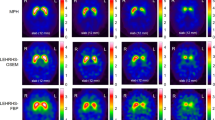
Abstract
Purpose
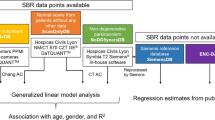
The purpose of this study was to create 123I-FP-CIT reference values for ultra-high-resolution fan beam collimators (UHR-FB) from a sample of subjects without dopaminergic degeneration and to compare them to a normal database -PPMI database- of a commercial software (DaTQUANT) obtained using high-resolution parallel-hole collimators (HR-PH).
Methods
A striatal phantom study was performed to compare UHR-FB with HR-PH and to obtain a correction factor between collimators. Normal 123I-FP-CIT studies from 177 subjects acquired using UHR-FB were retrospectively selected on the basis of visual and semi-quantitative analysis as well as of the neurological follow-up (range of 2–9 years). SPECT images were reconstructed using the same parameters of DaTQUANT normal database and SBR values were obtained for striatal structures. Correction factor was applied to the UHR-FB database to test differences against DaTQUANT database.
Results
Correction factor obtained from the phantom study was 0.84. Uncorrected SBR values of the local database were significantly higher than PPMI database values, but no significant differences were found using corrected values. Coefficients of variations of SBR values were significantly lower in a local database than PPMI database (15% vs 20%). Significant effects of age on SBR were observed in both databases with a reduction rate for a decade of 6% in the PPMI database and 4.5% in the local database. In the latter, women had slightly higher SBR values and a steeper decline with advancing age compared to men, whereas no significant gender differences were found in the PPMI database.
Conclusion
The SBR values obtained using UHR-FB have an age-related distribution comparable to that of healthy subjects but with lower variability. The reduction rate per decade was similar between the two databases but the gender effect was found only in the local database, probably related to the better performance of UHR-FB.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
SPECT imaging of dopamine transporter (DAT) is one of the most widely applied procedures to evaluate the integrity of presynaptic dopaminergic pathways and to differentiate degenerative parkinsonian syndromes (PS) from essential tremor and other causes of parkinsonism (normal pressure hydrocephalus, drug-induced, psychogenic or vascular parkinsonism) or dementia (e.g. Lewy Body Dementia from Alzheimer’s disease) [1]. DAT-SPECT was also used as a neuroimaging biomarker in clinical and research trials of Parkinson’s disease, the most frequent form of degenerative PS, for investigating the rate of degeneration and evaluating the efficacy of protective/reparative treatments [2,3,4]. Several studies have documented that the specific binding ratio (SBR) obtained with SPECT tracers (123I-beta-CIT, 123I-FP-CIT, 99mTc-TRODAT), although not an absolute measure of DAT density, represents a useful outcome measure being capable to detect subtle changes in DAT availability not only in the early motor stage of the disease but also in the preclinical and prodromal stages [5,6,7,8,9,10,11]. Based on a large scientific evidence, DAT-SPECT has been recently qualified as an enrichment biomarker for PD trials by EMA [12].
Nevertheless, some issues in SBR quantification need to be understood, since the high variability of data may have a significant impact on the study outcomes both in multicentre than in longitudinal single-centre trials. The quantitative values derived from SPECT depend on biological, technical and methodological factors. The effects of technical and methodological factors on SBR measurements, such as camera sensitivity, image reconstruction, region of interest sampling have been analysed in several studies [13,14,15] and standardization protocols have been provided to improve the image quality and quantification accuracy [16,17,18].
The application of these protocols has allowed the creation of normative databases from a large number of healthy subjects covering a large age range and, thus, to investigate the effects of biological factors (age, gender) which are in turn sources of variance [19,20,21]. An age-related decline of SBR values of about 5–8% per decade was found in healthy subjects and a higher DAT availability was observed in women even if clear evidence of gender influence was not consistent between the studies. Similar results were also reported in other studies with large series of subjects without degenerative parkinsonism [22,23,24].
Even so, the variance of SBR values remains high with coefficients of variation of about 20% in healthy subjects and of about 30% in PD patients, indicating that the line of demarcation between normal and abnormal findings can be unclear especially in the preclinical stages or changes in SBR measures may not be appreciable in longitudinal studies [25,26,27,28]. This issue may be influenced also by other technical factors, such as the limited spatial resolution of SPECT imaging. The majority of DAT-SPECT studies are performed using high-resolution parallel-hole (HR-PH) collimators that are affected by the trade-off between spatial resolution and sensitivity. Collimators with different geometry (fan beam, multi-pinhole) have been proposed to improve both spatial resolution and sensitivity. A recent study using multi-pinhole (MPH) collimators showed a significant improvement in intra- and between-rater agreement and visual interpretation of DAT images compared to conventional HR-PH collimators [29]. The putamen SBR estimates with MPH were higher in normal subjects and PS patients than with HR-PH and the difference between normal and reduced values was slightly superior even if the effect size was similar in MPH and HR-PH settings.
