Abstract
Purpose
Diffusion tensor magnetic resonance imaging (DTI) characterises tissue microstructure and provides proxy measures of myelination, axon diameter, fibre density and organisation. This may be valuable in the assessment of the roots of the brachial plexus in health and disease. Therefore, there is a need to define the normal DTI values.
Methods
The literature was systematically searched for studies of asymptomatic adults who underwent DTI of the brachial plexus. Participant characteristics, scanning protocols, and measurements of the fractional anisotropy (FA) and mean diffusivity (MD) of each spinal root were extracted by two independent review authors. Generalised linear modelling was used to estimate the effect of experimental conditions on the FA and MD. Meta-analysis of root-level estimates was performed using Cohen’s method with random effects.
Results
Nine articles, describing 316 adults (1:1 male:female) of mean age 35 years (SD 6) were included. Increments of ten diffusion sensitising gradient directions reduced the mean FA by 0.01 (95% CI 0.01, 0.03). Each year of life reduced the mean MD by 0.03 × 10–3 mm2/s (95% CI 0.01, 0.04). At 3-T, the pooled mean FA of the roots was 0.36 (95% CI 0.34, 0.38; I2 98%). The pooled mean MD of the roots was 1.51 × 10–3 mm2/s (95% CI 1.45, 1.56; I2 99%).
Conclusions
The FA and MD of the roots of the brachial plexus vary according to experimental conditions and participant factors. We provide summary estimates of the normative values in different conditions which may be valuable to researchers and clinicians alike.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
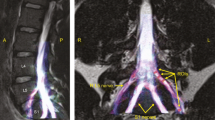
The brachial plexus is a network of nerves which supply the upper limb with movement and feeling (Fig. 1 and Supplementary Fig. 1). Magnetic resonance imaging (MRI) is generally considered the best non-invasive imaging modality for diagnosing various pathologies affecting the brachial plexus [1,2,3,4,5,6]. The roots of the brachial plexus are the most common site of injury [7] and typically, the status of the root dictates the prognosis and surgical reconstruction. Consequently, defining the health status of the roots is of paramount importance. Whilst MRI is more accurate than electrophysiology [2, 6], ultrasonography [3,4,5, 8] and computed tomography myelography [9], the diagnostic performance of conventional cross-sectional MRI for assessing the spinal nerve roots remains suboptimal [7]. Consequently, there has been a surge of research into diffusion tensor imaging (DTI) which may provide additional valuable information. DTI characterises tissue microstructure and provides reproducible [10,11,12,13] proxy measures of nerve health which are sensitive to myelination, axon diameter, fibre density and organisation [14, 15]. The parameters typically derived from DTI include the fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD). FA is a scalar value between zero and one; an FA of zero implies isotropic diffusion within a voxel, whilst (in the context of peripheral nerve imaging) a FA nearing one implies diffusion along a single axis i.e., either anterograde of retrograde diffusion along the length of the nerve. MD describes the average molecular diffusion rate of the tensor; AD describes the diffusion rate in the long axis and RD represents diffusion perpendicular to the long axis.
The roots of the brachial plexus emerging from the intervertebral foramina (upper left image) and their relationship to the scalene muscles and vasculature of the upper limb (upper right image). The lower image is a simplified schematic of the brachial plexus highlighting (in purple) the spinal roots.
Before researchers and clinicians can use DTI parameters to identify abnormal roots of the brachial plexus, there is a need to define the “normal” values. Numerous studies have examined aspects of DTI of the brachial plexus in healthy adults. This review aims to summarise the values observed in health and explore discrepancies in the reported measurements.
Methods
This review is registered with PROPSERO (ID CRD42019155788), it was designed and conducted in accordance with the Cochrane Handbook of Systematic Reviews [16] and has been authored in accordance with the PRISMA checklist [17].
Types of studies
We included all studies which reported the findings of diffusion tensor magnetic resonance imaging of the roots of the brachial plexus in healthy adults. Case reports were excluded.
Participants
Asymptomatic adults (aged ≥ 16 years) with no known pathology (past or present) affecting the spinal cord or brachial plexus were the population of interest.
