76 and 434 mg [52, 53], with no data on other alkaloids. Some of these studies have reported the association between long-term kratom use and health issues. This includes kratom’s impact on visual episodic memory and alteration of cholesterol level [54, 55], early stage of renal injury [50], dependence (which affects physical well-being if severe; [48, 56]), and craving with physical and psychological withdrawal symptoms [58, 59]. Such symptoms last about 3 days but are less severe than those described in the West or those related to classical opioids [57, 58, 59, 60]. However, until now, there is no evidence of kratom-related psychosis, social functioning alterations, long-term cognitive or biochemical/endocrinological impairment [53, 54, 61, 62, 63]. Furthermore, preliminary clinical data suggest kratom’s therapeutic potential as (i) an analgesic agent, with a randomized controlled trial (RCT) in kratom users showing enhanced pain tolerance after drinking kratom without any significant negative health consequences [51••], and (ii) a form of harm reduction, with kratom being reported to reduce regular drug (heroin, methamphetamine, amphetamine) use, opioids adverse effects, and HIV risk behaviors among illicit and opiates drug users [64, 65, 66]. Some evidence has also suggested that kratom possesses a favorable lipid profile and might be protective against metabolic syndrome, coronary heart, or cerebrovascular disease [63, 67, 68]. However, this data is in contrast with the slight increase in serum lipids linked to a higher frequency of kratom use reported by Leong Bin Abdullah et al. [69]. Finally, two studies evaluated the pharmacokinetic profile of MG in humans and suggest that it follows a two-compartment model with a 1-day terminal half-life [70••, 71]. As reported by the authors, such beneficial pharmacokinetic properties might be useful for its potential use as a medication (for a summary of clinical studies conducted on kratom users, see Table 2).
Clinical Issues/Health Hazards Linked to Kratom Use
An evaluation of kratom’s clinical implications should also consider the reported kratom-related negative clinical consequences. A large number of case reports and case series have been questioning kratom’s safety profile, especially when ingested outside controlled settings. Issues raised include the risks of kratom dependence or addiction with withdrawal symptoms, cases of neonates with abstinence syndrome born from mothers with or without kratom withdrawal, and neurological and psychiatric manifestations [76–78]. Recent evidence has reported episodes of mental and psychological distress, especially when kratom is used with nonmedical opioids and methamphetamine [79]. Other negative consequences to be investigated further include endocrinological damages, dermatological manifestations, electrolytic and kidney alterations, hepatic and gastrointestinal injuries, and respiratory and cardiological health hazards (e.g., [80–83]). Concerning kratom’s effects on cardiological functioning, data is still inconsistent. Trakulsrichai et al. [70••] found a temporary increase in pulse rate and blood pressure in kratom users, and Leong Bin Abdullah et al. [73] described a condition of sinus tachycardia without alterations of QTc intervals. However, in another study, kratom use has been associated with a dose-dependent prolonged QTc interval [49], highlighting the need for more investigation on the cardiological impact of kratom use. The serotonin syndrome, the autonomic nervous system dysfunction, an undifferentiated shock, conditions of multi-organ dysfunction [84–86], overdoses, and fatalities (e.g., [87, 88] must also be considered (for a summary of the main kratom-related toxicities and their clinical presentations, see Table 3).
While in Asia kratom has been used with a limited number of associated health hazards [25, 108], most of the safety issues have been reported in the West, mainly among users ingesting kratom on a daily basis in combination with other drugs, such as prescription medicines (e.g., antipsychotic, antidepressant, and hypnotic drugs, among others) and psychoactive substances (e.g., amphetamine, methamphetamine, cyclopropyl fentanyl, opioids, ethanol) [76, 88, 108]. Unscheduled contaminants/adulterants in kratom products (e.g., propyl-hexedrine, phenyl-ethylamine, O-desmethyl-tramadol, hydrocodone, and morphine) [90, 96•, 97•, 100], microbes (e.g., Salmonella, M. incognita), and toxic metals (lead and nickel) [109••, 110••, 111••] have also been found responsible for toxicities. Furthermore, MG was identified as a contributing factor to some fatalities [112]. More recently, a survey has suggested that kratom-related side effects might depend on the doses [113]. Thus, it is possible to hypothesize that low doses of kratom might come with smaller risks in the absence of other substances responsible for drug–drug interaction.
