Abstract
Introduction
Central obesity (CO), characterized by an increased waist circumference increases the risk of cardiovascular disease (CVD) and morbidity, yet the underlying mechanisms are not fully understood. CO is often associated with general obesity, hypertension, and abnormal glucose tolerance, confounding the independent contribution of CO to CVD.
Aim
We investigated the relationship of CO (without associated disorders) with left ventricular (LV) characteristics and intrathoracic adipose tissue (IAT) by cardiac magnetic resonance.
Methods
LV characteristics, epicardial (EAT), and mediastinal adipose tissue (MAT) were measured from 29 normoglycemic, normotensive males with CO but without general obesity (waist circumference >100 cm, body mass index (BMI) <30 kg/m2) and 18 non-obese male controls.
Results
LV maximal wall thickness (LVMWT) and IAT but not LV mass or volumes were increased in CO subjects compared to controls (LVMWT, 12.3±1.2 vs. 10.7±1.5 mm, p < 0.001; EAT, 5.5±3.0 vs. 2.2±2.0 cm2, p = 0.001; MAT, 31.0±12.8 vs. 15.4±10.7 cm2, p < 0.001). The LVMWT was ≥12 mm in 69% of subjects with CO and 22% of controls (p = 0.002). In CO suspects, EAT correlated inversely with LV end-diastolic volume index (r = − 0.403, p = 0.037) and LV stroke volume (SV) (r = − 0.425, p = 0.027). MAT correlated inversely with SV (r = − 0.427, p=0.026) and positively with LVMWT (r = 0.399, p = 0.035). Among CO subjects, the waist-to-hip ratio (WHR) was an independent predictor of LVMWT (B = 22.4, β = 0.617, p < 0.001). The optimal cut-off with Youden’s index for LV hypertrophy was identified at WHR 0.98 (sensitivity 85%, specificity 89%).
Conclusions
CO independent of BMI is associated with LV hypertrophy and intrathoracic adipose tissue contributing to cardiovascular burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Central obesity, characterized by excessive accumulation of abdominal fat, is a growing concern worldwide, affecting an estimated 42% of individuals aged 15 or older [1]. It is well-known that the risk of mortality increases with general obesity. Individuals with central obesity are at a particularly high risk of developing type 2 diabetes and cardiovascular disease (CVD) and facing a higher mortality rate [2, 3]. While previous studies have mainly focused on patients with overall obesity, as assessed by body mass index (BMI), there is a growing interest in understanding how central obesity and especially pericardial intrathoracic adipose tissue affects left ventricular (LV) characteristics [4, 5]. Moreover, a recent study has shown that waist-to-hip ratio (WHR) exhibits the most robust and consistent correlation with mortality, independent of BMI [6]. This phenomenon could be attributed to the diverse body compositions and discrepancies in adipose tissue distribution among individuals sharing similar BMI values. Central obesity, however, is often associated with general obesity, hypertension, and abnormal glucose tolerance, confounding the independent contribution of central obesity to CVD.
Intrathoracic adipose tissue (IAT), which includes mediastinal adipose tissue (MAT) and epicardial adipose tissue (EAT) located near the heart, can influence cardiovascular health through genetic and neural factors, inflammation, fibrosis, fatty infiltration, and atrial electrical or structural remodeling [7]. EAT, in particular, is unique due to its proximity to the heart and it undergoes metabolic changes as we age, shifting from a thermogenic role to primarily energy storage [8]. Additionally, pericardial fat and visceral adiposity tissue (VAT) have been linked to the risk of having coronary heart disease regardless of conventional measures of obesity [9]. Moreover, EAT affects myocardial electrophysiology through factors like intermyocyte disruption, tissue fibrosis, and gap junction remodeling [10]. Although both EAT and VAT have been found to correlate with LV mass and LV dimensions measured by cardiac magnetic resonance (CMR) imaging, the strength of these relationships is attenuated when adjusted for body weight [11]. While previous research indicates that IAT is linked to LV mass and LV diastolic dysfunction in cases of metabolic syndrome [12], further investigations are required to clarify these associations in individuals without a history of high blood pressure or diabetes.