Image acquisition
Studies must have reported diffusion tensor imaging parameters from the roots of the brachial plexus.
Search strategy
The NICE Healthcare Databases (hdas.nice.org.uk) was searched using the terms “diffusion tensor” OR “DTI” AND “brachial plexus”. This yielded 67 hits in PubMed, 36 in Embase, 8 in CINAHL, 2 in CENTRAL and 2 in ClinicalTrials.gov the on 13th November 2019. After de-duplication, 78 unique citations were independently screened by two review authors (RGW and AW). The full texts of all potentially relevant articles were obtained. The reference lists for included articles were also scrutinised for potentially relevant papers. The final lists of included articles were compared and disagreements resolved by discussion.
Study selection
Two review authors (RGW and AW) independently screened titles and abstracts for relevance, in accordance with the eligibility criteria. The full texts of potentially eligible articles were obtained and again independently assessed by the same two authors. Disagreements were resolved by discussion.
Data extraction
Two review authors (RGW and AW) independently extracted data concerning the demographics, scanner, pulse sequence, pre-processing, tensorial reconstruction, measurement conditions and the outcomes of interest. The spinal nerve root was the unit of analysis [7] and root-level estimates of DTI parameters were extracted. Where data was missing or unclear, the corresponding author was contacted by email and/or phone and if no reply was received, 4 weeks later all authors were contacted in addition to re-contacting the corresponding author. The authors of one study [18] provided additional information (measurements form the extraforaminal roots using identical methods) for the purposes of this review.
Outcomes
The primary outcome is to estimate the normal fractional anisotropy (FA) of the extraforaminal roots of the brachial plexus in healthy adults. The secondary outcomes include: (a) to estimate the normal mean diffusivity (MD) of the extraforaminal roots of the brachial plexus in healthy adults, and (b) to explore the associations between DTI parameters and participants age, the signal-to-noise (SNR) ratios and related factors such as the b value(s), echo time(s) (TE) and resolution (in cubic millimetres, mm3) and the number of diffusion sensitising gradient directions (ND) sampled per shell.
Methodological quality assessment
The risk of bias was assessed by two review authors (RGW and AW) using the ROBINS-I tool [19] and displayed graphically using robvis [20]. Disagreements were resolved by discussion.
Statistical analysis
Data were analysed in Stata/MP v15 (StataCop LLC, Texas). To estimate the effect of experimental/participant factors on the FA and MD, generalised linear modelling (GLM) was used with gaussian families. As the TE, b value and resolution are functions of SNR, the fixed effects were selected to be SNR, age in years and the ND all of which were handled as continuous variables. The random effects in the GLM varied by the study. Estimates were bootstrapped using lossless non-parametric resampling with replacement, with 1000 iterations. There was insufficient data to meaningfully assess the effect of different tensor fitting methods or components of the b value (diffusion time, magnitude or interval) on DTI parameters. To visualise the association of FA with ND, and MD with age, scatterplots of the aggregate estimates were generated using the metareg package; the circles are root-level estimates and the sizes are dependent on the precision (inverse variance) of the estimate. To estimate the pooled normal FA and MD of the spinal roots, meta-analyses were performed using the metan package. Cohen’s method was used because both FA and MD were homoscedastic. Dersimonian and Laird random effects were used given the clinical heterogeneity. Analyses were subgrouped by both the ND and spinal root (C5, C6, C7, C8, T1). Confidence intervals (CI) were generated to the 95% level. To assess the possibility of small-study effects we constructed a funnel plot using the metafunnel package, which is a scatterplot of the effect size against precision; symmetry implies the absence of small-study effects.
Results
After reviewing 27 full texts, 15 were excluded (Supplementary materials) and 9 articles (of 9 unique studies) were included [18, 21,22,23,24,25,26,27,28] (Fig. 2).
Study characteristics
Overall, 316 adults were included. The ratio of males:females was approximately 1:1 (156:154). The mean age of participants was 35 years (SD 6). The characteristics of included studies are shown in Table 1.