Clinical Consequences of Kratom Use in Naïve Users
Limited information is available regarding kratom quantity used and clinical consequences in naïve users. Very few clinical reports of toxicity have been recorded among individuals using kratom alone and/or for the first time [77, 105]. With no clinical studies conducted among such populations, doubts remain about its safety. More rigorous research on the clinical, pharmacodynamic, and pharmacokinetic aspects of kratom and MG is needed to clarify the therapeutically useful or risky doses of kratom in humans.
Discussion and Conclusion
With kratom research rapidly evolving, the most updated evidence on the clinical implications of kratom use has been reviewed in this work. Despite the growing amount of anecdotal self-reported evidence suggesting the therapeutic value of kratom in treating acute/chronic pain and psychiatric disorders, including SUD, in nonmedical settings, various studies report episodes of acute intoxications or the development of dependence linked to the heavy chronic use in such settings. Interestingly, most of the safety concerns derive from Western (non-native) countries, where kratom use frequently occurs in combination with other substances. Furthermore, kratom products are advertised online or elsewhere with captivating marketing strategies providing misleading or no information about the ingredients, dosage, type, and alkaloids, among others. These aspects make it difficult to determine the dose–response relationship and/or the causal relation with kratom exposure. On the contrary, most of the investigations that we found in native countries, mainly of cross-sectional nature, provide encouraging data on the potential therapeutic value of kratom, also supported by the initial preclinical evidence. Despite these discrepancies, it remains a priority to determine the dose of MG and other alkaloids that can be considered safe and clinically useful in medical treatment. Such insight might contribute to kratom’s risk assessment and give additional information to clinicians and regulatory agencies willing to recognize kratom as an agent for the treatment of pain, mental health, and other chronic/benign health conditions. Finally, controlled and longitudinal studies under careful clinical supervision, including healthy and naïve individuals and/or participants who consume other drugs or medicines outside the traditional context, will further contribute to the development of kratom research and provide a better understanding of the underlying clinical, pharmacological, and toxicological mechanisms necessary to inform future therapeutic applications of kratom.
Data Availability
All the information that was used is presented in the tables and in the manuscript.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kruegel AC, Grundmann O. The medicinal chemistry and neuropharmacology of kratom: a preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology. 2018;134(Pt A):108–20.
•• Swogger MT, Smith KE, Garcia-Romeu A, Grundmann O, Veltri CA, Henningfield JE, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855. This article is a complete and useful review for clinicians and researchers interested in kratom.
Covvey JR, Vogel SM, Peckham AM, Evoy KE. Prevalence and characteristics of self-reported kratom use in a representative US general population sample. J Addict Dis. 2020;38(4):506–13.
•• Grundmann O, Veltri CA, Morcos S, Smith KE, Singh D, Corazza O, et al. Correlations of kratom (Mitragyna speciosa Korth.) use behavior and psychiatric conditions from a cross-sectional survey. Exp Clin Psychopharmacol. 2023. https://doi.org/10.1037/pha0000632. Relevant paper about kratom use in the nonmedical self-treatment of psychiatric symptoms.
Prevete E, Catalani V, Singh D, Kuypers KPC, Theunissen EL, Townshend HD, Banayoty H, Ramaekers JG, Pasquini M, Corazza O. An inventory of kratom (Mitragyna speciosa) products and vendors on the darknet and cryptomarkets. Under review. 2022.
Cinosi E, Martinotti G, Simonato P, Singh D, Demetrovics Z, Roman-Urrestarazu A, et al. Following “the roots” of kratom (Mitragyna speciosa): the evolution of an enhancer from a traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in Western countries. Biomed Res Int. 2015;2015:968786.
Smith KE, Dunn KE, Rogers JM, Grundmann O, McCurdy CR, Garcia-Romeu A, et al. Kratom use as more than a “self-treatment.” Am J Drug Alcohol Abuse. 2022;48(6):684–94.
Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340–8.
•• Domnic G, Jeng-YeouChear N, Abdul Rahman SF, Ramanathan S, Lo KW, Singh D, et al. Combinations of indole based alkaloids from Mitragyna speciosa (kratom) and cisplatin inhibit cell proliferation and migration of nasopharyngeal carcinoma cell lines. J Ethnopharmacol. 2021;279:114391. Relevant preliminary evidence on the anticancer action of kratom.
• Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, et al. Mitragynine/corynantheidine pseudoindoxyls as opioid analgesics with Mu agonism and delta antagonism, which do not recruit β-arrestin-2. J Med Chem. 2016;59(18):8381–97. It was published in 2016 but still holds value for the data about the pharmacology of MG.
Henningfield JE, Rodricks JV, Magnuson AM, Huestis MA. Respiratory effects of oral mitragynine and oxycodone in a rodent model. Psychopharmacology. 2022;239(12):3793–804.
FDA. FDA and Kratom 2022; Available from: https://www.fda.gov/news-events/public-health-focus/fda-and-kratom. Accessed 18 Dec 2022.
EMCDDA. Kratom (Mitragyna speciosa) Drug Profile 2015; Available from: https://www.emcdda.europa.eu/publications/drug-profiles/kratom#control. Accessed 18 Dec 2022.
Prevete E, Hupli A, Marrinan S, Singh D, D’Udine B, Bersani G, et al. Exploring the use of kratom (Mitragyna speciosa) via the YouTube data tool: a novel netnographic analysis. Emerging Trends in Drugs, Addictions, and Health. 2021;1:100007.
Smith KE, Rogers JM, Schriefer D, Grundmann O. Therapeutic benefit with caveats?: analyzing social media data to understand the complexities of kratom use. Drug Alcohol Depend. 2021;226:108879.
•• Bath R, Bucholz T, Buros AF, Singh D, Smith KE, Veltri CA, et al. Self-reported health diagnoses and demographic correlates with kratom use: results from an online survey. J Addict Med. 2020;14(3):244–52. Paper providing a wide overview of anecdotal kratom use in several conditions.
•• Grundmann O, Veltri CA, Morcos D, Knightes D, 3rd, Smith KE, Singh D, et al. Exploring the self-reported motivations of kratom (Mitragyna speciosa Korth.) use: a cross-sectional investigation. Am J Drug Alcohol Abuse. 2022;48(4):433–44. The article is relevant since it provides a wide overview of anecdotal kratom use in several conditions.
Metastasio A, Prevete E, Singh D, Grundmann O, Prozialeck WC, Veltri C, et al. Can kratom (Mitragyna speciosa) alleviate COVID-19 pain? A case study Front Psychiatry. 2020;11:594816.
Network PN. Pain News Network 2017 ; Available from: https://www.painnewsnetwork.org/kratom-survey.
•• Coe MA, Pillitteri JL, Sembower MA, Gerlach KK, Henningfield JE. Kratom as a substitute for opioids: results from an online survey. Drug Alcohol Depend. 2019;202:24–32. Relevant paper providing a wide overview of anecdotal kratom use in several conditions
Singh D, Narayanan S, Vicknasingam B, Prozialeck WC, Smith KE, Corazza O, et al. The use of kratom (Mitragyna speciosa Korth.) among people who co-use heroin and methamphetamine in Malaysia. J Addict Med. 2022;16(2):223–8.
Singh D, YeouChear NJ, Narayanan S, Leon F, Sharma A, McCurdy CR, et al. Patterns and reasons for kratom (Mitragyna speciosa) use among current and former opioid poly-drug users. J Ethnopharmacol. 2020;249:112462.
Singh D, Grundmann O, Murugaiyah V, Rahim ABM, Chawarski M, Balasingam V. Improved sexual functioning of long-term daily users of Mitragyna speciosa (Korth). Journal of Herbal Medicine. 2020;19:100293.
Singh D, Narayanan S, Müller CP, Swogger MT, Chear NJY, Dzulkapli EB, et al. Motives for using kratom (Mitragyna speciosa Korth.) among regular users in Malaysia. J Ethnopharmacol. 2019;233:34–40.
Thewjitcharoen Y, Krittiyawong S, Nakasatien S, Himathongkam T. Kratom-associated mixed cholestatic-hepatocellular liver injury in a patient with long COVID: a case report. Clin Med Insights Case Rep. 2022;15:11795476221132824.
•• Prevete E, Kuypers KPC, Theunissen EL, Corazza O, Bersani G, Ramaekers JG. A systematic review of (pre)clinical studies on the therapeutic potential and safety profile of kratom in humans. Hum Psychopharmacol. 2022;37(1):e2805. Review providing (pre)clinical evidence on kratom’s therapeutic potential.
