Abstract
In the rapidly evolving field of nuclear medicine, imaging cardiac sympathetic innervation using both conventional nuclear medicine and PET tracers is a small but growing field of interest. Recent larger clinical trials have underlined the importance of imaging cardiac sympathetic innervation as well as the consequences of low regional tracer accumulation for patient outcomes. New developments have resulted in the introduction of novel PET tracers with high clinical potential, especially for imaging centers without an on-site cyclotron. Despite the generated guidelines, especially on MIBG scintigraphy, widespread compliance with standardization efforts for cardiac sympathetic innervation imaging has not been yet achieved. Compliance with standardization of imaging acquisition and data analysis is crucial to move forward towards refinement of clinical guidelines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Disturbances in cardiac sympathetic innervation can be consequence of different pathophysiological mechanisms. Myocardial perfusion abnormalities, such as ischemia and infarction, are the most prevalent causes of impaired cardiac sympathetic innervation. These result in a high risk of developing ventricular dysrhythmias and sudden cardiac death [1, 2]. However, also in non-ischemic cardiomyopathies (e.g., dilated and restrictive cardiomyopathy due to amyloid deposits and infiltration), cardiac sympathetic innervation abnormalities are known to translate into worse clinical outcomes [3, 4].
Similar to cardiac involvement in systemic amyloidosis, cardiac sympathetic innervation abnormalities can also be a result from systemic synucleinopathy [5]. In Lewy body dementia and Parkinson’s disease, α-synuclein aggregates are found in the heart as well as in the brain. As such, cardiac sympathetic innervation imaging enables reliable differentiation between Parkinson’s disease from syndromic Parkinsonism (as in multi-system atrophy) [6].
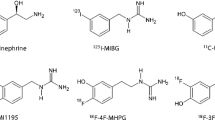
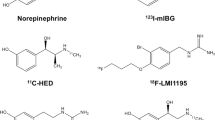
In the rapidly developing field of nuclear medicine, several tracers for cardiac sympathetic innervation imaging have been developed within the last 30 years. In conventional nuclear medicine, the most widely studied tracer is iodine-123-labeled metaiodobenzylguanidine (123I-MIBG), whereas in positron emission tomography (PET), most experience has been obtained using carbon-11-labeled meta-hydroxy-ephedrine (11C-mHED), which is the chemically preferred description over 11C-mHED. Both these tracers are used to visualize the presynaptic portion of sympathetic innervation. Details on cardiac sympathetic innervation imaging using both 123I-MIBG (in neurodegeneration) and 11C-mHED (in heart failure) are available in reviews published in this issue by Flotats et al. [7] and Popescu et al. [8].
As a complement to the aforementioned reviews, this mini-review addresses the advantages of PET over conventional nuclear medicine, the role of cardiac sympathetic innervation imaging in clinical decision-making, and the need for improvement of standardization of established techniques.
PET versus SPECT in cardiac sympathetic innervation
In the rapidly evolving field of nuclear medicine, cardiac sympathetic innervation imaging represents a discrete but growing field of interest. Cardiac sympathetic innervation using both conventional nuclear medicine and PET tracers is increasingly investigated, and recently, an overview of the currently available tracers was published in this journal [9].
123I-MIBG is the result of chemical modification of the false neurotransmitter analogue guanethidine and, therefore, an analogue of norepinephrine. The uptake of 123I-MIBG occurs similar to the uptake of norepinephrine: predominantly by a specific uptake system (“uptake-1”) and to a much lesser extent, by a non-specific uptake system (passive diffusion, “uptake-2”). Eventually, 123I-MIBG, like norepinephrine, is stored in the granules of the presynaptic nerve terminals. In a non-pathological situation, 123I-MIBG, unlike norepinephrine, is not bound to receptors on the myocyte membrane and does not become catabolized by monoamine oxidase (MOA). Therefore, 123I-MIBG is retained in these granules in healthy subjects [10, 11].
