Abstract
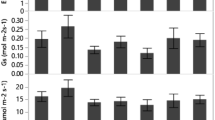
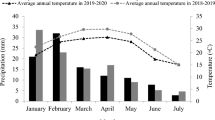
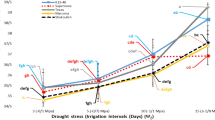
Stipagrostis ciliata (Desf.) De Winter is a pastoral C4 grass grown in arid regions. This research work focused on assessing the growth of S. ciliata accessions derived from two different climate regions (a wet arid region in the Bou Hedma National Park in the central and southern part of Tunisia (coded as WA), and a dry arid region from the Matmata Mountain in the south of Tunisia (coded as DA)) under water stress conditions. Specifically, the study aimed to investigate the phenological and physiological responses of potted S. ciliata seedlings under different water treatments: T1 (200 mm/a), T2 (150 mm/a), T3 (100 mm/a) and T4 (50 mm/a). Growth phenology, net photosynthesis (Pn), stomatal conductance (gs), midday leaf water potential (Ψmd), predawn leaf water potential (Ψpd), soil water content (SWC) and soil water potential (Ψs) were observed during the water stress cycle (from December 2016 to November 2017). The obtained results showed that the highest growth potential of the two accessions (WA and DA) was recorded under treatment T1. The two accessions responded differently and significantly to water stress. Photosynthetic parameters, such as Pn and gs, decreased sharply under treatments T2, T3 and T4 compared to treatment T1. The higher water stress increased the R/S ratio (the ratio of root dry biomass to shoot dry biomass), with values of 1.29 and 2.74 under treatment T4 for accessions WA and DA, respectively. Principal component analysis (PCA) was applied, and the separation of S. ciliata accessions on the first two axes of PCA (PC1 and PC2) suggested that accession DA was detected in the negative extremity of PC1 and PC2 under treatments T1 and T2. This accession was characterized by a high number of spikes. For treatments T3 and T4, both accessions were detected in the negative extremity of PC1 and PC2. They were characterized by a high root dry biomass. Therefore, S. ciliata accessions responded to water stress by displaying significant changes in their behaviours. Accession WA from the Bou Hedma National Park (wet arid region) showed higher drought tolerance than accession DA from the Matmata Mountain (dry arid region). S. ciliata exhibits a significant adaptation capacity for water limitation and may be an important species for ecosystem restoration.
Similar content being viewed by others
References
Adaawen S. 2021. Understanding climate change and drought perceptions, impact and responses in the Rural Savannah, West Africa. Atmosphere, 12(5): 594, doi: https://doi.org/10.3390/atmos12050594.
Anjum S A, Xie X Y, Wang L C, et al. 2011. Morphological, physiological and biochemical responses of plants drought stress. African Journal of Agriculture Research, 6(9): 2026–2032.
Aronson J, Goodwin N, Orlando L, et al. 2020. A world of possibilities: Six restoration strategies to support the United Nation’s Decade on Ecosystem Restoration. Restoration Ecology, 28(4): 730–736.
Batool T, Ali S, Seleiman M F, et al. 2020. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Scientific Reports, 10: 16975, doi: https://doi.org/10.1038/s41598-020-73489-z.
Ben Mariem H, Chaieb M. 2017. Climate change impacts on the distribution of Stipa tenacissima L. ecosystems in North African arid zones. Applied Ecology and Environmental Research, 15(3): 67–82.
Brocca L, Moramarco T, Melone F, et al. 2017. A new method for rainfall estimation through soil moisture observations. Geophysical Research Letters, 40(5): 853–858.
Chaieb M, Henchi B, Boukhris M. 1996. Impact of clipping on root systems of 3 grasses species in Tunisia. Journal of Range Management, 49(4): 336–339.
Christin P A, Colin P O. 2014. The evolutionary ecology of C4 plants. New Phytologist, 204(4): 765–781.
Daur I. 2012. Plant flora in the rangeland of western Saudi Arabia. Acta Physiologiae Plantarum, 44: 223–269.
Derbel D, Cortina J, Chaieb M. 2009. Acacia saligna plantation impact on soil surface properties and vascular plant species composition in central Tunisia. Arid Land Research and Management, 23(1): 28–46.
Ding Z, Ali E F, Elmahdy A M, et al. 2021. Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agricultural Water Management, 244: 106626, doi: https://doi.org/10.1016/j.agwat.2020.106626.
