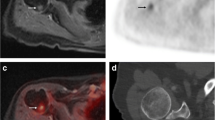
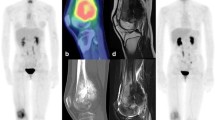
Abstract
Purpose
To review the existing literature on PET/MR for musculoskeletal malignancies.
Methods
PubMed (MEDLINE) was searched through July 2021. The search strategy focused on the keywords “PET/CT”, “PET/MR”, “MRI”, “attenuation correction”, “sarcoma”, “skeletal”, “metastases”, “myeloma”, “FDG”, “Sodium fluoride”, “PSMA”, “DOTATATE”, “fluciclovine”, and “FAPI”.
Results
97 relevant papers were considered for review. Studies selected were primarily retrospective and prospective cohort studies. There is limited literature comparing PET/CT to PET/MR for evaluation of primary musculoskeletal malignancies and musculoskeletal metastatic disease. Most studies involve relatively small numbers of patients.
Conclusions
Despite limited evidence and the lack of widespread application, PET/MR is a promising imaging technique for evaluating musculoskeletal malignancies. This is owing primarily to the strength of its constituent modalities. PET permits for acquisition of metabolic information using a variety of emerging novel radiopharmaceuticals. MR allows for assessment of bone marrow and for identification and characterization of soft-tissue lesions. Selection of appropriate radiopharmaceutical and MR protocol parameters is key for optimal evaluation. Although PET/MR has shown promise in evaluation of musculoskeletal involvement of multiple malignancies, it has not yet demonstrated broad superior to PET/CT. More research is needed to understand its value in the care of patients with such diseases.








Similar content being viewed by others
References
Hoh CK (2007) Clinical use of FDG PET. Nucl Med Biol 34(7):737–742. https://doi.org/10.1016/j.nucmedbio.2007.07.001
Bar-Shalom R, Yefremov N, Guralnik L et al (2003) Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med 44:1200–1209
Czernin J, Allen-Auerbach M, Schelbert HR (2007) Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med Off Publ Soc Nucl Med 48(Suppl 1):78S-88S
Yang H-L, Liu T, Wang X-M et al (2011) Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol 21:2604–2617
Costelloe CM (2009) Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol 10:606–614
Talbot JN, Paycha F, Balogova S (2011) Diagnosis of bone metastasis: recent comparative studies of imaging modalities. Q J Nucl Med Mol Imaging Publ Ital Assoc Nucl Med AIMN Int Assoc Radiopharm IAR Sect Soc Of 55:374–410
Buhmann Kirchhoff S, Becker C, Duerr HR et al (2009) Detection of osseous metastases of the spine: comparison of high resolution multi-detector-CT with MRI. Eur J Radiol 69:567–573
Tehranzadeh J, Mnaymneh W, Ghavam C et al (1989) Comparison of CT and MR imaging in musculoskeletal neoplasms. J Comput Assist Tomogr 13:466–472
Aisen AM (1986) MRI and CT evaluation of primary bone and soft-tissue tumors. AJR Am J Roentgenol 146:749–756
Rosenkrantz AB, Friedman K, Chandarana H et al (2015) Current status of hybrid PET/MRI in oncologic imaging. Am J Roentgenol 206:162–172. https://doi.org/10.2214/AJR.15.14968
Burger IA, Wurnig MC, Becker AS et al (2015) Hybrid PET/MR imaging: an algorithm to reduce metal artifacts from dental implants in Dixon-based attenuation map generation using a multiacquisition variable-resonance image combination sequence. J Nucl Med Off Publ Soc Nucl Med 56:93–97. https://doi.org/10.2967/jnumed.114.145862