Mat NH, Bakar SNS, Murugaiyah V, Chawarski MC, Hassan Z. Analgesic effects of main indole alkaloid of kratom, mitragynine in acute pain animal model. Behav Brain Res. 2022;439:114251.
•• Wilson LL, Harris HM, Eans SO, Brice-Tutt AC, Cirino TJ, Stacy HM, et al. Lyophilized kratom tea as a therapeutic option for opioid dependence. Drug Alcohol Depend. 2020;216:108310. Relevant paper on the therapeutic potential of kratom in opioid use disorder.
•• Farkas DJ, Foss JD, Ward SJ, Rawls SM. Kratom alkaloid mitragynine: inhibition of chemotherapy-induced peripheral neuropathy in mice is dependent on sex and active adrenergic and opioid receptors. IBRO Neurosci Rep. 2022;13:198–206. The article is important since it shows the potential of kratom in neuropathic pain.
• Foss JD, Nayak SU, Tallarida CS, Farkas DJ, Ward SJ, Rawls SM. Mitragynine, bioactive alkaloid of kratom, reduces chemotherapy-induced neuropathic pain in rats through α-adrenoceptor mechanism. Drug Alcohol Depend. 2020;209:107946. The article is important since it preliminarily suggests the potential of kratom in neuropathic pain.
•• Chen L, Fei S, Olatunji OJ. LC/ESI/TOF-MS Characterization, anxiolytic and antidepressant-like effects of Mitragyna speciosa Korth extract in diabetic rats. Molecules. 2022;27(7):2208. The article is important since it contributes to suggestion of the antidepressant and anxiolytic potential of kratom.
• Idayu NF, Hidayat MT, Moklas MA, Sharida F, Raudzah AR, Shamima AR, et al. Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine. 2011;18(5):402–7. It was published in 2011 but still holds value as preclinical evidence on kratom’s antidepressant activity.
Vázquez López JL, Schild L, Günther T, Schulz S, Neurath H, Becker A. The effects of kratom on restraint-stress-induced analgesia and its mechanisms of action. J Ethnopharmacol. 2017;205:178–85.
• Vijeepallam K, Pandy V, Kunasegaran T, Murugan DD, Naidu M. Mitragyna speciosa leaf extract exhibits antipsychotic-like effect with the potential to alleviate positive and negative symptoms of psychosis in mice. Front Pharmacol. 2016;7:464. It was published in 2016 but still holds value for the preclinical evidence on kratom’s antipsychotic activity.
•• Salim HM, Awwalia ES, Alam IP. Anti-inflammatory effects and potential mechanisms of Mitragyna speciosa methanol extract on λ-karagenan-induced inflammation model. Bali Med J. 2022;11(3):1172–5. Relevant preliminary evidence on the anti-inflammatory effect of kratom.
Chittrakarn S, Radenahmad N, Kaewsara S, Udomuksorn W, Keawpradub N, Phukpattaranont P. Gastroprotective effects of methanolic extract of kratom leaves on gastric ulcer and reflux esophagitis in rats. Songklanakarin Journal of Science & Technology. 2018;40(2)258–63.
Juanda E, Andayani S, Maftuch M. Phytochemical screening and antibacterial activity of kratom leaf (Mitragyna speciosa korth) against Aeromonas hydrophilla. J Exp Life Sci. 2019;9(3):155–8.
Yuniarti R, Nadia S, Alamanda A, Zubir M, Syahputra RA, Nizam M, editors. Characterization, phytochemical screenings and antioxidant activity test of kratom leaf ethanol extract (Mitragyna speciosa Korth) using DPPH method. J Phys Conf Ser. 2020;1462(1):012026.
•• Innok W, Hiranrat A, Chana N, Rungrotmongkol T, Kongsune P. In silico and in vitro anti-AChE activity investigations of constituents from Mytragyna speciosa for Alzheimer’s disease treatment. J Comput Aided Mol Des. 2021;35(3):325–36. Relevant paper about the therapeutic potential of MG.
•• Abidin KlR, Lesmana R, Syamsunarno MR, Anggun Adipurna Dharma KK. Potential role of mitragynine as lipolysis stimulator via adrenergic signalling: docking model study. Pharmacognosy Journal. 2022;14(5):527–531. Relevant preliminary evidence on the lipolytic effect of kratom.