Fan-beam collimators, particularly ultra-high-resolution fan beam (UHR-FB), have a superior trade-off between spatial resolution and sensitivity than HR-PH collimators and can provide more accurate measures. However, to our knowledge, the direct comparison between SBR values from HR-PH and UHR-FB collimators has not been investigated yet.
The aim of the present study was to create 123I-FP-CIT reference values using UHR-FB collimators from a sample of control subjects without dopaminergic degeneration and to compare and validate them against reference values derived from a normal database of DaTQUANT software (DaTQUANT™ 2.0 Stand Alone) including healthy volunteers enrolled in the Parkinson’s Progression Markers Initiative (PPMI).
Materials and methods
Striatal phantom
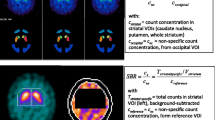
An anthropomorphic striatal phantom (Radiology Support Devices Inc., Long Beach, CA) was filled using solutions at different 123I concentrations, in particular 40 kBq/ml for the left striatum, 29 kBq/ml for the right striatum and 6.3 kBq/ml for the background, to achieve striatal to background ratios of 7:1 and 5:1 in left and right striatum, respectively.
SPECT scans were performed using a dual-head gamma camera (Discovery NM 630, GE Healthcare) equipped with HR-PH and UHR-FB collimators, in the same session with a time interval of about 80 min, a total scan time of 30 min for each scan and a rotation radius of 16 cm. The other acquisition parameters were the same as in the clinical setting (see Local database). The reconstruction protocol included ordered subset-expectation maximization (OSEM) algorithm with 2 iterations and 10 subsets, a 0.6 Butterworth post-filter and attenuation correction with the Chang method (coefficient 0.11 cm−1).
A CT scan of the phantom (90 slices of 512 × 512 pixels, pixel size of 0.49 mm, slice thickness of 2 mm) was acquired with the four striatal compartments filled with water and the brain shell cavity left empty. CT images were used for volume of interest (VOI) delineation, and not for attenuation correction purposes.
Data processing and analysis of the phantom images were conducted using Mango software (Research Imaging Institute, UTHSCSA). The HR-PH and UHR-FB images were co-registered and fused to the CT images using a point-matching method. Anatomical VOIs were automatically created for the left and right caudate nucleus and for left and right putamen (Fig. 1). Moreover, VOIs were manually drawn in the posterior region of the brain shell and extended over the same slices in which striata were present, keeping sufficiently far from them to avoid spillover effects. The latter VOIs were used as reference regions for determining the ratios between activity in the four striatal compartments and in the background as in the clinical setting:
We also performed the analysis of the HR-PH and UHR-FB phantom images with the DaTQUANT software using the factory default settings.
PPMI database
For this study, we used data from 117 healthy volunteers (44 women, 73 men, age range of 31–84 years) embedded in the DaTQUANT database, whom participated in PPMI [30]. Details of the PPMI 123I-FP-CIT SPECT protocol are given at https://www.ppmi-info.org/study-design/research-documents-and-sops. Briefly, imaging scans started 3–4 h after the intravenous injection of 123I-FP-CIT using a SPECT system equipped with low-energy HR-PH collimators, a circular step and shoot mode, 120 projections of 30 s, a 20% energy window centered on 159 keV (± 10%).
The reconstruction protocol set by the DaTQUANT manufacturer included OSEM algorithm with 2 iterations and 10 subsets, a 0.6 Butterworth post-filter and attenuation correction with the Chang method (coefficient 0.11 cm−1).
Local database
Among all the patients examined with 123I-FP-CIT SPECT for differential diagnosis of movement disorder or dementia and referred by the Neurology and Parkinson Units of our University Hospital between January 2013 and July 2020, we selected 177 subjects (105 women, 72 men, age range 20–90 years) on the basis of following criteria:
-
1.
normal SPECT scan at the visual inspection and semiquantitative analysis of SPECT image;
-
2.
caudate-to-putamen ratios and asymmetry indices within the corresponding ranges of the PPMI database to avoid subjects with pre-clinical degenerative PS;
-
3.
neurological evaluation at follow-up (range 2–9 years) excluding degenerative PS.