Gunzinger JM, Delso G, Boss A et al (2014) Metal artifact reduction in patients with dental implants using multispectral three-dimensional data acquisition for hybrid PET/MRI. EJNMMI Phys 1:102. https://doi.org/10.1186/s40658-014-0102-z
Fuin N, Pedemonte S, Catalano OA et al (2017) PET/MRI in the presence of metal implants: completion of the attenuation map from PET emission data. J Nucl Med Off Publ Soc Nucl Med 58:840–845. https://doi.org/10.2967/jnumed.116.183343
Dregely I, Lanz T, Metz S et al (2015) A 16-channel MR coil for simultaneous PET/MR imaging in breast cancer. Eur Radiol 25:1154–1161. https://doi.org/10.1007/s00330-014-3445-x
Oehmigen M, Lindemann ME, Lanz T et al (2016) Integrated PET/MR breast cancer imaging: attenuation correction and implementation of a 16-channel RF coil. Med Phys 43:4808. https://doi.org/10.1118/1.4959546
Eldib M, Bini J, Calcagno C et al (2014) Attenuation correction for flexible magnetic resonance coils in combined magnetic resonance/positron emission tomography imaging. Invest Radiol 49:63–69. https://doi.org/10.1097/RLI.0b013e3182a530f8
Eldib M, Bini J, Robson PM et al (2015) Markerless attenuation correction for carotid MRI surface receiver coils in combined PET/MR imaging. Phys Med Biol 60:4705–4717. https://doi.org/10.1088/0031-9155/60/12/4705
Frohwein LJ, Heß M, Schlicher D et al (2018) PET attenuation correction for flexible MRI surface coils in hybrid PET/MRI using a 3D depth camera. Phys Med Biol 63:025033. https://doi.org/10.1088/1361-6560/aa9e2f
Sander CY, Keil B, Chonde DB et al (2015) A 31-channel MR brain array coil compatible with positron emission tomography. Magn Reson Med 73:2363–2375. https://doi.org/10.1002/mrm.25335
Ahlawat S, Fayad LM (2018) Diffusion weighted imaging demystified: the technique and potential clinical applications for soft tissue imaging. Skelet Radiol 47:313–328. https://doi.org/10.1007/s00256-017-2822-3
Fisher SM, Joodi R, Madhuranthakam AJ et al (2016) Current utilities of imaging in grading musculoskeletal soft tissue sarcomas. Eur J Radiol 85:1336–1344. https://doi.org/10.1016/j.ejrad.2016.05.003
Kransdorf MJ, Murphey MD (2016) Imaging of soft-tissue musculoskeletal masses: fundamental concepts. Radiogr Rev Publ Radiol Soc N Am Inc 36:1931–1948. https://doi.org/10.1148/rg.2016160084
Zhang X, Chen Y-LE, Lim R et al (2016) Synergistic role of simultaneous PET/MRI-MRS in soft tissue sarcoma metabolism imaging. Magn Reson Imaging 34:276–279. https://doi.org/10.1016/j.mri.2015.10.027
Yokouchi M, Terahara M, Nagano S et al (2011) Clinical implications of determination of safe surgical margins by using a combination of CT and 18FDG-positron emission tomography in soft tissue sarcoma. BMC Musculoskelet Disord 12:166. https://doi.org/10.1186/1471-2474-12-166
Nanni C, Gasbarrini A, Cappelli A et al (2015) FDG PET/CT for bone and soft-tissue biopsy. Eur J Nucl Med Mol Imaging 42:1333–1334. https://doi.org/10.1007/s00259-015-3017-6
Kubo T, Furuta T, Johan MP, Ochi M (2016) Prognostic significance of (18)F-FDG PET at diagnosis in patients with soft tissue sarcoma and bone sarcoma; systematic review and meta-analysis. Eur J Cancer Oxf Engl 58:104–111. https://doi.org/10.1016/j.ejca.2016.02.007
Tateishi U, Hosono A, Makimoto A et al (2009) Comparative study of FDG PET/CT and conventional imaging in the staging of rhabdomyosarcoma. Ann Nucl Med 23:155–161. https://doi.org/10.1007/s12149-008-0219-z
Völker T, Denecke T, Steffen I et al (2007) Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol Off J Am Soc Clin Oncol 25:5435–5441. https://doi.org/10.1200/JCO.2007.12.2473