We hypothesize that central obesity, regardless of general adiposity, may instigate alterations in LV structure, potentially through its impact on IAT. These myocardial changes could potentially result in cardiovascular hypertrophy and further contribute to the heightened risk of cardiovascular mortality. For this reason, we conducted a study examining the influence of isolated central obesity (excluding general obesity, hypertension, or glucose intolerance) on LV characteristics and IAT using CMR.
2 Methods
2.1 Study Subjects
The subjects for the present CMR study were prospectively and consecutively recruited from the large METSIM (Metabolic Syndrome in Men) study [13]. The METSIM study is a population-based study including 10,197 Caucasian Finnish men aged 45 to 70 years at baseline living in Kuopio (population about 0,1 million), Eastern Finland. All the anthropometric and biochemical measurements were measured in the METSIM study. A total of 50 consecutive subjects studied in the METSIM study within a year before the onset of the CMR study and fulfilling the inclusion criteria were recruited for the CMR study.
METSIM study subjects with central obesity (n = 29) had to fulfill the following criteria to be included in the present CMR study: (1) age between 50 to 65 years, (2) waist >100 cm (to include those with clear-cut central obesity), (3) BMI <30 kg/m2 (to exclude those with clear-cut general obesity), (4) office measured systolic blood pressure <145 mmHg (mean of two measurements), and diastolic blood pressure <90 mmHg and no medication for elevated blood pressure, (5) no previously diagnosed diabetes and normal glucose tolerance in an oral glucose tolerance test, (6) serum alanine transaminase <80 U/l, and 7) no medication for CVD. Two-hour oral glucose tolerance test (2-h OGTT) and laboratory tests were carried out after at least 8 hours of fasting. A detailed description of biochemical assays is given in Supplementary data.
For the control subjects (n = 18), the inclusion criteria were identical except for the waist, which had to be <94 cm, a threshold of central obesity defined by the International Diabetes Federation and the World Health Organization for Europeans [14].
To exclude clinically significant coronary artery disease causing myocardial ischemia, the bicycle ergometer exercise tolerance test was carried out in all potential study subjects before enrollment for the CMR study. Exclusion criteria were defined as (1) slowly ascending ST segment depression >0.20 mV or (2) horizontal or downwards facing ST segment depression >0.10 mV in the exercise test. The details of the exercise study are shown in Supplementary data.
Three of the 50 subjects were excluded from the study. One subject had an aneurysm clip in his brain (contraindication for CMR), one subject had a myocardial scar in CMR late gadolinium enhancement (LGE) imaging indicating previous myocardial infarction, and one subject was claustrophobic. Thus, a total of 47 subjects (29 subjects with central obesity and 18 control subjects) were included in the final study population.
2.2 Anthropometric Measurements and Analyses of Body Composition at Baseline
The body weight of subjects wearing light clothing without shoes was measured at 0.1 kg precision in the METSIM study. The weight was measured again at the time of the CMR imaging, and the latter value was used for indices of CMR parameters. Height was measured to the nearest 0.5 cm. BMI was calculated as body weight in kilograms divided by height in meters squared. Waist circumference (WC) was measured as the average of two measurements taken after inspiration and expiration at the midpoint between the lowest rib and iliac crest. Systolic and diastolic blood pressures were measured three times using a standard sphygmomanometer. The mean values of the three measurements were used in the statistical analyses.