Risk of bias within studies
The risk of bias for the included studies are summarised in Fig. 3. Eight of the included studies [18, 21,22,23,24, 26, 28, 29] were at risk of bias due to confounding because there was no adjustment for a) effect modifiers such as age, weight and the experimental factors described above, and b) repeated measures, i.e. ten estimates of the FA (one from each spinal root, bilaterally) taken from the same individual will be highly correlated and without adjustment, the sample-level estimates will have falsely small variances. Seven studies [21,22,23,24,25, 28] provided no information about missing data and so the risk of bias is unclear. Two studies [21, 23] were judged to be at high risk of bias in the measurement of FA and MD because a bespoke region of interest was used to calculate the FA and the number of diffusion sensitising gradients used was not described [21]. Three studies [24, 25, 28] were at unclear risk of bias in the measurement of FA and MD because information was lacking about pre-processing, tensorial reconstruction or how the estimates of the FA and MD were derived from images (e.g. region of interest size and position). One study [23] was at high risk of reporting bias because three b values were tested (700, 900, 1100 mm/s) but it is unclear which yielded the estimates of FA and MD reported in the manuscript or whether they are an average of the three. The risk of bias due to selective outcome reporting was unclear in six studies [21,22,23,24, 26, 28], because no information was provided regarding the exclusion criteria, attrition due to scan intolerance, dataset exclusion (e.g. for uncorrectable motion artefact) or otherwise and there were no published protocols to consult.
Evidence synthesis
There were no clear associations between experimental factors and the FA or MD on univariable modelling (Table 2). Multivariable modelling showed that the angular resolution was strongly associated with FA, whereby every additional ten diffusion sensitising gradient directions sampled reduced the FA by 0.01 (95% CI 0.01, 0.03; Fig. 4). Furthermore, multivariable modelling showed that each year of life reduced the MD by 0.03 × 10–3 mm2/s (95% CI 0.01, 0.04; Fig. 5). Bootstrapping did not change these estimates.
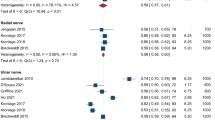
Seven studies [18, 21, 22, 24, 27,28,29] were included in the meta-analysis of the normal FA of the roots of the brachial plexus at 3 T; one study [26] did not provide estimates of the variance so could not be included. The pooled estimate of the normal FA of the root was 0.36 (95% CI 0.34, 0.38; Fig. 6). There were no statistically significant differences between the five roots. However, there was significant statistical heterogeneity between studies (I2 98%) which may be related to the experimental conditions described above. The pooled estimates of the normal FA subgrouped by spinal root (including the study performed at 1.5 T which had usable data [23]) are shown in Supplementary Fig. 2.
Six studies [18, 22, 24, 27,28,29] contributed to the meta-analysis of the normal MD of the roots of the brachial plexus at 3 T; one study [26] did not provide estimates of the variance and one study [21] did not report the MD of any roots. The pooled estimate of the normal MD of the roots of the brachial plexus was 1.51 × 10–3 mm2/s (95% CI 1.45, 1.56; Fig. 7 and Supplementary Fig. 3); however, there was high statistical heterogeneity between studies (I2 99%) which may be related to the experimental conditions described above. The pooled estimates of the normal MD subgrouped by spinal root (including the study performed at 1.5 T which had usable data [23]) is shown in Supplementary Fig. 3.
There was no evidence of small-study effects (Fig. 8).
Discussion
We have shown that the roots of the brachial plexus in adults have a mean fractional anisotropy of 0.36 (95% CI 0.34, 0.38) and mean diffusivity of 1.51 × 10–3 mm2/s (95% CI 1.45, 1.56). However, there is substantial variation and estimates are affected by experimental conditions such as the field strength, b value and ND as well as patient factors, such as age.
Although we provide no direct comparisons, this work reinforces the generally accepted concept that the DTI parameters of the peripheral nervous system are different to those of the central white matter tracts of the brain [27]. The systematic reviews by Label [30] and Yap [31] showed that in health, the white matter tracts of the brain have a mean FA of approximately 0.36–0.54 and mean MD of 0.84–1.43 × 10–3 mm2/s, depending on the fibre bundle measured and the age of the individual. We find that the roots of the brachial plexus have a substantially lower average FA (between 0.34 and 0.38 with 95% confidence) and higher MD (95% CI 1.45–1.56 mm2/s × 10–3), which are comparable to the lower bounds of the central white matter values. We believe that these discrepancies are likely to be related to differences in the axon density (the corpus callosum has 38,000 myelinated fibres per mm2, whereas the brachial plexus has approximately 8348 fibres per mm2) [32, 33] and extensive intraneural fascicular sharing/crossing [34, 35].