Kneisl JS, Patt JC, Johnson JC, Zuger JH (2006) Is PET useful in detecting occult nonpulmonary metastases in pediatric bone sarcomas? Clin Orthop 450:101–104. https://doi.org/10.1097/01.blo.0000229329.06406.00
Erfanian Y, Grueneisen J, Kirchner J et al (2017) Integrated 18F-FDG PET/MRI compared to MRI alone for identification of local recurrences of soft tissue sarcomas: a comparison trial. Eur J Nucl Med Mol Imaging 44:1823–1831. https://doi.org/10.1007/s00259-017-3736-y
Bosma SE, Vriens D, Gelderblom H et al (2019) 18F-FDG PET-CT versus MRI for detection of skeletal metastasis in Ewing sarcoma. Skeletal Radiol 48:1735–1746. https://doi.org/10.1007/s00256-019-03192-2
Billingsley KG, Burt ME, Jara E et al (1999) Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 229:602–610. https://doi.org/10.1097/00000658-199905000-00002 (Discussion 610-612)
García Franco CE, Torre W, Tamura A et al (2010) Long-term results after resection for bone sarcoma pulmonary metastases. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg 37:1205–1208. https://doi.org/10.1016/j.ejcts.2009.11.026
Tabacchi E, Fanti S, Nanni C (2016) The possible role of PET imaging toward individualized management of bone and soft tissue malignancies. PET Clin 11:285–296. https://doi.org/10.1016/j.cpet.2016.02.011
Iagaru A, Chawla S, Menendez L, Conti PS (2006) 18F-FDG PET and PET/CT for detection of pulmonary metastases from musculoskeletal sarcomas. Nucl Med Commun 27:795–802. https://doi.org/10.1097/01.mnm.0000237986.31597.86
Raad RA, Friedman KP, Heacock L et al (2016) Outcome of small lung nodules missed on hybrid PET/MRI in patients with primary malignancy. J Magn Reson Imaging JMRI 43:504–511. https://doi.org/10.1002/jmri.25005
Benjamin MS, Drucker EA, McLoud TC, Shepard J-AO (2003) Small pulmonary nodules: detection at chest CT and outcome. Radiology 226:489–493. https://doi.org/10.1148/radiol.2262010556
Hanamiya M, Aoki T, Yamashita Y et al (2012) Frequency and significance of pulmonary nodules on thin-section CT in patients with extrapulmonary malignant neoplasms. Eur J Radiol 81:152–157. https://doi.org/10.1016/j.ejrad.2010.08.013
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176. https://doi.org/10.1053/ctrv.2000.0210
Riedl CC, Pinker K, Ulaner GA et al (2017) Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur J Nucl Med Mol Imaging 44:1428–1437. https://doi.org/10.1007/s00259-017-3703-7
Liu T, Xu J-Y, Xu W et al (2011) Fluorine-18 deoxyglucose positron emission tomography, magnetic resonance imaging and bone scintigraphy for the diagnosis of bone metastases in patients with lung cancer: which one is the best?–a meta-analysis. Clin Oncol R Coll Radiol G B 23:350–358. https://doi.org/10.1016/j.clon.2010.10.002
Beiderwellen K, Huebner M, Heusch P et al (2014) Whole-body [18F]FDG PET/MRI vs. PET/CT in the assessment of bone lesions in oncological patients: initial results. Eur Radiol 24:2023–2030. https://doi.org/10.1007/s00330-014-3229-3
Eiber M, Takei T, Souvatzoglou M et al (2014) Performance of whole-body integrated 18F-FDG PET/MR in comparison to PET/CT for evaluation of malignant bone lesions. J Nucl Med Off Publ Soc Nucl Med 55:191–197. https://doi.org/10.2967/jnumed.113.123646
Samarin A, Hüllner M, Queiroz MA et al (2015) 18F-FDG-PET/MR increases diagnostic confidence in detection of bone metastases compared with 18F-FDG-PET/CT. Nucl Med Commun 36:1165–1173. https://doi.org/10.1097/MNM.0000000000000387