11C-mHED is a PET radiopharmaceutical for imaging cardiac sympathetic innervation. 11C-mHED is derived from the false norepinephrine analogue metaraminol, which is taken up by the presynaptic nerve terminals similar to norepinephrine (through an uptake-1 mechanism) [12, 13]. After intravenous injection, 11C-mHED is rapidly cleared from the blood and is taken up by presynaptic nerve terminals. Like 123I-MIBG, 11C-mHED is not metabolized by catechol-O-methyl transferase or oxidatively deaminated by MOA. Unlike 123I-MIBG, 11C-mHED rapidly leaks out of the presynaptic vesicles and diffuses into the interstitial space and will dynamically recycle into the neurons again [3]. During increased sympathetic tone, there is an increase in spillover of 11C-mHED from the nerve terminal, leading to a decreased re-uptake [14]. Notably, when released into the synaptic cleft by sympathetic nerve stimulation, both radiopharmaceuticals have no significant postsynaptic effect.
PET imaging has several advantages over conventional nuclear medicine techniques, the most important being the better spatial resolution: 8–10 mm for conventional nuclear medicine versus approximately 3–4 mm for PET. Another advantage of using PET as the imaging modality over conventional nuclear medicine techniques is the possibility of absolute quantification of tracer uptake. The uptake of 11C-mHED is expressed by the retention index, which is defined as the myocardial activity divided by the integral of the time-activity curve in plasma [15]. Since retention of 11C-mHED is dependent of myocardial perfusion, a nitrogen-13-labeled ammonia (13N-NH3) PET is always performed before the 11C-mHED acquisition. Distribution of 11C-mHED throughout left ventricular myocardium in healthy normal individuals is regionally homogeneous with high uptake in all myocardial segments [16]. Therefore, the PET tracer 11C-mHED is an attractive non-invasive method to quantify the activity and distribution of sympathetic innervation.
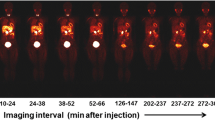
Performing head-to-head comparisons within the same patient cohort would provide insights into which modality outperforms the other. However, these comparisons between 123I-MIBG and 11C-mHED for cardiac sympathetic innervation in humans are rather scarce. In patients with left ventricular dysfunction, defect size (defined as < 60% of maximum) assessed with 123I-MIBG scintigraphy and 11C-mHED PET are closely correlated: r = 0.88 based on late heart-to-mediastinum ratio [17]. Since 123I-MIBG scintigraphy underestimates the tracer uptake in septal and inferior wall segments, this modality overestimates defect size in those areas compared to 11C-mHED. The inferior to inferoapical (or even inferolateral) defect in 123I-MIBG accumulation is probably not a consequence from heart failure, since the same distribution is found in healthy controls [18]. At present, the explanation for this defect has not been fully elucidated, but may be caused by the so-called liver–heart artifact and filtered back projection algorithm. Therefore, it is likely that 11C-mHED outperforms 123I-MIBG in the assessment of regional cardiac sympathetic innervation abnormalities. Despite the advantage of 11C-mHED over 123I-MIBG in tracer uptake quantification, 11C-mHED does not provide in washout kinetics, in contrast to 123I-MIBG. To overcome the challenges of applying 11C-labeled PET sympathetic innervation imaging radiotracers, an 18F-labeled benzylguanidine [18F-N-(3-bromo-4-(3-fluoro-propoxy)-benzyl)-guanidine (18F-LMI1195)] was developed resembling the structure of 123I-MIBG with the additional benefit of high-sensitivity PET imaging and the extended half-life of 18F. 18F-LMI1195 has similar binding affinity and transport kinetics to endogenous norepinephrine [19] and similar tracer kinetics to 123I-MIBG [20, 21]. These initial clinical studies also showed excellent image quality of 18F-LMI1195, which was superior to 123I-MIBG and equal to 11C-mHED [22]. Future studies are needed to assess the potential of MIBG-like PET tracers in the evaluation of PET-derived washout kinetics.
The role of cardiac sympathetic de-innervation in clinical decision-making
There is increasing evidence that a diminished cardiac 123I-MIBG uptake and 11C-mHED retention are associated with worse clinical outcome in patients with ischemic and non-ischemic cardiomyopathies (especially dilated cardiomyopathy) [23,24,25]. The ADMIRE-HF trial showed that low late heart-to-mediastinum ratio (HMR) was associated with a higher incidence of appropriate implantable cardioverter–defibrillator (ICD) discharges as well as with the composite end-point consisting of progression of heart failure, ventricular arrhythmia, and sudden cardiac death. Unfortunately, a meta-analysis of 600 + heart failure patients could not identify a low HMR as an independent prognostic factor for the development of ventricular arrhythmia [26]. However, in transthyretin-derived cardiac amyloidosis, multivariate analyses did reveal that a low late HMR is as an independent prognostic marker for 5-year mortality [4]. Despite the evidence for dichotomizing late 123I-MIBG HMR to predict survival, survival rates are linearly correlated with decreasing late 123I-MIBG HMR. This dichotomizations may have limitations, since the presence of both intra- and inter-observer variabilities in drawing the regions-of-interest. This uncertainty results in a ‘measurement grey zone’ with potentially important consequences for both treatment and prognosis [27].
