Abstract
Purpose
Asthma is the most common chronic disorder in childhood. Inhaled corticosteroid therapy is currently the most effective treatment for Asthma. The oral cavity complications related to this treatment may be in terms of the changes in the innate immune system of mouth. Salivary defensin has many immunomodulatory properties. The expression of beta-defensin 2 was measured before and after inhaled corticosteroid treatment in children with asthma to determine the potential impact of corticosteroids on defensin expression.
Methods
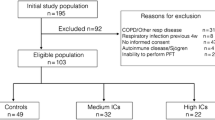
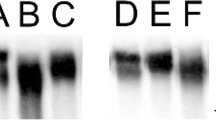
The present study was a cohort study conducted on the patients referred to Children’s Medical Center for whom a diagnosis of Asthma was confirmed, and inhaled corticosteroid therapy was prescribed. Saliva was sampled once at the stage of diagnosis and before receiving any treatment. Another salivary sample was collected 4 weeks after receiving corticosteroids. ELISA was performed to assess beta-defensin 2.
Results
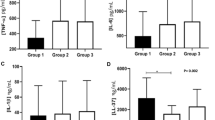
The beta-defensin 2 salivary level after inhaled corticosteroid therapy was significantly lower than before treatment. There is no significant difference in the salivary flow rate before and after treatment.
Conclusions
Considering the limitations of the present study, the following conclusions can be made salivary beta-defensin 2 is decreased in children with asthma after treatment with a corticosteroid inhaler. Regular dental and oral soft tissue examinations in Asthmatic children under corticosteroid therapy could be suggested.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Allen DB. Inhaled Corticosteroids and Endocrine Effects in Childhood. Endocrinol Metab Clin North Am. 2020;49:651–65. https://doi.org/10.1016/j.ecl.2020.07.003.
Arafa A, Aldahlawi S, Fathi A. Assessment of the oral health status of asthmatic children. Eur J Dent. 2017;11:357–63. https://doi.org/10.4103/ejd.ejd_65_17.
Cherkasov SV, Popova LY, Vivtanenko TV, Demina RR, Khlopko YA, Balkin AS, Plotnikov AO. Oral microbiomes in children with asthma and dental caries. Oral Dis. 2019;25:898–910. https://doi.org/10.1111/odi.13020.
Cogen AL, Walker SL, Roberts CH, Hagge DA, Neupane KD, Khadge S, Lockwood DN. Human beta-defensin 3 is up-regulated in cutaneous leprosy type 1 reactions. PLoS Negl Trop Dis. 2012;6: e1869.
Del-Rio-navarro BE, Corona-Hernandez L, Fragoso-Rios R, Berber A, Torres-Alcantara S, Cuairan-Ruidiaz V, Sienra-Monge JJ. Effect of salmeterol and salmeterol plus beclomethasone on saliva flow and IgA in patients with moderate-persistent chronic asthma. Ann Allergy Asthma Immunol. 2001;87:420–3. https://doi.org/10.1016/s1081-1206(10)62925-0.
Ektah K, Arun RJ, Elza T. Oral findings in asthmatic children. Amrita J Med. 2014;10:1–4.
Gani F, Caminati M, Bellavia F, Baroso A, Faccioni P, Pancera P, Batani V, Senna G. Oral health in asthmatic patients: a review : asthma and its therapy may impact on oral health. Clin Mol Allergy. 2020;18:22. https://doi.org/10.1186/s12948-020-00137-2.
Ghosh SK, McCormick TS, Weinberg A. Human beta defensins and cancer: contradictions and common ground. Front Oncol. 2019;9:341.
Hernandez-Pacheco N, Gorenjak M, Li J, Repnik K, Vijverberg SJ, Berce V, Jorgensen A, Karimi L, Schieck M, Samedy-Bates L-A. Identification of ROBO2 as a potential locus associated with inhaled corticosteroid response in childhood asthma. J Person Med. 2021;11:733.
Kulhava L, Eckhardt A, Pataridis S, Foltan R, Miksik I. Proteomic analysis of whole saliva in relation to dental caries resistance. Folia Biol (Praha). 2020;66:72–80.
Kulkarni NN, Gunnarsson HI, Yi Z, Gudmundsdottir S, Sigurjonsson OE, Agerberth B, Gudmundsson GH. Glucocorticoid dexamethasone down-regulates basal and vitamin D3 induced cathelicidin expression in human monocytes and bronchial epithelial cell line. Immunobiology. 2016;221:245–52. https://doi.org/10.1016/j.imbio.2015.09.001.
Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int. 2014;111:847–55. https://doi.org/10.3238/arztebl.2014.0847.
Lotan I, Ganelin-Cohen E, Tartakovsky E, Khasminsky V, Hellmann MA, Steiner I, Ben-Zvi I, Livneh A, Golderman S, Kaplan B. Saliva immunoglobulin free light chain analysis for monitoring disease activity and response to treatment in multiple sclerosis. Mult Scler Relat Disord. 2020;44:102339. https://doi.org/10.1016/j.msard.2020.102339.