Lee SM (2016) Preoperative staging of non-small cell lung cancer: prospective comparison of PET/MR and PET/CT. Eur Radiol 26:3850–3857
Fraioli F (2015) Non-small-cell lung cancer resectability: diagnostic value of PET/MR. Eur J Nucl Med Mol Imaging 42:49–55
Melsaether AN (2016) Comparison of whole-body (18)F FDG PET/MR imaging and whole-body (18)F FDG PET/CT in terms of lesion detection and radiation dose in patients with breast cancer. Radiology 281:193–202
Catalano OA (2015) Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR in the evaluation of osseous metastases in breast cancer patients. Br J Cancer 112:1452–1460
Shigematsu Y, Hirai T, Kawanaka K et al (2014) Distinguishing imaging features between spinal hyperplastic hematopoietic bone marrow and bone metastasis. AJNR Am J Neuroradiol 35:2013–2020. https://doi.org/10.3174/ajnr.A4012
Shortt CP (2009) Whole-Body MRI versus PET in assessment of multiple myeloma disease activity. AJR Am J Roentgenol 192:980–986
Caldarella C, Treglia G, Isgrò MA et al (2012) The role of fluorine-18-fluorodeoxyglucose positron emission tomography in evaluating the response to treatment in patients with multiple myeloma. Int J Mol Imaging 2012:e175803. https://doi.org/10.1155/2012/175803
Shortt CP, Gleeson TG, Breen KA et al (2009) Whole-Body MRI versus PET in assessment of multiple myeloma disease activity. AJR Am J Roentgenol 192:980–986. https://doi.org/10.2214/AJR.08.1633
Zamagni E, Nanni C, Patriarca F et al (2007) A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica 92:50–55. https://doi.org/10.3324/haematol.10554
Spinnato P, Bazzocchi A, Brioli A et al (2012) Contrast enhanced MRI and 18F-FDG PET-CT in the assessment of multiple myeloma: a comparison of results in different phases of the disease. Eur J Radiol 81:4013–4018. https://doi.org/10.1016/j.ejrad.2012.06.028
Cascini GL, Falcone C, Console D et al (2013) Whole-body MRI and PET/CT in multiple myeloma patients during staging and after treatment: personal experience in a longitudinal study. Radiol Med (Torino) 118:930–948. https://doi.org/10.1007/s11547-013-0946-7
Pawlyn C, Fowkes L, Otero S et al (2016) Whole-body diffusion-weighted MRI: a new gold standard for assessing disease burden in patients with multiple myeloma? Leukemia 30:1446–1448. https://doi.org/10.1038/leu.2015.338
Sachpekidis C, Hillengass J, Goldschmidt H et al (2015) Comparison of (18)F-FDG PET/CT and PET/MRI in patients with multiple myeloma. Am J Nucl Med Mol Imaging 5:469–478
Shah SN, Oldan JD (2017) PET/MR imaging of multiple myeloma. Magn Reson Imaging Clin N Am 25:351–365. https://doi.org/10.1016/j.mric.2017.01.003
Blake GM, Park-Holohan SJ, Cook GJ, Fogelman I (2001) Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin Nucl Med 31:28–49. https://doi.org/10.1053/snuc.2001.18742
Piert M, Zittel TT, Becker GA et al (2001) Assessment of porcine bone metabolism by dynamic [18F] fluoride ion PET: correlation with bone histomorphometry. J Nucl Med 42:1091–1100
Hawkins RA, Choi Y, Huang SC et al (1992) Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med Off Publ Soc Nucl Med 33:633–642
Schiepers C, Nuyts J, Bormans G et al (1997) Fluoride kinetics of the axial skeleton measured in vivo with fluorine-18-fluoride PET. J Nucl Med Off Publ Soc Nucl Med 38:1970–1976
Czernin J, Satyamurthy N, Schiepers C (2010) Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med Off Publ Soc Nucl Med 51:1826–1829. https://doi.org/10.2967/jnumed.110.077933