Decreased cardiac 11C-mHED retention is inversely related to severity of heart failure symptoms in patients with dilated cardiomyopathy and associated with an increased risk of ventricular arrhythmia in patients with ischemic cardiomyopathy.
Besides global tracer accumulation, the size of a regionally diminished cardiac tracer accumulation (defined as < 60% of maximum) has been studied in relation with clinical outcome. A prospective multicenter center identified defect size as the only independent prognostic factor for ventricular arrhythmia in patients with ischemic cardiomyopathy [28]. In addition, in patients who underwent ICD implantation due to (non-)ischemic cardiomyopathy, a large late 123I-MIBG sized defect was an independent prognostic predictor of appropriate ICD discharge and sudden cardiac death [29]. Although in another study, neither late HMR nor 123I-MIBG defect size was associated with appropriate ICD therapy, the combination of late HMR and LVEF was significantly associated with the absence of appropriate ICD discharge [30]. In addition, comparable results were presented in the PAREPET trail using 11C-mHED for cardiac sympathetic innervation imaging [23]. PET-derived defect size was considered to be the only independent predictive marker for sudden cardiac death [23]. Based on these findings, future studies should focus on cost-effectiveness of ICDs in patients with ischemic cardiomyopathy. Despite that ICD is the most effective treatment option to prevent death from ventricular arrhythmia and superior compared to the use of anti-arrhythmic drugs alone [31, 32], not all patients eventually suffer from ventricular arrhythmia. In fact, it seems that only a minority of patients (approximately 30%) benefit from prophylactic ICD treatment.
Cardiac resynchronization therapy (CRT) is a potential treatment option for patients with heart failure (HF), particularly in those with a left ventricular ejection fraction (LVEF) < 35%, a wide QRS complex (≥ 150 ms), and a New York Heart Association (NYHA) class of II–IV [31]. CRT has been shown to improve HF symptoms and reduce hospitalizations and life-threatening arrhythmias in some patients [33]. However, about one-third of patients receiving CRT do not respond to therapy. In a case series (n = 10), patients with dilated cardiomyopathy and moderate severity HF (LVEF ≤ 35%; NHYA II–III) underwent 11C-mHED PET imaging prior to and early (1 week, respectively, 3 months) after CRT implantation [34]. Patients that responded to CRT therapy (reduction in left ventricle end systolic volume of ≥ 15% at the 3-month follow-up) had higher 11C-mHED SUVs and less regional heterogeneity in tracer uptake at baseline compared to non-responders.
Therefore, future studies should focus on: (1) the need for additional therapy in patients with larger innervation defect sizes to decrease the risk of sudden cardiac death; (2) confirmation that patients with a small sized defect on either 123I-MIBG or any PET tracers are likely to benefit from postponing ICD implantation; and (3) better identification of those patients who will benefit from CRT.
Improvements in standardization of acquisition and analysis
With the recent introduction of new and improved tracers for cardiac sympathetic innervation, there is an increasing demand for standardization of both image acquisition and analysis. Imaging cardiac sympathetic innervation has been reported to have potential in clinical decision-making, especially regarding the identification of those patients who will benefit from ICD implantation. However, this potential may not proceed into incorporation to the guidelines as long as there is heterogeneity in image acquisition and data analysis [35]. Despite proposed standardization for 123I-MIBG imaging, substantial differences in late HMR and washout have been reported due to unaddressed heterogeneity in imaging acquisition and semi-quantitative calculations, respectively. In particular, both early and late HMR are vendor dependent and are determined by the choice for either low-energy or medium-energy collimators [36]. HMRs determined on images acquired with low-energy collimators are generally lower than those acquired with medium-energy collimators [36]. Although the use of medium-energy collimators is preferred, many studies report image acquisition using low-energy collimators, and conversion algorithms have been published to overcome these differences [37, 38]. In addition, meta-analyses of data from larger prospective studies using these conversion factors in the evaluation of outcome parameters are now being published. Especially, in discriminating dementia with Lewy bodies from Alzheimer’s disease, the conversion factors were used to re-assess 123I-MIBG HMR [39]. In addition to these conversion factors, cross-calibration phantom studies have been performed in both Japan and Europe, to convert clinically established HMR into standardized HMR [40, 41]. Very recently, recalculation of previously published 123I-MIBG HMR and washout data was used to validate a 2-year mortality risk model in patients with chronic heart failure [42]. The model showed a good correlation with actual cardiac mortality, but underestimated cardiac mortality for the quartile of patients with the highest risk of cardiac death. Data from these cross-calibration studies may also be helpful in comparing future multicenter 123I-MIBG databases [43].