•• Limcharoen T, Pouyfung P, Ngamdokmai N, Prasopthum A, Ahmad AR, Wisdawati W, et al. Inhibition of α-glucosidase and pancreatic lipase properties of Mitragyna speciosa (Korth.) Havil. (kratom) leaves. Nutrients. 2022;14(19):3909. Relevant preliminary evidence on the antidiabetic effect of kratom.
Vijeepallam K, Pandy V, Murugan DD, Naidu M. Methanolic extract of Mitragyna speciosa Korth leaf inhibits ethanol seeking behaviour in mice: involvement of antidopaminergic mechanism. Metab Brain Dis. 2019;34(6):1713–22.
•• Gutridge AM, Chakraborty S, Varga BR, Rhoda ES, French AR, Blaine AT, et al. Evaluation of kratom opioid derivatives as potential treatment option for alcohol use disorder. Front Pharmacol. 2021;12:764885. The article is important since it preliminarily describes the therapeutic potential of kratom in alcohol use disorder.
•• Hassan R, Pike See C, Sreenivasan S, Mansor SM, Müller CP, Hassan Z. Mitragynine attenuates morphine withdrawal effects in rats-a comparison with methadone and buprenorphine. Front Psychiatry. 2020;11:411. The article is important since it preliminarily suggests the therapeutic potential of kratom in opioid use disorder.
Johari IS, Harun N, Sofian ZM, Shoaib M. Pentylenetetrazol-like stimulus is not produced following naloxone-precipitated mitragynine withdrawal in rats. Psychopharmacology. 2021;238(11):3183–91.
•• You CY, Hassan Z, Müller CP, Suhaimi FW. Mitragynine improves cognitive performance in morphine-withdrawn rats. Psychopharmacology. 2022;239(1):313–25. The article is important since it shows that MG has a positive effect on cognitive functioning.
Nukitram J, Cheaha D, Sengnon N, Wungsintaweekul J, Limsuwanchote S. Kumarnsit E. Ameliorative effects of alkaloid extract from Mitragyna speciosa (Korth.) Havil. leaves on methamphetamine conditioned place preference in mice. J Ethnopharmacol. 2022;284:114824.
Leong Bin Abdullah MFI, Singh D, Narayanan S, Rahim AA, Vicknasingam B. Socio-demographic characteristics, kratom use and quality of life (QoL) of regular kratom (Mitragyna speciosa Korth) users. Malaysian Journal Of Medicine And Health Sciences. 2019;15:4–9.
Leong Bin Abdullah MFI, Singh D. Assessment of cardiovascular functioning among regular kratom (Mitragyna speciosa Korth) users: a case series. Front Pharmacol. 2021;12: 527–31.
Jasim RK, Hassan Z, Singh D, Boyer E, Gam LH. Characterization of urinary protein profile in regular kratom (Mitragyna speciosa Korth.) users in Malaysia. J Addict Dis. 2022;40(2):235–46.
•• Vicknasingam B, Chooi WT, Rahim AA, Ramachandram D, Singh D, Ramanathan S, et al. Kratom and pain tolerance: a randomized, placebo-controlled, double-blind study. Yale J Biol Med. 2020;93(2):229–38. This is the only RCT on kratom to date, which preliminarily suggests kratom’s potential as an analgesic agent.
Leong Abdullah MFI, Tan KL, Narayanan S, Yuvashnee N, Chear NJY, Singh D, et al. Is kratom (Mitragyna speciosa Korth.) use associated with ECG abnormalities? Electrocardiogram comparisons between regular kratom users and controls. Clin Toxicol (Phila). 2021;59(5):400–8.
Singh D, Murugaiyah V, Hamid SBS, Kasinather V, Chan MSA, Ho ETW, et al. Assessment of gonadotropins and testosterone hormone levels in regular Mitragyna speciosa (Korth.) users. J Ethnopharmacol. 2018;221:30–6.
Singh DP, Narayanan SP, Müller CPP, Vicknasingam BP, Yücel MP, Ho ETWP, et al. Long-term cognitive effects of kratom (Mitragyna speciosa Korth.) use. J Psychoactive Drugs. 2019;51(1):19–27.
Singh D, Müller CP, Murugaiyah V, Hamid SBS, Vicknasingam BK, Avery B, et al. Evaluating the hematological and clinical-chemistry parameters of kratom (Mitragyna speciosa) users in Malaysia. J Ethnopharmacol. 2018;214:197–206.