SPECT scans were performed starting 3–4 h after intravenous administration of 123I-FP-CIT (dose of 185 MBq) using a dual-head gamma camera (Discovery NM 630, GE Healthcare) equipped with UHR-FB collimators, 120 projections in step and shoot mode, a 20% energy window centered on 159 keV (± 10%). The radius of rotation varied between 15 and 17 cm and the scanning time lasted 40 min.
The projection data downloaded from the PACS of the Diagnostic Imaging Department were reconstructed and processed using the DaTQUANT software, using OSEM algorithm with 2 iterations and 10 subsets, a 0.6 Butterworth post-filter and attenuation correction with Chang method (coefficient 0.11 cm−1). After image reconstruction, DaTQUANT automatically registers SPECT images to an MRI template in MNI space and positions predefined VOIs in the target regions, including striatum, caudate nucleus, putamen, and in occipital cortex. The software automatically computes bilateral specific to non-displaceable binding ratios (SBRs) in putamen and caudate nucleus using the occipital cortex as a reference region.
Statistical analysis
SPSS Statistics (IBM version 29, SPSS Inc.) was used for all statistical analyses.
Differences between SBR ratios using HR-PH and UHR-FB were evaluated by paired T test. The relationship between SBR ratios with HR-PH and UHR-FB collimators of the phantom study was visualised using scatter plots and described by simple linear regression:
where CF is the correction factor between the two collimators.
To compensate for the effects of different collimators, the correction factor was applied to the original SBR values of the local database; both uncorrected and corrected values were used for the comparison with the PPMI database. Differences between databases were evaluated by the analysis of variance (ANOVA) and Bonferroni correction for multiple comparisons.
Linear regression analysis was performed to evaluate differences in the distribution of SBR values as a function of age between the PPMI and local database. Differences in regression coefficients were evaluated according to the following formula [31]:
where \({r}_{1}^{\prime}\) and \({r}_{2}^{\prime}\) are the Fisher’s r to z transformation of the coefficients r1 and r2 and n1 and n2 are the corresponding sample sizes. Moreover, multiple linear regression analysis was performed to analyse the effects of age and gender on SBR values in both databases.
Level of statistical significance was set at P value less than 0.05.
Results
In the phantom study a linear relationship was found between the SBR ratios obtained with the two collimators both in the analysis with Mango software, where the VOIs were created for left and right caudate and putamen based on the anatomical shape on the co-registered CT and in that made with DaTQUANT software, with automatically pre-defined VOIs, as shown in Fig. 2A and B, respectively, where the HR-PH are plotted against those with UHR-FB. The regressions were constrained through the origin, although the lines were practically passing through the origin even without constraint. In addition, in both cases the measures using UHR-FB were significantly higher than using HR-PH (mean values 2.35 ± 0.46 vs 1.96 ± 0.41, P < 0.005 and 1.86 ± 0.38 vs 1.54 ± 0.46, P < 0.05), with resulting CF of 0.84 and 0.837, respectively.
Demographic data of local and PPMI databases are summarized in Table 1. The first one included 177 subjects, of whom 107 were women (59%) and 72 men (41%), with a mean age 68.9 ± 13.4. Final clinical diagnoses were essential tremor (50%), drug-induced parkinsonism (15%), vascular parkinsonism (10%), normal pressure hydrocephalus (7%), Alzheimer’s disease (6%) and various conditions not associated with parkinsonism (12%).
SBR values in each striatal region of the two databases are reported in Table 2. The uncorrected UHR-FB SBR values of the local database were significantly higher than the HR-PH SBR values of the PPMI database in all striatal regions (P < 0.001). No significant differences were found between the SBR values of the PPMI database and the corrected SBR values of the local database, indicating a good harmonization of the different imaging systems.
The coefficients of variations of SBR values were significantly lower for the local database than the PPMI database (15% vs 20%, P < 0.001). Moreover, in each database there were no significant differences between the right and left regions, nor between the right and left caudate-to-putamen ratios.
Figure 3 illustrates the distribution of SBR values as a function of age and the regression lines observed in the two databases. No significant differences in regression coefficients were found between the two databases, indicating that the slopes of the two regression lines were similar and the different data distribution was only due to a higher intercept value for the uncorrected values of the local database (Fig. 3a). Indeed, no significant differences in the intercept values were found between the SBR values of the PPMI database and the corrected ones of the local database and the distribution of two datasets was similar with almost complete overlapping of 95% confidence intervals (Fig. 3b).