Finally, comparison of results from different studies would benefit from robust software applications for analysis of both PET and 123I-MIBG-based data. At present, there is no dedicated application for quantification of cardiac sympathetic function available.
Conclusions
Imaging cardiac sympathetic innervation is a small but growing field of interest. It has a large potential in clinical decision-making, particular in risk stratification to define patients that would benefit from ICD therapy or in predicting the response to CRT in HF patients with reduced LVEF. Compliance to standardization of imaging acquisition and data analysis is needed to move forward towards incorporation into clinical guidelines.
References
Gradel C, Jain D, Batsford WP, Wackers FJ, Zaret BL (1997) Relationship of scar and ischemia to the results of programmed electrophysiological stimulation in patients with coronary artery disease. J Nucl Cardiol 4:379–386
Wichter T, Hindricks G, Lerch H, Bartenstein P, Borggrefe M, Schober O, Breithardt G (1994) Regional myocardial sympathetic dysinnervation in arrhythmogenic right ventricular cardiomyopathy. An analysis using 123I-meta-iodobenzylguanidine scintigraphy. Circulation 89:667–683
Hartmann F, Ziegler S, Nekolla S, Hadamitzky M, Seyfarth M, Richardt G, Schwaiger M (1999) Regional patterns of myocardial sympathetic denervation in dilated cardiomyopathy: an analysis using carbon-11 hydroxyephedrine and positron emission tomography. Heart 81:262–270
Coutinho MC, Cortez-Dias N, Cantinho G, Conceição I, Oliveira A, e Sá AB et al (2013) Reduced myocardial 123-iodine metaiodobenzylguanidine uptake: a prognostic marker in familial amyloid polyneuropathy. Circ Cardiovasc Imaging 6:627–636
Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ et al (2014) Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord 29:1010–1018
Braune S, Reinhardt M, Schnitzer R, Riedel A, Lücking CH (1999) Cardiac uptake of [123I]mIBG separates Parkinson’s disease from multiple system atrophy. Neurology 53:1020–1025
Flotats A (2018) Role of myocardial innervation imaging in the diagnosis of neurodegenerative diseases. Clin Transl Imaging 6:449–458
Popescu CE, Cuzzocrea M, Monaco L, Caobelli F (2018) Assessment of myocardial sympathetic innervation by PET in patients with heart failure: a review of the most recent advances and future perspectives. Clin Transl Imaging 6:459–470
Werner RA, Chen X, Hirano M, Rowe SP, Lapa C, Javadi MS, Higuchi T (2018) SPECT vs. PET in cardiac innervation imaging: clash of the titans. Clin Transl Imaging 6:293–303
Kline RC, Swanson DP, Wieland DM, Thrall JH, Gross MD, Pitt B, Beierwaltes WH (1981) Myocardial imaging in man with I-123 meta-iodobenzylguanidine. J Nucl Med 22:129–132
Wieland DM, Brown LE, Rogers WL, Worthington KC, Wu JL, Clinthorne NH, Otto CA, Swanson DP, Beierwaltes WH (1981) Myocardial imaging with a radioiodinated norepinephrine storage analog. J Nucl Med 22:22–31
Rosenspire KC, Haka MS, Van Dort ME, Jewett DM, Gildersleeve DL, Schwaiger M, Wieland DM (1990) Synthesis and preliminary evaluation of carbon-11-meta-hydroxyephedrine: a false transmitter agent for heart neuronal imaging. J Nucl Med 31:1328–1334
Crout JR, Alpers HS, Tatum EL, Shore PA (1964) Release of metaraminol (aramine) from the heart by sympathetic nerve stimulation. Science 145:828–829
Vesalainen RK, Pietilä M, Tahvanainen KU, Jartti T, Teräs M, Någren K, Lehikoinen P, Huupponen R, Ukkonen H, Saraste M, Knuuti J, Voipio-Pulkki LM (1999) Cardiac positron emission tomography imaging with [11C]hydroxyephedrine, a specific tracer for sympathetic nerve endings, and its functional correlates in congestive heart failure. Am J Cardiol 84:568–574