Several experimental factors [36] are known to affect DTI parameter estimates, including: (a) scanning conditions such as the SNR [37], components of the b value [38, 39], ND [40, 41]; (b) software pipelines for denoising, correcting artefacts arising from susceptibility, motion and eddy currents [42, 43] and tensor fitting methods [37], as well as (c) the size and position of ROIs which may contribute to partial volume effects [44]. Despite these limitations, the TraCED challenge [10] and several phantom studies [11,12,13] have demonstrated very high reproducibility across scanners, sequences and sessions for tractography from DTI. With more sophisticated diffusion sequences, the reasons for the disparities between central and peripheral fibre diffusion parameters may become apparent. Nonetheless, we have shown DTI parameters (and thus, probably, tractograms derived from these datasets) are related to numerous experimental conditions and, therefore, we suggest that researchers and clinicians interpret our summary values with both caution and respect to their particular circumstances.
In this study, the ND was strongly related to the FA whereby fewer directions were associated with higher estimates of the FA. This is a well-known phenomenon [41] and likely to be explained by the association between noise (which can couple to give rise to anisotropy) and artefactually high estimates of the FA [37]. Whilst the observed FA values in the studies with relatively fewer directions and lower SNR appear plausible (rather than e.g. 0.9 which would clearly be artefactual), it is possible that such studies are more susceptible to bias and their estimates of FA are falsely high. Equally, our model may still be subject to collinearity because studies with higher ND tended to also have a higher SNR. Conversely, as MD measures the size of the diffusion ellipsoid [45], we expected [41] MD to be independent of the ND and this is what we observed.
As humans age, axons lose their integrity, peripheral nerves demyelinate and there is a corresponding increase in extra-cellular fluid. Consequently, advancing aging is typically associated with reduced FA and increased diffusivity in white matter structures [31, 46]. Prior work by Kronlage et al. [47] on the peripheral nerves in the forearm showed that the FA reduced with age (as in the brain [31]). We observed no statistically significant association between age and the FA of the roots of the brachial plexus but this may be due to the narrow age range of participants in our study. Whilst Kronlage [47] found that MD increased with age, we found that MD was slowed by 0.03 × 10–3 mm2/s with each year of life (Fig. 5). Our findings are in agreement with the wider literature on age-related diffusivity changes in the brain [46] and compatible with the biological mechanisms of aging. Specifically, we observed that the MD of the roots of the brachial plexus slowed in the 3rd and 4th decade of life, which has also been observed in the healthy senescent adult brain [46]. It is unclear why Kronlage’s work differs but this might be due to discrepancies in the age range of the sample (adults in this review were aged 28–45 years versus 20–80 years in Kronlage’s [47]) and aspects of the scanning because Kronlage [47] used non-isotropic voxels (4.0 mm through plane, 1.5 mm2 in plane) which might underestimate the FA and overestimate the MD [43].
Limitations
The main limitation of this review is the pooled estimation of FA and MD. We decided to perform meta-analyses in the presence of high statistical heterogeneity because (a) the generated outputs provide an important graphical representation of the variability of measurements in relation to experimental conditions which are easy to interpret, and (b) the forest plots provide a pictorial representation of the deleterious effects of failing to adjust study-level estimates for repeated measures. Figures 6 and 7 and Supplementary Figs. 2 and 3 show that in the eight studies [18, 21,22,23,24, 26, 28, 29] which did not use multilevel models, the standard errors of the mean (and thus, their CIs) are falsely small. We believe that if studies had appropriately adjusted for clustering/repeated measures, the CIs would be wider, so overlaping the aggregate means from other studies and the measures of statistical heterogeneity (e.g. I2) would fall.
We show a negative association between MD and age; however, readers should note that the range of aggregate ages in the included studies is narrow (28–45 years) and so the estimates may not be generalisable to the population.