Scatterplot of the SBR measures versus age in the PPMI (full circles) and local database (open circles). The uncorrected (a) and corrected (b) values of the local database are reported. The full lines represent the linear fit for the PPMI (black) and local (red) database. The respective 95% confidence intervals are also shown following the same colour code
Multiple regression analysis of SBR was performed using age and gender as independent variables and for the local database the corrected measures were used as dependent variable. The relationship between age and SBR in women and men is shown in Fig. 4. Data of DAT availability and rate of reduction in SBR per decade are reported in Table 3.
In the PPMI database, there were no gender differences in SBR values in the caudate nucleus and putamen (2.87 ± 0.53 and 2.54 ± 0.48 in women, 2.76 ± 0.53 and 2.44 ± 0.43 in men; Table 3). A significant linear decline with advancing age was found in both caudate nucleus and putamen (r = − 0.483 and r = − 0.508, respectively, P < 0.001; Fig. 4a) with a similar rate of reduction per decade for women and men (6.3% and 6.4% in women and 6.6% and 6.2% in men; Table 3).
In the local database, women showed significantly higher SBR values in the caudate nucleus and putamen (2.87 ± 0.45 and 2.46 ± 0.38, P < 0.005) than men (2.65 ± 0.40 and 2.29 ± 0.33; Table 3). The percentage difference in mean SBR values between women and men was about 12.5% in the 20–39 age group and decreased to about 5.1% in the 70–89 age group. A significant linear decline with advancing age was found in the caudate nucleus and putamen.
(r = -0.492 and r = -0.482 respectively, P < 0.001; Fig. 4b) with a slightly higher, although not significant, rate of reduction per decade for women (5.0% and 5.0%) than for men (4.1% and 4.4%; Table 3).
Discussion
This study was designed to generate a large reference database of SBR values obtained using SPECT and UHR-FB collimators from a cohort of patients with normal 123I-FP-CIT scans and without nigrostriatal degeneration as confirmed at neurological follow-up of 2–9 years. The validity of our local database was tested by means of comparison with a normal database from healthy subjects enrolled in PPMI after harmonization of imaging system performance.
To compare the SBR values of the local database with those of the PPMI database, a phantom study was performed to obtain a correction factor between UHR-FB and HR-PH collimators. A linear relationship between the measured ratios with the two collimators showed an accurate fit of data and the correction factor was calculated from the regression equation. The phantom study demonstrated a better performance of UHR-FB collimators providing higher estimates of SBR ratios, an expected result considering higher spatial resolution and sensitivity of UHR-FB collimators. However, these results cannot be considered actual estimates of the quantification accuracy of the two collimators due to the limited number of filling ratios performed in our phantom study.
In line with the phantom study, the uncorrected SBR measures obtained with UHR-FB collimators for the local database were significantly higher and showed lower coefficients of variation (− 25%) than with HR-PH for the PPMI database. By applying the correction factor for the system response, harmonization of quantitative metrics was obtained and the corrected SBR measures of the local database completely overlapped with those of the PPMI database, but the 25% reduction in coefficients of variation remained unchanged. These results indicate that our group of patients without degenerative parkinsonism may be used as a normal database and the lower variability in SBR may be related to the better spatial resolution of UHR-FB collimators.
The corrected SBR measures of our local database are also consistent with the uncalibrated data from healthy volunteers in the ENC-DAT multicentre study obtained with analogous reconstruction, attenuation correction and ROI delineation methods [19]. Our data are not comparable to the findings of the Japanese multicentre study [20], in which, however, ROI analysis was performed with the Southampton method that is less dependent on partial volume effects with respect to anatomical VOI analysis. Similarly, the comparison with other databases obtained from patients without degenerative parkinsonism and with normal SPECT images [22,23,24] is difficult since in these studies procedures of compensation for resolution recovery, attenuation with CT and/or scatter were used for correcting image-degrading effects and increasing quantification accuracy of SBR measurements [32, 33]. However, the variability of 15% reported in our study using only attenuation correction is comparable to the variability of 14–16% with two or more compensation methods in the latter studies as well as of 13.8% in the ENC-DAT dataset after re-evaluation of the reconstruction procedure [18]. Taking into account the different contributions of degrading factors [31], it is possible to hypothesize that the variability obtained in our study can be reduced to less than 10% by the application of compensation methods for scatter and septal penetration, such as transmission-dependent convolution subtraction (TDCS) avoiding enhancement of statistical noise [34]. These observations evidenced that spatial resolution is the major factor contributing to variability in brain imaging [35] and that using of high-resolution SPECT systems may be one way to reduce variability in SBR measurements. This may have important implications in diagnostics and clinical trials, as to help to more accurately identify patients in the early or premotor stage and select those for follow-up. Moreover, assessing statistically significant changes in less variable SBR values could require smaller sample sizes or shorter follow-up times than currently.