Leong Bin Abdullah MFI, Yuvashnee N, Singh D. Effect of regular kratom (Mitragyna speciosa Korth.) use on quality of life of people who use kratom. Subst Abus. 2021;42(4):444–9.
Singh D, Damodaran T, Prozialeck WC, Grundmann O, Karunakaran T, Vicknasingam B. Constipation prevalence and fatigue severity in regular kratom (Mitragyna speciosa Korth.) users. Journal of Substance Use. 2019;24(3):233–9.
Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132–7.
Singh D, Narayanan S, Müller CP, Swogger MT, Rahim AA, Leong Bin Abdullah MFI, et al. Severity of kratom (Mitragyna speciosa Korth.) psychological withdrawal symptoms. J Psychoactive Drugs. 2018;50(5):445–50.
Singh D, Narayanan S, Vicknasingam BK, Prozialeck WC, Ramanathan S, Zainal H, et al. Severity of pain and sleep problems during kratom (Mitragyna speciosa Korth.) cessation among regular kratom users. J Psychoactive Drugs. 2018;50(3):266–74.
Leong Bin Abdullah MFI, Singh D, Swogger MT, Rahim AA, Vicknasingam B. The prevalence of psychotic symptoms in kratom (Mitragyna speciosa Korth.) Users in Malaysia. Asian J Psychiatr. 2019;43:197–201.
Singh D, Müller CP, Vicknasingam BK, Mansor SM. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125–31.
Ramachandram D, Sangarran Chia Siang K. Comparison of biochemical and safety parameters of regular kratom (Mitragyna speciosa Korth.) users at two different time periods. Journal of Substance Use. 2023;28(1):20–5.
Saref A, Suraya S, Singh D, Grundmann O, Narayanan S, Swogger MT, et al. Self-reported prevalence and severity of opioid and kratom (Mitragyna speciosa korth.) side effects. J Ethnopharmacol. 2019;238:111876.
Singh D, Narayanan S, Abdullah M, Vicknasingam B. Effects of kratom (Mitragyna speciosa Korth.) in reducing risk-behaviors among a small sample of HIV positive opiate users in Malaysia. J Ethn Subst Abuse. 2022;16:1–11.
Saref A, Suraya S, Singh D, Grundmann O, Narayanan S, Swogger MT, et al. Self-report data on regular consumption of illicit drugs and HIV risk behaviors after kratom (Mitragyna speciosa korth.) initiation among illicit drug users in Malaysia. J Psychoactive Drugs. 2020;52(2):138–44.
La-Up A, Saengow U, Aramrattana A. High serum high-density lipoprotein and low serum triglycerides in kratom users: a study of kratom users in Thailand. Heliyon. 2021;7(4):e06931.
La-Up A, Wongrith P, Chaichan W, Aramrattana A, Saengow U. Association between kratom (Mitragyna speciosa) use and metabolic syndrome. Heliyon. 2022;8(5):e09468.
Leong Bin Abdullah MFI, Tan KL, Mohd Isa S, Yusoff NS, Chear NJY, Singh D. Lipid profile of regular kratom (Mitragyna speciosa Korth.) users in the community setting. PLoS ONE 2020;15(6):e0234639.
•• Trakulsrichai S, Sathirakul K, Auparakkitanon S, Krongvorakul J, Sueajai J, Noumjad N, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015;9:2421–9. It was published in 2015 but still holds value as it provides data on the pharmacokinetics of kratom in humans.
•• Tanna RS, Nguyen JT, Hadi DL, Manwill PK, Flores-Bocanegra L, Layton ME, et al. Clinical pharmacokinetic assessment of kratom (Mitragyna speciosa), a botanical product with opioid-like effects, in healthy adult participants. Pharmaceutics. 2022;14(3):620. Among the very few clinical studies on the pharmacokinetics of kratom in humans.
• Grewal KS. Mytragynine in man. The psychoanalytic review (1913–1957). 1932;12(1):41–58. It was published in 1932 but still holds value as it is the first clinical experiment on MG in humans.
Leong Abdullah MFI, Tan KL, Narayanan S, Yuvashnee N, Chear NJY, Singh D, et al. Is kratom (Mitragyna speciosa Korth.) use associated with ECG abnormalities? Electrocardiogram comparisons between regular kratom users and controls. Clin Toxicol (Phila). 2021;59(5):400–8.