Conclusions
The roots of the brachial plexus in adults appear to have a pooled mean fractional anisotropy of 0.36 (95% CI 0.34, 0.38) and pooled mean diffusivity of 1.51 × 10–3 mm2/s (95% CI 1.45, 1.56), although these parameters are dependent on experimental conditions and vary slightly from C5 to T1.
Availability of data
The extracted data and statistical syntax are available from the first author upon request.
References
Vargas MI, Viallon M, Nguyen D, Beaulieu JY, Delavelle J, Becker M (2010) New approaches in imaging of the brachial plexus. Eur J Radiol 74:403–410
Nardin RA, Patel MR, Gudas TF, Rutkove SB, Raynor EM (1999) Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve 22:151–155
Zhu Y-S, Mu N-N, Zheng M-J, Zhang Y-C, Feng H, Cong R et al (2014) High-resolution ultrasonography for the diagnosis of brachial plexus root lesions. Ultrasound Med Biol 40:1420–1426
Mallouhi A, Meirer R, Bodner G (2003) Sonographic features of brachial plexus traumatic rupture. J Neurosurg 99:432–432
Lapegue F, Faruch-Bilfeld M, Demondion X, Apredoaei C, Bayol MA, Artico H et al (2014) Ultrasonography of the brachial plexus, normal appearance and practical applications. Diagn Interv Imaging 95:259–275
Sureka J, Cherian RA, Alexander M, Thomas BP (2009) MRI of brachial plexopathies. Clin Radiol 64:208–218
Wade RG, Takwoingi Y, Wormald JCR, Ridgway JP, Tanner S, Rankine JJ et al (2019) MRI for detecting root avulsions in traumatic adult brachial plexus injuries: a systematic review and meta-analysis of diagnostic accuracy. Radiology 293:125–133
Chin B, Ramji M, Farrokhyar F, Bain JR (2017) Efficient imaging: examining the value of ultrasound in the diagnosis of traumatic adult brachial plexus injuries, a systematic review. Neurosurgery 83:323–332. https://doi.org/10.1093/neuros/nyx483
Van der Linde E, Naidu V, Mitha A, Rocher A (2015) Diagnosis of nerve root avulsion injuries in adults with traumatic brachial plexopathies: MRI compared with CT myelography. S Afr J Radiol 19:1–9
Nath V, Schilling KG, Parvathaneni P, Huo Y, Blaber JA, Hainline AE et al (2020) Tractography reproducibility challenge with empirical data (TraCED): the 2017 ISMRM diffusion study group challenge. J Magn Reson Imaging 51:234–249
Vavasour IM, Meyers SM, Mädler B, Harris T, Fu E, Li DKB et al (2019) Multicenter measurements of T1 relaxation and diffusion tensor imaging: intra and intersite reproducibility. J Neuroimaging 29:42–51
Prohl AK, Scherrer B, Tomas-Fernandez X, Filip-Dhima R, Kapur K, Velasco-Annis C et al (2019) Reproducibility of structural and diffusion tensor imaging in the TACERN multi-center study. Front Integr Neurosci 13:1–15
Kimura M, Yabuuchi H, Matsumoto R, Kobayashi K, Yamashita Y, Nagatomo K et al (2020) The reproducibility of measurements using a standardization phantom for the evaluation of fractional anisotropy (FA) derived from diffusion tensor imaging (DTI). Magn Reson Mater Physics, Biol Med. 33(2):293–298
Heckel A, Weiler M, Xia A, Ruetters M, Pham M, Bendszus M et al (2015) Peripheral nerve diffusion tensor imaging: assessment of axon and myelin sheath integrity. PLoS ONE 10:e0130833
Andersson G, Orädd G, Sultan F, Novikov LN (2018) In vivo diffusion tensor imaging, diffusion kurtosis imaging, and tractography of a sciatic nerve injury model in rat at 9.4 T. Sci Rep 8:12911
Higgins JPT, Green S (eds) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collab
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Systematic reviews and meta-analyses: the PRISMA statement. Annu Intern Med 151:264–269
Wade RG, Tanner SF, Teh I, Ridgway JP, Shelley D, Chaka B et al (2020) Diffusion tensor imaging for diagnosing root avulsions in traumatic adult brachial plexus injuries: a proof-of-concept study. Front Surg 7:19. https://doi.org/10.3389/fsurg.2020.00019