Ueda CE, Duarte PS, de Castroneves LA et al (2020) Comparison of 18F-NaF PET/CT with other imaging methods in the detection of bone metastases in patients with medullary thyroid cancer: a report of a series of 31 cases. Nucl Med Mol Imaging 54:281–291. https://doi.org/10.1007/s13139-020-00666-3
Bucklan D, Muzic R, Faulhaber P, Jones R (2017) 18F NaF PET/MR for the evaluation of bone metastases in breast cancer patients: comparison to NaF PET/CT, FDG PET/CT, and MDP bone scan. J Nucl Med 58:472–472
Wallitt KL (2017) Clinical PET imaging in prostate cancer. Radiogr Rev Publ Radiol Soc N Am Inc 37:1512–1536
Maurer T, Beer AJ, Wester H-J et al (2014) Positron emission tomography/magnetic resonance imaging with 68Gallium-labeled ligand of prostate-specific membrane antigen: promising novel option in prostate cancer imaging? Int J Urol 21:1286–1288. https://doi.org/10.1111/iju.12577
Kabasakal L, Demirci E, Ocak M et al (2015) Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl Med Commun 36:582–587. https://doi.org/10.1097/MNM.0000000000000290
Perera M, Papa N, Christidis D et al (2016) Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol 70:926–937. https://doi.org/10.1016/j.eururo.2016.06.021
De Coster L, Sciot R, Everaerts W et al (2017) Fibrous dysplasia mimicking bone metastasis on 68GA-PSMA PET/MRI. Eur J Nucl Med Mol Imaging 44:1607–1608. https://doi.org/10.1007/s00259-017-3712-6
Artigas C, Otte F-X, Lemort M et al (2017) Vertebral hemangioma mimicking bone metastasis in 68Ga—PSMA ligand PET/CT. Clin Nucl Med 42:368–370. https://doi.org/10.1097/RLU.0000000000001631
Sasikumar A, Joy A, Pillai MRA et al (2017) 68Ga-PSMA PET/CT Imaging in multiple myeloma. Clin Nucl Med 42:e126–e127. https://doi.org/10.1097/RLU.0000000000001479
Sasikumar A, Joy A, Nanabala R et al (2016) 68Ga-PSMA PET/CT false-positive tracer uptake in paget disease. Clin Nucl Med 41:e454. https://doi.org/10.1097/RLU.0000000000001340
Rhee H, Blazak J, Tham CM et al (2016) Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. https://doi.org/10.1186/s13550-016-0231-6
Kranzbühler B (2018) Clinical performance of 68Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging 45:20–30
Freitag MT (2016) Comparison of hybrid (68)Ga-PSMA PET/MRI and (68)Ga-PSMA PET/CT in the evaluation of lymph node and bone metastases of prostate cancer. Eur J Nucl Med Mol Imaging 43:70–83
Domachevsky L (2020) Comparison between pelvic PSMA-PET/MR and whole-body PSMA-PET/CT for the initial evaluation of prostate cancer: a proof of concept study. Eur Radiol 30:328–336
Oka S (2007) A preliminary study of anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J Nucl Med Publ Soc Nucl Med 48:46–55
Shoup TM (1999) Synthesis and evaluation of [18F]1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J Nucl Med Publ Soc Nucl Med 40:331–338
Parent EE, Schuster DM (2018) Update on 18F-fluciclovine PET for prostate cancer imaging. J Nucl Med 59:733–739. https://doi.org/10.2967/jnumed.117.204032
Oka S, Kanagawa M, Doi Y et al (2017) PET tracer 18F-fluciclovine can detect histologically proven bone metastatic lesions: a preclinical study in rat osteolytic and osteoblastic bone metastasis models. Theranostics 7:2048–2064. https://doi.org/10.7150/thno.19883
Amorim BJ (2020) Performance of 18F-fluciclovine PET/MR in the evaluation of osseous metastases from castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 47:105–114
Schuster DM, Nanni C, Fanti S et al (2014) Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: physiologic uptake patterns, incidental findings, and variants that may simulate disease. J Nucl Med Off Publ Soc Nucl Med 55:1986–1992. https://doi.org/10.2967/jnumed.114.143628