Saengmolee W, Cheaha D, Sa-Ih N, Kumarnsit E. Exploring of cardiac autonomic activity with heart rate variability in long-term kratom (Mitragyna speciosa Korth.) users: a preliminary study. PeerJ. 2022;10:e14280.
Singh D, Narayanan S, Grundmann O, Dzulkapli EB, Vicknasingam B. Effects of kratom (Mitragyna Speciosa Korth.) use in regular users. Subst Use Misuse. 2019;54(14):2284–9.
Settle AG, Yang C. A case of severe kratom addiction contributing to a suicide attempt. Cureus. 2022;14(9):e29698.
Afzal H, Esang M, Rahman S. A case of kratom-induced seizures Cureus. 2020;12(1):e6588.
Eldridge WB, Foster C, Wyble L. Neonatal abstinence syndrome due to maternal kratom use. Pediatrics. 2018;142(6):e20181839.
Smith KE, Rogers JM, Strickland JC. Associations of lifetime nonmedical opioid, methamphetamine, and kratom use within a nationally representative US sample. J Psychoactive Drugs. 2022;54(5):429–39.
Powell LR, Ryser TJ, Morey GE, Cole R. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145–8.
Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought ? BMJ Case Rep. 2019;12(7):e229778.
LaBryer L, Sharma R, Chaudhari KS, Talsania M, Scofield RH. Kratom, an emerging drug of abuse, raises prolactin and causes secondary hypogonadism: case report. J Investig Med High Impact Case Rep. 2018;6:2324709618765022.
Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546–7.
Eudaley ST, Brooks SP, Hamilton LA. Case report: possible serotonin syndrome in a patient taking kratom and multiple serotonergic agents. J Pharm Pract. 2022:8971900221116009.
Zuberi M, Guru PK, Bansal V, Diaz-Gomez J, Grieninger B, Alejos D. Undifferentiated shock and extreme elevation of procalcitonin related to kratom use. Indian J Crit Care Med. 2019;23(5):239–41.
Sangani V, Sunnoqrot N, Gargis K, Ranabhotu A, Mubasher A, Pokal M. Unusual presentation of kratom overdose with rhabdomyolysis, transient hearing loss, and heart failure. J Investig Med High Impact Case Rep. 2021;9:23247096211005068.
Tobarran N, Wolf C, Cumpston KL, Wills BK. Pressure necrosis requiring fasciotomy after kratom overdose. J Addict Med. 2022;16(2):252–3.
Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110–3.
Matos-Casano HA, Nanduri S. Transient paralysis: a novel expression of kratom toxicity in humans. Neurol Clin Pract. 2021;11(1):e28–9.
Nacca N, Schult RF, Li L, Spink DC, Ginsberg G, Navarette K, et al. Kratom adulterated with phenylethylamine and associated intracerebral hemorrhage: linking toxicologists and public health officials to identify dangerous adulterants. J Med Toxicol. 2020;16(1):71–4. https://doi.org/10.1007/s13181-019-00741-y.
Cutlip HA, Bushman E, Thottumari L, Mogallapu R, Ang-Rabanes M. A case report of kratom-induced psychosis. Cureus. 2021;13(6):e16073.
Garrels E, Gill G, Mitra S, Korenis P. A case report of kratom-induced psychiatric decompensation. European Psychiatry. 2022;65(S1):S826-S.
Bowe A, Kerr PL. A complex case of kratom dependence, depression, and chronic pain in opioid use disorder: effects of buprenorphine in clinical management. J Psychoactive Drugs. 2020;52(5):447–52.
Anand A, Hosanagar A. The addictive potential and challenges with use of the “Herbal Supplement” kratom: a case report and literature review. Pain Med. 2022;23(1):4–9.
Davidson L, Rawat M, Stojanovski S, Chandrasekharan P. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom.’ J Neonatal Perinatal Med. 2019;12(1):109–12.
• Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54–9. It was published in 2011 but still holds value as it contributes to showing the role of contaminants in mixed toxicities linked to kratom use.
• Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242–7. It was published in 2011 but still holds value as it contributes to showing the role of contaminants in mixed toxicities linked to kratom use.