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
McGuinness LA, Higgins JPT (2020) Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. https://doi.org/10.1002/jrsm.1411(in press)
Ho MJ, Ciritsis A, Manoliu A, Stieltjes B, Marcon M, Andreisek G et al (2019) Diffusion tensor imaging of the brachial plexus: a comparison between readout-segmented and conventional single-shot echo-planar imaging. Magn Reson Med Sci 18:150–157
Ho MJ, Manoliu A, Kuhn FP, Stieltjes B, Klarhöfer M, Feiweier T et al (2017) Evaluation of reproducibility of diffusion tensor imaging in the brachial plexus at 3.0 T. Invest Radiol. 52:482–487
Chen M, Li X, Chen J, Sun C, He J (2012) Quantitative analysis of the normal brachial plexus with diffusion tensor imaging. Chin J Med Imaging Technol 28:77–81
Oudeman J, Verhamme C, Engbersen MP, Caan MWA, Maas M, Froeling M et al (2018) Diffusion tensor MRI of the healthy brachial plexus. PLoS ONE 13:e0196975
Su X, Kong X, Liu D, Kong X, Alwalid O, Wang J et al (2019) Multimodal magnetic resonance imaging of peripheral nerves: establishment and validation of brachial and lumbosacral plexi measurements in 163 healthy subjects. Eur J Radiol 117:41–48
Vargas MI, Viallon M, Nguyen D, Delavelle J, Becker M (2010) Diffusion tensor imaging (DTI) and tractography of the brachial plexus: feasibility and initial experience in neoplastic conditions. Neuroradiology 52:237–245
Wade RG, Teh I, Andersson G, Fang-Cheng Y, Wiberg M, Bourke G (2020) Diffusion tensor imaging of the brachial plexus: defining the fractional anisotropy threshold for deterministic tractography of the roots. FigShare Prepr. https://doi.org/10.6084/m9.figshare.12173184
Tagliafico A, Calabrese M, Puntoni M, Pace D, Baio G, Neumaier CE et al (2011) Brachial plexus MR imaging: accuracy and reproducibility of DTI-derived measurements and fibre tractography at 3.0-T. Eur Radiol 21:1764–1771
Samardzic M, Rasulic L, Grujicic D, Bacetic D, Milicic B (2011) Nerve transfers using collateral branches of the brachial plexus as donors in patients with upper palsy-thirty years’ experience. Acta Neurochir (Wien) 153:2009–2019
Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C (2012) Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60:340–352
Yap QJ, Teh I, Fusar-Poli P, Sum MY, Kuswanto C, Sim K (2013) Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. J Neural Transm 120:1369–1395
Gesslbauer B, Hruby LA, Roche AD, Farina D, Blumer R, Aszmann OC (2017) Axonal components of nerves innervating the human arm. Ann Neurol 82:396–408
Aboitiz F, Scheibel AB, Fisher RS, Zaidel E (1992) Fiber composition of the human corpus callosum. Brain Res 598:143–153
Yokoyama I (1989) Study on the intraneural topography of the brachial plexus. Nihon Seikeigeka Gakkai Zasshi Japan 63:1085–1102
Sinha S, Prasad GL, Lalwani S (2016) A cadaveric microanatomical study of the fascicular topography of the brachial plexus. J Neurosurg 125:355–362
Helmer KG, Chou M-C, Preciado RI, Gimi B, Rollins NK, Song A et al (2016) Multi-site study of diffusion metric variability: effects of site, vendor, field strength, and echo time on regions-of-interest and histogram-bin analyses. In: Gimi B, Krol A (eds) Physiol Behav, p 97882U
Jones DK, Basser PJ (2004) “Squashing peanuts and smashing pumpkins”: how noise distorts diffusion-weighted MR data. Magn Reson Med 52:979–993
Schilling KG, Nath V, Blaber J, Harrigan RL, Ding Z, Anderson AW et al (2017) Effects of b-value and number of gradient directions on diffusion MRI measures obtained with Q-ball imaging. In: Styner MA, Angelini ED (eds) Proc SPIE Int Soc Opt Eng., p 101330N
Qin W, Shui YuC, Zhang F, Du XY, Jiang H, Xia Yan Y et al (2009) Effects of echo time on diffusion quantification of brain white matter at 15T and 30T. Magn Reson Med 61:755–760