2.3 Cardiac Imaging and Image Analysis
CMR was performed with a 1.5 T clinical CMR unit (Magnetom Avanto; Siemens Medical Systems, Erlangen, Germany) by one radiologist (J.M.) with three years of experience in CMR at the beginning of the study. A phased-array body coil was used as a receiver. After scout views were obtained, 8 mm sections with a 10% gap were acquired in the transaxial plane using a Half-Fourier acquisition single-shot turbo spin echo (HASTE) sequence covering the whole heart (repetition time 700 msec/echo time 40 msec, flip angle 160°, data matrix 192 × 256, field of view 255 × 340). The HASTE sequence was used for the evaluation of EAT and MAT.
For the anatomical and functional evaluation, 8 mm sections with a 25% gap were acquired in the short axis plane using a multisection True-Fisp sequence extending from the apex of the heart to the base including the atria (repetition time 34.86 msec/echo time 1.08 msec, 65° flip angle; 168x192 data matrix; 297 × 340 field of view). Eight to twelve 8 mm slices were assessed to cover the whole LV. Imaging was performed during multiple breath holds.
The end-diastolic image was the first image acquired after the R-wave of the electrocardiographic signal, and the end-systolic image was the image with the smallest LV area at the mid-ventricular level. The LV end-diastolic volume (LVEDV), end-systolic volume (LVESV), and stroke volume (SV) were computed using manually traced short-axis end-diastolic and end-systolic areas respectively, excluding the papillary muscles and trabeculations.
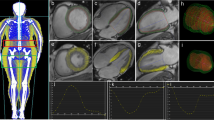
All anatomic measurements on CMR images were performed with Syngo Argus postprocessing software provided with the CMR system (Syngo MMWP VE27A, Siemens, Erlangen, Germany) by the same radiologist (J.M). To evaluate LV mass, the endocardium, and epicardium (excluding papillary muscles and trabeculations) were manually traced using end-diastolic images. The total myocardial mass of the LV was calculated by using the Argus software. LV mass, LVEDV, and LVESV were indexed by dividing the values by the body surface area. To determine the maximal end-diastolic wall thickness of the LV (LVMWT), all LV short-axis plane images were evaluated. The LVMWT was measured manually from every image, excluding right ventricular trabeculation and LV papillary muscles (Fig. 1A, B). The maximal value of all LV wall thickness measurements was defined as LVMWT. The location of LVMWT was noted according to a 16-segment system [15].
Assessment of left ventricular maximal wall thickness (LMWT), mediastinal, and epicardial fat. LMWT is measured from the cardiac magnetic resonance (CMR) short-axis end-diastolic image (Images A and B). Image A shows a normal LMWT of 10 mm in the interventricular septum in a control subject. Image B shows an increased LMWT of 15 mm in a subject with central obesity. White arrows show a typical location for LMWT in both study groups. The transaxial CMR image at the level of the coronary ostium shows the mediastinal fat (MFat, red arrows) and epicardial fat (EFat, yellow arrows) separated by the pericardium, in a control subject (Image C) and a subject with central obesity (Image D)
To measure IAT, the EAT and MAT areas were evaluated from a single 8 mm slice at the level of the coronary sinus ostium using the HASTE sequence, which provides high tissue contrast between fat and other mediastinal structures. The EAT area was defined as the adipose tissue area between the myocardium and pericardium (Fig. 1C, D). The MAT area was defined as the adipose tissue area outside the pericardium and limited to the anterior bony thorax, diaphragm, and posteriorly thoracic spine (Fig. 1C, D). The IAT area measurements were performed with an IDS5 diagnostic workstation (version 10.2P4; Sectra Imtec, Linköping, Sweden).
To evaluate the intraobserver variability of LVMWT measurements, the same radiologist (J.M.) repeated all LVMWT measurements 3 months after the completion of the first assessments blinded to his original measurements. To evaluate interobserver variability, another radiologist (P.S.) with fifteen years of experience in CMR performed 30 random LVMWT measurements independently, blinded to the study group and prior measurements.