Allman KC, Wieland DM, Muzik O, Degrado TR, Wolfe ER Jr, Schwaiger M (1993) Carbon-11 hydroxyephedrine with positron emission tomography for serial assessment of cardiac adrenergic neuronal function after acute myocardial infarction in humans. J Am Coll Cardiol 22:368–375
Schwaiger M, Kalff V, Rosenspire K, Haka MS, Molina E, Hutchins GD, Deeb M, Wolfe E Jr, Wieland DM (1990) Noninvasive evaluation of sympathetic nervous system in human heart by positron emission tomography. Circulation 82:457–464
Matsunari I, Aoki H, Nomura Y, Takeda N, Chen WP, Taki J, Nakajima K, Nekolla SG, Kinuya S, Kajinami K (2010) Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ Cardiovasc Imaging 3:595–603
Morozumi T, Kusuoka H, Fukuchi K, Tani A, Uehara T, Matsuda S, Tsujimura E, Ito Y, Hori M, Kamada T, Nishimura T (1997) Myocardial iodine-123-metaiodobenzylguanidine images and autonomic nerve activity in normal subjects. J Nucl Med 38:49–52
Yu M, Bozek J, Lamoy M et al (2011) Evaluation of LMI1195, a novel 18F-labeled cardiac neuronal PET imaging agent, in cells and animal models. Circ Cardiovasc Imaging 4:435–443
Werner RA, Rischpler C, Onthank D et al (2015) Retention kinetics of the 18F-labeled sympathetic nerve PET tracer LMI1195: comparison with 11C-hydroxyephedrine and 123I-MIBG. J Nucl Med 56:1429–1433
Higuchi T, Yousefi BH, Reder S et al (2015) Myocardial kinetics of a novel [F]-labeled sympathetic nerve PET tracer LMI1195 in the isolated perfused rabbit heart. J Am Coll Cardiol Cardiovasc Imaging 8:1229–1231
Zelt J, Renaud JM, Mielniczuk L, Gerrard L, Robinson S, Orlandi C, Beanlands RS, de Kemp R (2018) Tracer kinetics for fluorine-18 LMI-1195 compared to carbon-11 hydroxyephedrine for PET imaging of sympathetic innervation. Can J Cardiol 34:S117–118
Fallavollita JA, Heavey BM, Luisi AJ Jr, Michalek SM, Baldwa S, Mashtare TL Jr, Hutson AD, Dekemp RA, Haka MS, Sajjad M, Cimato TR, Curtis AB, Cain ME, Canty JM Jr (2014) Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol 63:141–149
Pietilä M, Malminiemi K, Ukkonen H, Saraste M, Någren K, Lehikoinen P, Voipio-Pulkki LM (2001) Reduced myocardial carbon-11 hydroxyephedrine retention is associated with poor prognosis in chronic heart failure. Eur J Nucl Med 28:373–376
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J, ADMIRE-HF Investigators (2010) Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 55:2212–2221
Verschure DO, Veltman CE, Manrique A, Somsen GA, Koutelou M, Katsikis A et al (2014) For what endpoint does myocardial 123I-MIBG scintigraphy have the greatest prognostic value in patients with chronic heart failure? Results of a pooled individual patient data meta-analysis. Eur H J Cardiovasc Imaging 15:996–1003
Petretta M, Pellegrino T, Cuocolo A (2014) Cardiac neuronal imaging with 123I-meta-iodobenzylguanidine in heart failure: implications of endpoint selection and quantitative analysis on clinical decisions. Eur J Nucl Med Mol Imaging 41(9):1663–1665
Bax JJ, Kraft O, Buxton AE, Fjeld JG, Parizek P, Agostini D et al (2008) 123I-mIBG scintigraphy to predict inducibility of ventricular arrhythmias on cardiac electrophysiology testing: a prospective multicenter pilot study. Circ Cardiovasc Imaging 1:131–140
Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E et al (2010) Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol 55:2769–2777
Verschure DO, de Groot JR, Mirzaei S, Gheysens O, Nakajima K, van Eck-Smit BLF, Aernout Somsen G, Verberne HJ (2017) Cardiac 123I-mIBG scintigraphy is associated with freedom of appropriate ICD therapy in stable chronic heart failure patients. Int J Cardiol 248:403–408