Macredmond RE, Greene CM, Dorscheid DR, McElvaney NG, O’Neill SJ. Epithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smoke. Respir Res. 2007;8:1–12.
Maneechotesuwan K, Singh D, Fritscher LG, Dursunoglu NPGA, Phansalkar A, Aggarwal B, Pizzichini E, Chorazy J, Burnett H. Impact of inhaled fluticasone propionate/salmeterol on health-related quality of life in asthma: a network meta-analysis. Respir Med. 2022;203:106993. https://doi.org/10.1016/j.rmed.2022.106993.
Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121:121S-126S. https://doi.org/10.1378/chest.121.5_suppl.121s.
Marsh PD, Do T, Beighton D, Devine DA. Influence of saliva on the oral microbiota. Periodontol. 2016;2000(70):80–92. https://doi.org/10.1111/prd.12098.
Navazesh M, Kumar SK. Measuring salivary flow: challenges and opportunities. J Am Dent Assoc. 2008;139:35S-40S.
O’Byrne P, Fabbri LM, Pavord ID, Papi A, Petruzzelli S, Lange P. Asthma progression and mortality: the role of inhaled corticosteroids. Eur Respir J. 2019;54:1900491. https://doi.org/10.1183/13993003.00491-2019.
Peona V, Amici MD, Quaglini S, Bellaviti G, Castellazzi AM, Marseglia G, Ciprandi G. Serum eosinophilic cationic protein: is there a role in respiratory disorders? J Asthma. 2010;47:131–4.
Sag C, Ozden FO, Acikgoz G, Anlar FY. The effects of combination treatment with a long-acting beta2-agonist and a corticosteroid on salivary flow rate, secretory immunoglobulin A, and oral health in children and adolescents with moderate asthma: a 1-month, single-blind clinical study. Clin Ther. 2007;29:2236–42. https://doi.org/10.1016/j.clinthera.2007.10.014.
Salem A, Almahmoudi R, Hagstrom J, Stark H, Nordstrom D, Salo T, Eklund KK. Human beta-defensin 2 expression in oral epithelium: potential therapeutic targets in oral lichen planus. Int J Mol Sci. 2019;20:1780. https://doi.org/10.3390/ijms20071780.
Salvatori O, Puri S, Tati S, Edgerton M. Innate immunity and saliva in candida albicans-mediated oral diseases. J Dent Res. 2016;95:365–71. https://doi.org/10.1177/0022034515625222.
Singanayagam A, Glanville N, Cuthbertson L, Bartlett NW, Finney LJ, Turek E, Bakhsoliani E, Calderazzo MA, Trujillo-Torralbo MB, Footitt J, James PL, Fenwick P, Kemp SV, Clarke TB, Wedzicha JA, Edwards MR, Moffatt M, Cookson WO, Mallia P, Johnston SL. Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease. Sci Transl Med. 2019;11:eaav3879. https://doi.org/10.1126/scitranslmed.aav3879.
Terai K, Sano Y, Kawasaki S, Endo K, Adachi W, Hiratsuka T, Ihiboshi H, Nakazato M, Kinoshita S. Effects of dexamethasone and cyclosporin A on human beta-defensin in corneal epithelial cells. Exp Eye Res. 2004;79:175–80. https://doi.org/10.1016/j.exer.2004.03.006.
van der Velden HM, Pasch MC, van Erp PE, van Lingen RG, Otero ME, de Boer-van Huizen RT, van de Kerkhof PC. Treatment of plaque psoriasis with the two-compound product calcipotriol/betamethasone dipropionate versus both monotherapies: an immunohistochemical study. J Dermatol Treat. 2010;21:13–22.
Wozniak M, Paluszkiewicz C, Kwiatek W. Saliva as a non-invasive material for early diagnosis. Acta Biochim Pol. 2019;66:383–8.
Yang D, Liu ZH, Tewary P, Chen Q, de la Rosa G, Oppenheim JJ. Defensin participation in innate and adaptive immunity. Curr Pharm Des. 2007;13:3131–9. https://doi.org/10.2174/138161207782110453.
Funding
None.
Author information
Authors and Affiliations
Contributions
MSM and MSH: contributed to conception and design, drafted manuscript, gave final approval, PSH: contributed to acquisition and interpretation, drafted manuscript, gave final approval and GP: contributed to analysis and interpretation, drafted manuscript, gave final approval.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The study protocol was reviewed and approved by the Ethics Committee of the Tehran University of Medical Sciences (No.IR.TUMS.DENTISTRY.REC.1398.090) and all methods were carried out in accordance with relevant guidelines and regulations (declaration of Helsinki).
Consent to participate
Written informed consent was also obtained from the children's parents before entering the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moosavi, MS., Hosseinizade, PS., Panahi, G. et al. Decreased salivary beta-defensin 2 in children with asthma after treatment with corticosteroid inhaler. Eur Arch Paediatr Dent 24, 249–254 (2023). https://doi.org/10.1007/s40368-022-00776-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40368-022-00776-w