2.4 Statistical Analyses
Data are presented as mean ± SD, or median and interquartile range if the variable was not normally distributed. The independent samples t-test was used to investigate the differences between the study groups when analyzing variables with normal distribution. Mean workload during the last 4 minutes during exercise (WLAST4), maximal heart rate (HRmax), total triglycerides, high-sensitivity CRP, and plasma adiponectin were log-transformed for statistical analyses due to their skewed distribution. Age, BMI, waist, diastolic blood pressure, fasting plasma glucose, and EAT area were not normally distributed after logarithmic transformation and were therefore compared between the study groups using the Mann-Whitney U test. Univariate regression analysis was used to investigate the correlates of LV characteristics in subjects with central obesity. Nonparametric Spearman’s correlation was used to evaluate the correlation between the parameters not normally distributed after logarithmic transformation.
To show the difference in LVMWT between the study groups, mean and 95% confidence intervals were used. Power analysis (R statistical software version 3.0.2) was performed to verify adequate sample size in LVMWT analysis. In power analysis, 80% power was achieved with 12 subjects. We analyzed 47 study subjects with 97% power. Analysis of covariance (ANCOVA) was used to control for the effects of BMI and age when comparing the LVMWT and the amount of IAT between centrally obese and control subjects. BMI standardized LVMWT was calculated by regression analysis.
A multivariable regression analysis was performed for centrally obese subjects, using a stepwise approach, was utilized to identify associations with selected parameters (which differed significantly between centrally obese and control patients) and LVMWT and the Receiver Operating Characteristic (ROC) curve was utilized to analyze the sensitivity and specificity for predicting LVH. The optimal cut-off points were determined using Youden's index. Intra- and interobserver variability of the LVMWT assessments were examined by calculating intraclass correlation coefficients (ICC) and coefficient of variability (CV%). Data analyses were performed with SPSS (16.0 and 29.0).
3 Results
Of clinical characteristics, age, height, weight, BMI, waist, and hip circumference were higher in the central obesity group compared to the control group (p<0.001) (Table 1), whereas office-measured systolic and diastolic blood pressure did not differ between the groups. In the bicycle ergometer exercise test (Table 1, Supplement 1), there was no significant difference in systolic or diastolic blood pressure at rest or maximal exercise, mean workload during the last 4 minutes of exercise (WLAST4), or maximal oxygen consumption (VO2max) between the study groups. The maximal heart rate (HRmax) was significantly lower in the central obesity group compared to the control group.
In laboratory tests, there was no statistically significant difference in total cholesterol, LDL, HDL cholesterol, or fasting glucose levels between the control subjects and centrally obese subjects (Table 2). In the central obesity group, the total triglyceride level was significantly higher compared to that in the control group (p = 0.007). Fasting serum insulin level was significantly higher (p = 0.004) and the insulin sensitivity index was significantly lower (p = 0.002) in the central obesity group than in the control group. In subjects with central obesity, high-sensitive CRP was significantly higher compared to the controls (p < 0.001). There was no statistically significant difference in plasma adiponectin levels between the two groups. Fasting serum insulin, insulin resistance index, high-sensitive CRP, total cholesterol, LDL, HDL cholesterol, fasting glucose levels, total triglyceride levels or plasma adiponectin did not correlate with CMR findings in centrally obese subjects (data not shown) except for the borderline correlation between Matsuda index and SV in centrally obese (r = 0.39, p = 0.04).
In CMR imaging, subjects with central obesity had significantly increased LVMWT compared to control subjects (12.3±1.2 (8–14) vs. 10.7±1.5 (10–15) mm, p < 0.001) (Figs. 1 and 2; Table 3). The LVMWT exceeded the normal range (6-11 mm) in 20 of 29 subjects with central obesity and four of 18 control subjects (69 vs. 22 %, p=0.002). The maximal wall thickness in the centrally obese subjects was located in the interventricular septum at basal and midventricular levels (Fig. 1B).