In our study, a significant linear effect of age on SBR values was found in the striatal regions of PPMI and local database with mean reduction rates per decade of about 6% and 4.5%, respectively, consistent with findings of previous studies, in which the overall age-related decline varied between about 4% and 7% per decade [19,20,21,22,23, 36,37,38]. Moreover, we observed higher DAT availability in women than in men of the local database, but gender differences were not found in the PPMI database. Similar conflicting findings have been reported in the literature with some studies describing higher DAT availability in women than in men [19, 20, 22] and with others showing no significant gender differences [36,37,38]. We hypothesize that similar discrepancies may depend on reconstruction and compensation methods that, by reducing the variability in SBR values, highlight different effects on DAT availability in women and men.
Another interesting aspect that we have reflected upon during the analysis that we have carried out is the possibility of reducing the small differences that have been found in the SBR values for the two different collimators, for example by modifying the reconstruction parameters, including Chang attenuation correction coefficient; however, this investigation goes beyond the scope of our study but constitutes a starting point for future analysis.
This study has some limitations. First, we selected a large cohort of patients with normal 123I-FP-CIT SPECT, having a wide age range (20–90 years) and a well-balanced gender distribution of 59% women and 41% men. However, there was a prevalence of women aged 60–90 years. This slight difference might have contributed to the different gender-related declines, although only in part since the major difference in SBR values between women and men was found in the 20–39 age group. The gender-related difference is in agreement with some previous studies [19,20,21,22,23] and, as suggested, might be related to higher dopamine transmission or turnover in women than in men due to hormonal effects or to the larger volume of the striatum in men compared with women. Recently, Honkanen et al. [39] indicated the need to implement sex correction in automated analysis software to reduce the risk of false-positive findings for men. This aspect may play an important role not only in the clinical setting to avoid inaccurate diagnoses but also in preclinical and prodromal trials to better identify subjects at risk for degenerative parkinsonism.
Moreover, our local database was established using data from subjects with clinically uncertain parkinsonism. Diagnoses were based on clinical criteria and neurological follow-up of 2–9 years that excluded the presence of degenerative parkinsonism, even if it may be possible that in our local database some subjects might be affected by pre-clinical degenerative conditions. However, the complete overlap between the corrected SBR measures of our local database with those of the PPMI database and the similar distribution across age tend to demonstrate the goodness of our local database.
Finally, to harmonize the differences in performance between HR-PH and UHR-FB collimators we performed a phantom study using the same camera model. In this way, differences in performance between the SPECT cameras used for our local database and for PPMI database are not incorporated into the correction factor so determined and, as a result, the corrected measures for our local database could be over- or underestimated. Tossici-Bolt et al. [15] examined the camera/collimator performance of 17 SPECT systems and found that the underestimation of the measured SBR ratios with attenuation correction varied between 11 and 31% with a higher difference in inter-system performance of 22%. Therefore, it is possible that the remaining differences in performance between SPECT systems used for local database and the PPMI database may not lead to substantial changes in the data. In any case, the true correction factor being a multiplicative factor would have no effect on the variability of the corrected SBR measures and on the variations according to age or gender found in our local database.