Jaliawala HA, Abdo T, Carlile PV. Kratom; a potential cause of acute respiratory distress syndrome. D35 DRUG INDUCED LUNG DISEASE: CASE REPORTS: American Thoracic Society. 2018;A6604–A .
ELJack A, Beasley M, Ibrahim H, Taha M, Werns S. Kratom-associated ventricular fibrillation. Am J Ther. 2020;28(6):e792–5.
LeSaint KT, Yin S, Sharma A, Avery BA, McCurdy CR, Waksman JC. Acute renal insufficiency associated with consumption of hydrocodone- and morphine-adulterated kratom (Mitragyna speciosa). J Emerg Med. 2022;63(1):e28–30.
Martin G, Collins DP, Valenzuela H. Life-threatening hyponatremia secondary to chronic kratom use: a case presentation. Cureus. 2022;14(9):e29073.
Torres-Ortiz A, Al Zein S, Alqudsi M. A case of hyperkalemia induced by kratom (Mitragyna speciosa). Cureus. 2022;14(4):e24036.
• Sheleg SV, Collins GB. A coincidence of addiction to “Kratom” and severe primary hypothyroidism. J Addict Med. 2011;5(4):300–1. It was published in 2011 but still holds value as it is the only case of hypothyroidism linked to kratom use described in literature to date.
Khan MZ, Saleh MA, Alkhayyat M, Roberts DE, Lindenmeyer CC. Multiorgan dysfunction related to kratom ingestion. ACG Case Rep J. 2021;8(8):e00647.
Singh V, Mulla N, Wilson JL, Umansky A, Lee J, Stead T, et al. Intractable nausea and vomiting in naïve ingestion of kratom for analgesia. Int J Emerg Med. 2020;13(1):42.
Hall A, Hall D. Kratom ingestion and emergency care: summary and a case report. J Emerg Nurs. 2021;47(4):551-6.e1.
Sekar A, Velani S, Katzman S, O’Donnell M, Conway KS. Suspected Fanconi syndrome from cadmium toxicity exacerbated by heavy kratom use. A rare occurrence Clin Toxicol (Phila). 2022;60(7):888–9.
Jittasopa W, Srisont S. The causes of death and pathological findings of kratom users: a 5-year retrospective analysis. Am J Forensic Med Pathol. 2021;42(4):335–40.
•• Prozialeck WC, Edwards JR, Lamar PC, Plotkin BJ, Sigar IM, Grundmann O, et al. Evaluation of the mitragynine content, levels of toxic metals and the presence of microbes in kratom products purchased in the Western suburbs of Chicago. Int J Environ Res Public Health. 2020;17(15):5512. Relevant paper as it contributes to showing the role of contaminants in kratom products.
•• Prozialeck W, Fowler A, Edwards J. Public health implications and possible sources of lead (Pb) as a contaminant of poorly regulated kratom products in the United States. Toxics. 2022;10(7):398. Among the articles contributing to showing the role of contaminants in kratom products.
•• Habteweld A, Davidson W, Desaeger J, Crow WT. First report of Meloidogyne incognita infecting Mitragyna speciosa in the United States. J Nematol. 2022;54(1):20220021. Among the articles contributing to showing the role of contaminants in kratom products.
Domingo O, Roider G, Stöver A, Graw M, Musshoff F, Sachs H, et al. Mitragynine concentrations in two fatalities. Forensic Sci Int. 2017;271:e1–7.
Smith KE, Rogers JM, Dunn KE, Grundmann O, McCurdy CR, Schriefer D, et al. Searching for a signal: self-reported kratom dose-effect relationships among a sample of US adults with regular kratom use histories. Front Pharmacol. 2022;13:765917.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: OC, EP, MP. Searches: EP. Writing—original draft: EP. Writing—review and editing: OC, GE, KK, EP, MP, JR, ET. Visualization: ALL. Co-supervision: MP, JR. Supervision: OC. Project administration: EP.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Authors’ Note
The authors confirm that this work is original and has not been published elsewhere, neither is currently under consideration for publication elsewhere, or in press at other journals.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prevete, E., Kuypers, K.P.C., Theunissen, E.L. et al. Clinical Implications of Kratom (Mitragyna speciosa) Use: a Literature Review. Curr Addict Rep 10, 317–334 (2023). https://doi.org/10.1007/s40429-023-00478-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40429-023-00478-3