Du N, Guo W H, Zhang X R, et al. 2010. Morphological and physiological responses of Vitex negundo L. var. heterophylla (Franch.) Rehd. to drought stress. Acta Physiologiae Plantarum, 32: 839–848.
Fahad S, Ali A B, Usman N, et al. 2017. Crop production under drought and heat stress: plant responses and management options. Frontiers of Plant Science, 8: 1147, doi: https://doi.org/10.3389/fpls.2017.01147.
Farman A, Bano A, Fazal A. 2017. Recent methods of drought stress tolerance in plants. Plant Growth Regulation, 82(1): 363–375.
Farooq M, Kobayashi N, Ito O, et al. 2010. Broader leaves result in better performance of indica rice under drought stress. Journal of Plant Physiology, 167(13): 1066–1075.
Fernandez R J, Reynolds J F. 2000. Potential growth and drought tolerance of eight desert grasses: lack of a trade-off? Oecologia, 123: 90–98.
Frontier S. 1973. Etude statistique de la dispersion du zooplancton. Journal of Experimental Marine Biology and Ecology, 12(3): 229–262.
Gazanchian A, Hajheidari M, Sima N A K, et al. 2007. Proteome response of Elymus elongatum to severe water stress and recovery. Journal of Experimental Botany, 58(2): 291–300.
Ghannoum O. 2009. C4 photosynthesis and water stress. Annals of Botany, 103(4): 635–644.
Guo W, Li B, Zhang X, el al. 2007. Architectural plasticity and growth responses of Hippophae rhamnoides and Caragana intermedia seedlings to simulated water stress. Journal of Arid Environments, 69(3): 385–399.
Hamdani M, Krichen K, Chaieb M. 2019. Predicting leaf trait variability as a functional descriptor of the effect of climate change in three perennial grasses. Diversity, 11(12): 233, doi: https://doi.org/10.3390/d11120233.
Henschel J R, Burke A, Seely M. 2005. Temporal and spatial variability of grass productivity in the central Namib desert. African Studies Monographs, 30: 43–56.
Hillel D. 1982. Introduction to Soil Physics. San Diego: Academic Press, 392.
IPCC. 2014. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Jones H G. 2007. Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance. Journal of Experimental Botany, 58(2): 119–130.
Kadioglu A, Terzi R. 2007. A dehydration avoidance mechanism: leaf rolling. Botanical Review, 73: 290–302.
Kellogg E A. 2013. C4 photosynthesis. Current Biology, 23(14): R594–R599.
Kharrat-Souissi A, Baumel A, Torre F, et al. 2012. Genetic differentiation of the dominant perennial grass Cenchrus ciliaris L. contributes to response to water deficit in arid lands. Rangeland Journal, 34(1): 55–62.
Kharrat-Souissi A, Siljak-Yakovlev S, Brown S C, et al. 2013. Cytogeography of Cenchrus ciliaris (Poaceae) in Tunisia. Folia Geobotanica, 48(1): 95–113.
Kharrat-Souissi A, Siljak-Yakovlev S, Brown S C, et al. 2014. The polyploid nature of Cenchrus ciliaris L. (Poaceae) has been overlooked: new insights for the conservation and invasion biology of this species-a review. Rangeland Journal, 36(1): 11–23.
Liu S, Liu J, Cao J, et al. 2006. Stomatal distribution and character analysis of leaf epidermis of jujube under drought stress. Journal of Anhui Agricultural University, 34: 1315–1318. (in Chinese)
Ma Y, Maria C D, Helena F. 2020. Drought and salinity stress responses and microbe-induced tolerance in plants. Frontiers of Plant Science, 13: 1–18.
Maestre F T, Blas M B, Miguel B, et al. 2021. Biogeography of global drylands. New Phytologist, 231(2): 540–558.
Mnif Fakhfakh L, Anjum N A, Chaieb M. 2018. Assessment of temperature and water limitation effects on the germination of Stipagrostis ciliata seeds collected from Bou Hedma, Central South Tunisia. Journal of Arid Land, 10(2): 304–315.
Mnif Fakhfakh L, Jeddi K, Anjum N A, et al. 2020. Plant traits and phenotypic variability effect on the phytomass production of Stipagrostis ciliata (Desf.) De Winter. Saudi Journal of Biological Sciences, 27(6): 1553–1561.