Conclusion
This study provides a large local database of 123I-FP-CIT SPECT using UHR-Fan Beam collimators having age-related distribution comparable to that of healthy subjects of the PPMI database. Our quantitative results obtained with OSEM reconstruction and attenuation correction show that UHR-Fan Beam collimators provide reduced variability of the SBR measures compared to HR parallel-hole collimators. We expect that using compensation procedures for scatter and/or other degrading factors, the variability in SBR may be reduced to less than 10%.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Morbelli S, Esposito G, Arbizu J, Barthel H, Boellaard R, Bohnen NI, Brooks DJ, Darcourt J, Dickson JC, Douglas D, Drzezga A, Dubroff J, Ekmekcioglu O, Garibotto V, Herscovitch P, Kuo P, Lammertsma A, Pappata S, Peñuelas I, Seibyl J, Semah F, Tossici-Bolt L, Van de Giessen E, Van Laere K, Varrone A, Wanner M, Zubal G, Law I (2020) EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-020-04817-8
Marek K, Innis R, van Dyck C, Fussell B, Early M, Eberly S, Oakes D, Seibyl J (2001) [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology. https://doi.org/10.1212/wnl.57.11.2089
Parkinson Study Group (2002) Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. https://doi.org/10.1001/jama.287.13.1653
Parkinson Study Group (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med. https://doi.org/10.1056/NEJMoa033447
Winogrodzka A, Bergmans P, Booij J, van Royen EA, Stoof JC, Wolters EC (2003) [(123)I]beta-CIT SPECT is a useful method for monitoring dopaminergic degeneration in early stage Parkinson’s disease. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp.74.3.294
Jennings D, Siderowf A, Stern M, Seibyl J, Eberly S, Oakes D, Marek K, PARS Investigators (2014) Imaging prodromal Parkinson disease: the Parkinson Associated Risk Syndrome Study. Neurology. https://doi.org/10.1212/WNL.0000000000000960
Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters ECh, Berendse HW (2004) Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol. https://doi.org/10.1002/ana.20160
Postuma RB, Iranzo A, Hu M, Högl B, Boeve BF, Manni R, Oertel WH, Arnulf I, Ferini-Strambi L, Puligheddu M, Antelmi E, Cochen De Cock V, Arnaldi D, Mollenhauer B, Videnovic A, Sonka K, Jung KY, Kunz D, Dauvilliers Y, Provini F, Lewis SJ, Buskova J, Pavlova M, Heidbreder A, Montplaisir JY, Santamaria J, Barber TR, Stefani A, St Louis EK, Terzaghi M, Janzen A, Leu-Semenescu S, Plazzi G, Nobili F, Sixel-Doering F, Dusek P, Bes F, Cortelli P, Ehgoetz Martens K, Gagnon JF, Gaig C, Zucconi M, Trenkwalder C, Gan-Or Z, Lo C, Rolinski M, Mahlknecht P, Holzknecht E, Boeve AR, Teigen LN, Toscano G, Mayer G, Morbelli S, Dawson B, Pelletier A (2019) Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. https://doi.org/10.1093/brain/awz030
Arnaldi D, Chincarini A, Hu MT, Sonka K, Boeve B, Miyamoto T, Puligheddu M, De Cock VC, Terzaghi M, Plazzi G, Tachibana N, Morbelli S, Rolinski M, Dusek P, Lowe V, Miyamoto M, Figorilli M, Verbizier D, Bossert I, Antelmi E, Meli R, Barber TR, Trnka J, Miyagawa T, Serra A, Pizza F, Bauckneht M, Bradley KM, Zogala D, McGowan DR, Jordan L, Manni R, Nobili F (2021) Dopaminergic imaging and clinical predictors for phenoconversion of REM sleep behaviour disorder. Brain. https://doi.org/10.1093/brain/awaa365
Chahine LM, Brumm MC, Caspell-Garcia C, Oertel W, Mollenhauer B, Amara A, Fernandez-Arcos A, Tolosa E, Simonet C, Hogl B, Videnovic A, Hutten SJ, Tanner C, Weintraub D, Burghardt E, Coffey C, Cho HR, Kieburtz K, Poston KL, Merchant K, Galasko D, Foroud T, Siderowf A, Marek K, Simuni T, Iranzo A (2021) Dopamine transporter imaging predicts clinically-defined α-synucleinopathy in REM sleep behavior disorder. Ann Clin Transl Neurol. https://doi.org/10.1002/acn3.51269
Janzen A, Vadasz D, Booij J, Luster M, Librizzi D, Henrich MT, Timmermann L, Habibi M, Sittig E, Mayer G, Geibl F, Oertel W (2022) Progressive olfactory impairment and cardiac sympathetic denervation in REM sleep behavior disorder. J Parkinsons Dis. https://doi.org/10.3233/JPD-223201
Conrado DJ, Nicholas T, Tsai K, Macha S, Sinha V, Stone J, Corrigan B, Bani M, Muglia P, Watson IA, Kern VD, Sheveleva E, Marek K, Stephenson DT, Romero K (2018) Dopamine transporter neuroimaging as an enrichment biomarker in early Parkinson’s disease clinical trials: a disease progression modeling analysis. Clin Transl Sci. https://doi.org/10.1111/cts.12492
Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS (2006) Quantification of [(123)I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-006-0155-x
Dickson JC, Tossici-Bolt L, Sera T, Erlandsson K, Varrone A, Tatsch K, Hutton BF (2010) The impact of reconstruction method on the quantification of DaTSCAN images. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-009-1212-z
Tossici-Bolt L, Dickson JC, Sera T, de Nijs R, Bagnara MC, Jonsson C, Scheepers E, Zito F, Seese A, Koulibaly PM, Kapucu OL, Koole M, Raith M, George J, Lonsdale MN, Münzing W, Tatsch K, Varrone A (2011) Calibration of gamma camera systems for a multicentre European 123I-FP-CIT SPECT normal database. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-011-1801-5
Tossici-Bolt L, Dickson JC, Sera T, Booij J, Asenbaun-Nan S, Bagnara MC, Borght TV, Jonsson C, de Nijs R, Hesse S, Koulibaly PM, Akdemir UO, Koole M, Tatsch K, Varrone A (2017) [(123)I]FP-CIT ENC-DAT normal database: the impact of the reconstruction and quantification methods. EJNMMI Phys. https://doi.org/10.1186/s40658-017-0175-6
Dickson JC, Tossici-Bolt L, Sera T, de Nijs R, Booij J, Bagnara MC, Seese A, Koulibaly PM, Akdemir UO, Jonsson C, Koole M, Raith M, Lonsdale MN, George J, Zito F, Tatsch K (2012) Proposal for the standardisation of multi-centre trials in nuclear medicine imaging: prerequisites for a European 123I-FP-CIT SPECT database. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-011-1884-z
Buchert R, Kluge A, Tossici-Bolt L, Dickson J, Bronzel M, Lange C, Asenbaum S, Booij J, Atay Kapucu LÖ, Svarer C, Koulibaly PM, Nobili F, Pagani M, Sabri O, Sera T, Tatsch K, Vander Borght T, Van Laere K, Varrone A, Iida H (2016) Reduction in camera-specific variability in [123I]FP-CIT SPECT outcome measures by image reconstruction optimized for multisite settings: impact on age dependence of the specific binding ratio in the ENC-DAT database of healthy controls. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-016-3309-5
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, Kapucu OL, Kluge A, Knudsen GM, Koulibaly PM, Nobili F, Pagani M, Sabri O, Vander Borght T, Van Laere K, Tatsch K (2013) European multicentre database of healthy controls for [123 I] FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-012-2276-8
Matsuda H, Murata M, Mukai Y, Sako K, Ono H, Toyama H, Inui Y, Taki Y, Shimomura H, Nagayama H, Tateno A, Ono K, Murakami H, Kono A, Hirano S, Kuwabara S, Maikusa N, Ogawa M, Imabayashi E, Sato N, Takano H, Hatazawa J, Takahashi R (2018) Japanese multicenter database of healthy controls for [123 I] FP-CIT SPECT. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-018-3976-5
Schmitz-Steinkrüger H, Lange C, Apostolova I, Mathies FL, Frings L, Klutmann S, Hellwig S, Meyer PT, Buchert R (2021) Impact of age and sex correction on the diagnostic performance of dopamine transporter SPECT. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-020-05085-2
Nicastro N, Garibotto V, Poncet A, Badoud S, Burkhard PR (2016) Establishing on-site reference values for 123 I-FP-CIT SPECT (DaTscan®) using a cohort of individuals with non-degenerative conditions. Mol Imaging Biol. https://doi.org/10.1007/s11307-015-0889-6
Fahmi R, Platsch G, Sadr AB, Gouttard S, Thobois S, Zuehlsdorff S, Scheiber C (2020) Single-site 123I-FP-CIT reference values from individuals with non-degenerative parkinsonism-comparison with values from healthy volunteers. Eur J Hybrid Imaging. https://doi.org/10.1186/s41824-020-0074-2
Schmitz-Steinkrüger H, Lange C, Apostolova I, Amthauer H, Lehnert W, Klutmann S, Buchert R (2020) Impact of the size of the normal database on the performance of the specific binding ratio in dopamine transporter SPECT. EJNMMI Phys. https://doi.org/10.1186/s40658-020-00304-z
Marek K, Seibyl J, Eberly S, Oakes D, Shoulson I, Lang AE, Hyson C, Jennings D, Parkinson Study Group PRECEPT Investigators (2014) Longitudinal follow-up of SWEDD subjects in the PRECEPT Study. Neurology. https://doi.org/10.1212/WNL.0000000000000424
Simuni T, Siderowf A, Lasch S, Coffey CS, Caspell-Garcia C, Jennings D, Tanner CM, Trojanowski JQ, Shaw LM, Seibyl J, Schuff N, Singleton A, Kieburtz K, Toga AW, Mollenhauer B, Galasko D, Chahine LM, Weintraub D, Foroud T, Tosun D, Poston K, Arnedo V, Frasier M, Sherer T, Chowdhury S, Marek K, Parkinson’s Progression Markers Initiative (2018) Longitudinal change of clinical and biological measures in early Parkinson’s disease: Parkinson’s progression markers initiative cohort. Mov Disord. https://doi.org/10.1002/mds.27361