Scatterplot of the relationship of left ventricular maximal wall thickness and body mass index (BMI) in centrally obese and control subjects (A). Left ventricular maximal wall thickness (mean and 95 % confidence intervals) in study groups before and after standardization for BMI (B). Stdzd standardized
There was no significant difference in LVEDV index, LVESV index, LV ejection fraction, LV SV, or LV mass index between centrally obese and control subjects (Table 3). There was, however, a trend for smaller LVEDV in subjects with central obesity compared to that in controls, but the difference between the groups was not statistically significant.
LVMWT in the centrally obese did not correlate with BMI (r = 0.06, p = 0.757; Figure 2A) or age (r = 0.243, p = 0.100). The difference in LVMWT between the study groups remained significant when adjusted for BMI (p = 0.015), age (p < 0.001), or both (p = 0.012) (Table 3). The BMI-standardized LVMWTs in the study groups are shown in Figure 2B.
EAT area, MAT area, and VAT as well as SAT areas were significantly larger in the central obesity group than in the control group (Figure 3). The difference in IAT area (epicardial plus mediastinal adipose tissue area) between the study groups remained even after adjustment for BMI (p = 0.012), age (p < 0.001), or both (p = 0.021).
EAT area correlated inversely with LVEDV index (r = − 0.403, p = 0.037) and LV SV (r = − 0.425, p = 0.027), and there was a trend for a positive correlation between EAT area and LVMWT (r = 0.368, p = 0.054). MAT area correlated inversely with LV SV (r = − 0.427, p = 0.027) and positively with LVMWT (r = 0.399, p = 0.035). EAT area or MAT area did not correlate with LV mass, LV end-systolic volume, or ejection fraction (data not shown). There was no correlation between EAT area or MAT area and CMR findings in the control group (data not shown).
For the measurement of LVMWT, the intraobserver ICC was 0.82 and the corresponding intraobserver CV was 8.0 %. The interobserver ICC was 0.87 and the interobserver CV was 8.0 %.
The univariate and multivariate analyses were conducted on the centrally obese population (n = 29) related to LVMWT. In the univariate model WHR, hip measurement, and total blood triglyceride level correlated significantly with LVMWT (p<0.05 for all). BMI did not correlate with LVMWT (p = 0.260) (Table 4). The WHR (β = 0.617, p < 0.001) was an independent predictor of LVMWT, explaining together 36% of its variation. Additionally, the optimal WHR was calculated to predict LVH (LVMWT ≥12mm) and presented as a ROC curve (Fig. 4). Utilizing Youden’s index, the optimal cut-off for WHR was 0.98, with a sensitivity of 85% and specificity of 89% (Fig. 4).
A Receiver operating characteristic (ROC) curve for left ventricular maximal wall thickness (LVMWT) ≥12 mm representing of left ventricle hypertrophy (LVH) among centrally obese subjects (n = 29). B The correlation between waist-to-hip ratio (WHR) and LVMWT among centrally obese. The blue dotted line indicates the threshold distinguishing normal myocardial thickness from LVH, while the red dotted line signifies the WHR cut-off (0.98) derived from ROC analysis. AUC Area Under the Curve (95% confidential interval), Sens sensitivity, Spec specificity
4 Discussion
4.1 Principal Findings
The present study shows that central obesity independently of BMI is associated with LVH characterized by increased LV maximal wall thickness in CMR. LV maximal wall thickness, EAT area, and MAT area in centrally obese were significantly increased compared to those in control subjects. Moreover, in subjects with central obesity, IAT area correlated inversely with LVEDV index and SV, and positively with LVMWT, suggesting that IAT may play a significant role in LV remodeling in central obesity.