Niu F, Nathan A P, Steven R A, et al. 2021. Germination and early establishment of dryland grasses and shrubs on intact and wind-eroded soils under greenhouse conditions. Plant and Soil, 465: 245–260.
Ouled Belgacem A, Neffati M, Papanastasis V P, et al. 2006. Effects of seed age and seeding depth on growth of Stipa lagascae R. & Sch. Seedlings. Journal of Arid Environments, 65(4): 682–687.
Raza A, Razzaq A, Mehmood S S, et al. 2019. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants, 8(2): 34, doi: https://doi.org/10.3390/plants8020034.
Ritchie G A, Hinckley T M. 1975. The pressure chamber as an instrument for ecological research. Advances in Ecological Research, 9: 165–254.
Roy A, Núñez Delgado A, Sultana S, et al. 2021. Additions of optimum water, spent mushroom compost and wood biochar to improve the growth performance of Althaea rosea in drought-prone coal-mined spoils. Journal of Environmental Management, 295: 113076, doi: https://doi.org/10.1016/j.jenvman.2021.113076.
Sage R F. 2004. The evolution of C4 photosynthesis. New Phytologist, 161(2): 341–370.
Santos F D, Stigter T Y, Faysse N, et al. 2014. Impacts and adaptation to climate change in the Mediterranean coastal areas: the CIRCLE-MED initiative. Regional Environmental Change, 14(Suppl. 1): 1–3.
Sayed O H. 2003. Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica, 41: 321–330.
Scholander P F, Bradstreet E D, Hemmingsen E A, et al. 1965. Sap pressure in vascular plants. Science, 148(3668): 339–346.
Seleiman M F, Refay Y, Al-Suhaibani N, et al. 2019. Integrative effects of rice-straw biochar and silicon on oil and seed quality, yield and physiological traits of Helianthus annuus L. grown under water deficit stress. Agronomy, 9(10): 637, doi: https://doi.org/10.3390/agronomy9100637.
Shao H, Chu L Y, Jaleel C A, et al. 2008. Water deficit stress induced anatomical changes in higher plants. Comptes Rendus Biologies, 331(3): 215–225.
Thioulouse J, Chessel D, Dolédec S, et al. 1997. ADE-4: multivariate analyses and graphical display for environmental data. Statistics and Computing, 7(1): 75–83.
Turner N C. 1988. Measurement of plant water status by the pressure chamber technique. Irrigation Sciences, 9(4): 289–308.
van Rooyen N, Bredenkamp G J, Theron G K. 1991. Kalahari vegetation: veld condition trends and ecological status of species. Koedoe-African Protected Area Conservation and Science, 34(1): 61–72.
Wellstein C, Poschlod P, Gohlke A, et al. 2017. Effects of extreme drought on specific leaf area of grassland species: A meta-analysis of experimental studies in temperate and sub-Mediterranean systems. Global Change Biology, 23(6): 2473–2481.
West A G, Hultine K R, Sperry J S, et al. 2008. Transpiration and hydraulic strategies in a piñon-juniper woodland. Ecological Applications, 18(4): 911–927.
Xiong L, Wang R G, Mao G, et al. 2006. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiology, 142: 1065–1074.
Xu B C, Gichuki P, Shan L, et al. 2006. Aboveground biomass production and soil water dynamics of four leguminous forages in semiarid region, northwest China. South African Journal of Botany, 72(4): 507–516.
Yu M, Gao Q, Epstein H E, et al. 2009. Quantification of leaf gas exchange characteristics of dominant C3/C4 plants at the Kalahari transect. South African Journal of Botany, 75(3): 518–525.
Zhao W, Lui L, Shen Q, et al. 2020. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water, 12(8): 2127, doi: https://doi.org/10.3390/w12082127.
Acknowledgements
This research was supported by Tunisian Minister of Research and High Education in particular Laboratory of Ecosystems and Biodiversity in Arid Land of Tunisia (LEBIOMAT), University of Sfax.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mnif Fakhfakh, L., Chaieb, M. Effects of water stress on growth phenology photosynthesis and leaf water potential in Stipagrostis ciliata (Desf.) De Winter in North Africa. J. Arid Land 15, 77–90 (2023). https://doi.org/10.1007/s40333-022-0082-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40333-022-0082-0