Vavougios GD, Doskas T, Kormas C, Krogfelt KA, Zarogiannis SG, Stefanis L (2018) Identification of a prospective early motor progression cluster of Parkinson’s disease: data from the PPMI study. J Neurol Sci. https://doi.org/10.1016/j.jns.2018.01.025
Aleksovski D, Miljkovic D, Bravi D, Antonini A (2018) Disease progression in Parkinson subtypes: the PPMI dataset. Neurol Sci. https://doi.org/10.1007/s10072-018-3522-z
Mathies F, Apostolova I, Dierck L, Jacobi J, Kuen K, Sauer M, Schenk M, Klutmann S, Forgács A, Buchert R (2022) Multiple-pinhole collimators improve intra- and between-rater agreement and the certainty of the visual interpretation in dopamine transporter SPECT. EJNMMI Res. https://doi.org/10.1186/s13550-022-00923-w
Marek K, Chowdhury S, Siderowf A, Lasch S, Coffey CS, Caspell-Garcia C, Simuni T, Jennings D, Tanner CM, Trojanowski JQ, Shaw LM, Seibyl J, Schuff N, Singleton A, Kieburtz K, Toga AW, Mollenhauer B, Galasko D, Chahine LM, Weintraub D, Foroud T, Tosun-Turgut D, Poston K, Arnedo V, Frasier M, Sherer T, Parkinson’s Progression Markers Initiative (2018) The Parkinson’s progression markers initiative (PPMI)—establishing a PD biomarker cohort. Ann Clin Transl Neurol. https://doi.org/10.1002/acn3.644
Cohen J, Cohen P, West S, Aiken L (2013) Applied multiple regression/correlation analysis for the behavioural sciences, 3rd edn. Taylor and Francis, Milton Park
Cot A, Falcón C, Crespo C, Sempau J, Pareto D, Bullich S, Lomeña F, Calviño F, Pavía J, Ros D (2005) Absolute quantification in dopaminergic neurotransmission SPECT using a Monte Carlo-based scatter correction and fully 3-dimensional reconstruction. J Nucl Med 46:1497–1504
Crespo C, Gallego J, Cot A, Falcón C, Bullich S, Pareto D, Aguiar P, Sempau J, Lomeña F, Calviño F, Pavía J, Ros D (2008) Quantification of dopaminergic neurotransmission SPECT studies with 123I-labelled radioligands. A comparison between different imaging systems and data acquisition protocols using Monte Carlo simulation. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-007-0711-z
Iida H, Narita Y, Kado H, Kashikura A, Sugawara S, Shoji Y, Kinoshita T, Ogawa T, Eberl S (1998) Effect of scatter and attenuation correction on quantitative assessment of regional cerebral blood flow with SPECT. J Nucl Med 39:181–189
Joshi A, Koeppe RA, Fessler JA (2009) Reducing between scanner differences in multi-center PET studies. Neuroimage. https://doi.org/10.1016/j.neuroimage.2009.01.057
van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Wallace E, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Charney DS, Hoffer PB (1995) Age-related decline in striatal dopamine transporter binding with iodine-123-beta-CITSPECT. J Nucl Med 36:1175–1181
van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, Innis RB (2002) Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry 10:36–43
Ryding E, Lindstrom M, Brådvik B, Grabowski M, Bosson P, Träskman-Bendz L, Rosén I (2004) A new model for separation between brain dopamine and serotonin transporters in 123I-beta-CIT SPECT measurements: normal values and sex and age dependence. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-004-1489-x
Honkanen EA, Noponen T, Hirvilammi R, Lindholm K, Parkkola R, Joutsa J, Varrone A, Kaasinen V (2021) Sex correction improves the accuracy of clinical dopamine transporter imaging. EJNMMI Res. https://doi.org/10.1186/s13550-021-00825-3
Acknowledgements
We thank the nursing and technical staff of the Nuclear Medicine Unit at Florence University Hospital for their assistance to patients and for the acquisition of SPECT data.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. Work supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006)—A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by MTRDC, MP and VB. The first draft of the manuscript was written by FL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University of Florence CEAVC 25316 of 2024). This is an observational retrospective study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Cristofaro, M.T.R., Linguanti, F., Pepponi, M. et al. Reduction in variability of dopamine transporter measures using SPECT and ultra-high-resolution fan beam collimators: comparison with parallel-hole collimators in healthy controls for a clinical reference database. Clin Transl Imaging 12, 347–357 (2024). https://doi.org/10.1007/s40336-023-00613-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-023-00613-0