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators (2005) Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 352:225–237
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial II Investigators (2002) Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346:877–883
Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, Kapa S, Kremers MS, Lindsay BD, Stevenson LW (2013) ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 61:1318–1368
Martignani C, Diemberger I, Nanni C, Biffi M, Ziacchi M, Boschi S, Corzani A, Fanti S, Sambuceti G, Boriani G (2015) Cardiac resynchronization therapy and cardiac sympathetic function. Eur J Clin Invest 45:792–799
Nakajima K, Okuda K, Matsuo S, Agostini D (2016) The time has come to standardize 123I-MIBG heart-to-mediastinum ratios including planar and SPECT methods. Eur J Nucl Med Mol Imaging 43:386–388
Flotats A, Carrio I, Agostini D et al (2010) Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging 37:1802–1812
Nakajima K, Okuda K, Yoshimura M, Matsuo S, Wakabayashi H, Imanishi Y, Kinuya S (2014) Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J Nucl Cardiol 21:970–978
Travin MI, Matsunari I, Thomas GS, Nakajima K, Yoshinaga K (2018) How do we establish cardiac sympathetic nervous system imaging with 123I-mIBG in clinical practice? Perspectives and lessons from Japan and the US. J Nucl Cardiol. https://doi.org/10.1007/s12350-018-1394-5 (Epub ahead of print)
Komatsu J, Samuraki M, Nakajima K, Arai H, Arai H, Arai T, Asada T, Fujishiro H, Hanyu H, Iizuka O, Iseki E, Kashihara K, Kosaka K, Maruno H, Mizukami K, Mizuno Y, Mori E, Nakamura H, Nakano S, Nakashima K, Nishio Y, Orimo S, Takahashi A, Taki J, Tokuda T, Urakami K, Utsumi K, Wada K, Washimi Y, Yamashina S, Yamasaki J, Yoshita M, Yamada M (2018) 123I-MIBG myocardial scintigraphy for the diagnosis of DLB: a multicentre 3-year follow-up study. J Neurol Neurosurg Psychiatry 89:1167–1173
Nakajima K, Okuda K, Yokoyama K, Yoneyama T, Tsuji S, Oda H, Yoshita M, Kubota K (2017) Cross calibration of 123I-meta-iodobenzylguanidine heart-to-mediastinum ratio with D-SPECT planogram and Anger camera. Ann Nucl Med 31:605–615
Verschure DO, Poel E, Nakajima K, Okuda K, van Eck-Smit BLF, Somsen GA, Verberne HJ (2018) A European myocardial 123I-mIBG cross-calibration phantom study. J Nucl Cardiol 25:1191–1197
Nakajima K, Nakata T, Doi T, Kadokami T, Matsuo S, Konno T, Yamada T, Jacobson AF (2018) Validation of 2-year 123I-meta-iodobenzylguanidine-based cardiac mortality risk model in chronic heart failure. Eur Heart J Cardiovasc Imaging 19:749–756
Nakajima K, Verschure DO, Okuda K, Verberne HJ (2017) Standardization of 123I-meta-iodobenzylguanidine myocardial sympathetic activity imaging: phantom calibration and clinical applications. Clin Transl Imaging 5:255–263
Author information
Authors and Affiliations
Contributions
WN literature search, manuscript writing, final approval. AWJMG content planning, critical revision, final approval, LEJ-O critical revision, and final approval. RHJAS content planning, critical revision, and final approval.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest. No funding was received for this article.
Ethical approval (research involving human participants and/or animals)
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Noordzij, W., Glaudemans, A.W.J.M., Juarez-Orozco, L.E. et al. Towards consensus in acquisition and image analysis of PET and SPECT in the assessment of cardiac sympathetic innervation: a mini-review. Clin Transl Imaging 7, 33–38 (2019). https://doi.org/10.1007/s40336-018-00309-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-018-00309-w