Hofman MS, Lau WFE, Hicks RJ (2015) Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiogr Rev Publ Radiol Soc N Am Inc 35:500–516. https://doi.org/10.1148/rg.352140164
Subramaniam RM (2018) ACR practice parameter for the performance of gallium-68 DOTATATE PET/CT for neuroendocrine tumors. Clin Nucl Med 43:899–908
Geijer H, Breimer LH (2013) Somatostatin receptor PET/CT in neuroendocrine tumours: update on systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 40:1770–1780
Mojtahedi A, Thamake S, Tworowska I et al (2014) The value of 68Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging 4:426–434
Sadowski SM, Neychev V, Millo C et al (2016) Prospective study of 68 Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol 34:588–596. https://doi.org/10.1200/JCO.2015.64.0987
Panagiotidis E (2017) Comparison of the impact of 68Ga-DOTATATE and 18F-FDG PET/CT on clinical management in patients with neuroendocrine tumors. J Nucl Med Publ Soc Nucl Med 58:91–96
Mackie EJ, Trechsel U, Bruns C (1990) Somatostatin receptors are restricted to a subpopulation of osteoblast-like cells during endochondral bone formation. Dev Camb Engl 110:1233–1239
Sonmezoglu K, Vatankulu B, Elverdi T et al (2017) The role of 68Ga-DOTA-TATE PET/CT scanning in the evaluation of patients with multiple myeloma: preliminary results. Nucl Med Commun 38:76–83. https://doi.org/10.1097/MNM.0000000000000610
Klinaki I, Al-Nahhas A, Soneji N, Win Z (2013) 68Ga DOTATATE PET/CT uptake in spinal lesions and MRI correlation on a patient with neuroendocrine tumor: potential pitfalls. Clin Nucl Med 38:449–453
Brogsitter C, Hofmockel T, Kotzerke J (2014) 68Ga DOTATATE uptake in vertebral hemangioma. Clin Nucl Med 39:462–463
Clifton-Bligh RJ, Hofman MS, Duncan E et al (2013) Improving diagnosis of tumor-induced osteomalacia with gallium-68 DOTATATE PET/CT. J Clin Endocrinol Metab 98:687–694. https://doi.org/10.1210/jc.2012-3642
Hamson EJ, Keane FM, Tholen S et al (2014) Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. PROTEOMICS Clin Appl 8:454–463. https://doi.org/10.1002/prca.201300095
Kratochwil C, Flechsig P, Lindner T et al (2019) 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 60:801–805. https://doi.org/10.2967/jnumed.119.227967
Chen H, Pang Y, Wu J et al (2020) Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging 47:1820–1832. https://doi.org/10.1007/s00259-020-04769-z
Funding
There is no source of funding for this manuscript.
Author information
Authors and Affiliations
Contributions
JSH: literature search and review, writing, and editing; RB: literature search and review, writing, and editing; LE: editing and image acquisition; LGC: editing and image acquisition; OC: content planning, writing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
Dr Catalano has been a consultant for Siemens Healthineers and IBM, and receives research support from Bayer. He is a recipient of an IBM fellowship. The Martinos center receives research support from Siemens Healthineers and GE Healthcare. Drs Husseini, Balza, Evangelista, and García Cañamaque have no financial disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Husseini, J.S., Balza, R., Evangelista, L. et al. PET/MR for evaluation of musculoskeletal malignancies. Clin Transl Imaging 10, 71–83 (2022). https://doi.org/10.1007/s40336-021-00470-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-021-00470-9