Haakma W, Pedersen M, Froeling M, Uhrenholt L, Leemans A, Boel LWT (2016) Diffusion tensor imaging of peripheral nerves in non-fixed post-mortem subjects. Forensic Sci Int 263:139–146
Giannelli M, Cosottini M, Michelassi MC, Lazzarotti G, Belmonte G, Bartolozzi C et al (2010) Dependence of brain DTI maps of fractional anisotropy and mean diffusivity on the number of diffusion weighting directions. J Appl Clin Med Phys 11:176–190
Taylor PA, Alhamud A, van der Kouwe A, Saleh MG, Laughton B, Meintjes E (2016) Assessing the performance of different DTI motion correction strategies in the presence of EPI distortion correction. Hum Brain Mapp 37:4405–4424
Haddad SMH, Scott CJM, Ozzoude M, Holmes MF, Arnott SR, Nanayakkara ND et al (2019) Comparison of quality control methods for automated diffusion tensor imaging analysis pipelines. PLoS ONE 14:e0226715
Vos SB, Jones DK, Viergever MA, Leemans A (2011) Partial volume effect as a hidden covariate in DTI analyses. Neuroimage 55:1566–1576
Le Bihan D, Mangin J-F, Poupon C, Clark CA, Pappata S, Molko N et al (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13:534–546
Watanabe M, Sakai O, Ozonoff A, Kussman S, Jara H (2013) Age-related apparent diffusion coefficient changes in the normal brain. Radiology 266:575–582
Kronlage M, Schwehr V, Schwarz D, Godel T, Uhlmann L, Heiland S et al (2018) Peripheral nerve diffusion tensor imaging (DTI): normal values and demographic determinants in a cohort of 60 healthy individuals. Eur Radiol 28:1801–1808
Acknowledgements
We thank Yuan Chi, Researcher at the Institute for Complementary and Integrative Medicine, University Hospital Zurich, Switzerland for translating an included article from Chinese to English and Helena Roess for translating an article we screened from Czech to English, which was facilitated through Cochrane’s TaskExchange platform (https://community.cochrane.org/help/tools-and-software/taskexchange). We also wish to thank Yemisi Takwoingi DVM MSc PhD, Senior Research Fellow in Biostatistics in the Test Evaluation Research Group within the Institute of Applied Health Research, University of Birmingham for her advice on the statistical approach. We thank Donald Sammut FRCS FRCS(Plast) for generously permitting the reproduction of his artwork in this manuscript (https://www.donaldsammut.com).
Funding
Ryckie Wade is a Doctoral Research Fellow funded by the National Institute for Health Research (NIHR, DRF-2018-11-ST2-028). This research is also supported by the NIHR Leeds Biomedical Research Centre and University of Leeds Advanced Imaging Centre, which is funded by the Medical Research Council with support from the British Heart Foundation and Arthritis Research UK. Funding was also given by the Wallenberg Centre for Molecular Medicine and the Västerbotten County Council, Sweden. The views expressed are those of the author(s) and not necessarily those of the United Kingdom’s National Health Service, NIHR or Department of Health.
Author information
Authors and Affiliations
Contributions
RGW conceived and designed the study, registered the protocol, extracted and analysed data, performed risk of bias assessments and co-authored the manuscript. AW extracted data, performed risk of bias assessments and co-authored the manuscript. IT, GA, FY and MW supervised the analysis of data analysis, interpretation and co-authored the manuscript. GB had oversight of the entire project, supervising the design, analysis and interpretations, and co-authored the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wade, R.G., Whittam, A., Teh, I. et al. Diffusion tensor imaging of the roots of the brachial plexus: a systematic review and meta-analysis of normative values. Clin Transl Imaging 8, 419–431 (2020). https://doi.org/10.1007/s40336-020-00393-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-020-00393-x