4.2 Findings in the Context of the Current Literature
It is widely recognized that overall obesity is associated with LV dilatation, hypertrophy, and increased LV mass assumed to result from increased blood volume creating a high cardiac output state [16]. More recently, attention has shifted towards the exploration of central obesity, revealing a distinct link to unfavorable cardiac structure and function. Some studies have demonstrated that pericardial fat is associated with cardiac remodeling [17]. Yet, it has been also suggested that the systemic impact of obesity might have a more substantial effect on cardiac structure and function than the localized pathogenic effects of pericardial fat [11]. Nevertheless, a significant gap exists in the research, as prospective studies have not been conducted specifically in patients with isolated central obesity without the presence of other metabolic syndrome-related factors like hypertension, hypercholesterolemia, or dyslipidemia. The prevalence of central obesity is often closely associated with these components, which can make it challenging to find individuals with central obesity who do not also have hypertension, hyperlipidemia, or diabetes.
A study from an Asian cohort (n = 2471, 51% women) free from known CVD showed that elevated pericardial adipose tissue has a significant association with higher LV mass index [5]. Similarly, another study (n = 4234, 52% of women) by Shah et al. confirmed an association between pericardial fat and elevated LV mass index [4]. Moreover, they showed that pericardial fat was predictive of an adverse cardiovascular prognosis over a 12-year follow-up period. These findings showed an association between pericardial fat and the manifestation of unfavorable cardiovascular outcomes. Notably, in both studies [4, 5] the patients with higher pericardial fat were more frequently male and had a higher prevalence of dysglycemia, dyslipidemia, and hypertension. In our research, we found that isolated central obesity was not linked to LV dilatation or an increase in LV mass. Instead, it was associated with LV wall thickening. This intriguing distinction may, in part, be attributed to the lack of hypertensive patients in our cohort, a factor that appears to exert a particularly noteworthy effect on LV mass [18]. The thickest area in our subjects with central obesity was located in the interventricular septum and exceeded the normal upper limit (≥12 mm) in the majority of subjects [19]. Our study suggests that in isolated central obesity, LV remodeling is characterized mostly by increased LV thickness.
4.3 Potential Mechanisms
This study is cross-sectional in nature, thus limiting our ability to definitively determine the underlying mechanisms. However, we propose potential explanations behind these findings. In the present study, the amount of MAT significantly correlated with LV thickness, and there was a near-significant correlation between EAT and LV thickness. Adipose tissue adjacent to the heart may have a direct local effect on the myocardium by adipokines and inflammatory mediators inducing remodeling of the LV [20, 21]. Alternatively, there may be lipid infiltration in the myocardium leading to LVH in centrally obese subjects, a phenomenon already described in general obesity and patients with metabolic syndrome [22, 23]. Furthermore, individuals with central obesity displayed higher insulin levels, which act as a growth factor, alongside diminished insulin sensitivity compared to the control group (Table 2). Insulin levels and insulin resistance have been shown to be associated independently with LVH [24]. Nevertheless, owing to the relatively small dataset, the absence of a clear correlation may be limited in effectively demonstrating a direct relationship with LV mass. This limitation could be due to statistical constraints (beta error).
Overall obesity, hypertension, or abnormal glucose tolerance are less likely to account for the observed LVH because subjects with clear-cut obesity, hypertension, and abnormal glucose tolerance were excluded from the present study. BMI was higher in the centrally obese group, but the difference in LVMWT remained between the study groups after standardization for BMI. Finally, fasting plasma glucose, lipids, insulin, insulin sensitivity index, hS-CPR, or adiponectin levels were not associated with LWMWT or other LV characteristics in centrally obese subjects, suggesting that these biomarkers do not directly cause LVH.
Other mechanisms, like aortic stiffness, might explain to some small extent the association between central obesity and LVH [25]. Aortic arch stiffness is associated with LV mass in isolated hypertension [25] and systolic LV function in patients with type 1 diabetes [26] even in young persons. Insulin metabolism disorders, characteristic of central obesity, might increase aortic stiffness and subsequently LV afterload, resulting in LVH. Finally, genetic factors might contribute to the development of LVH in central obesity. Lately, several susceptibility loci for component traits of metabolic syndrome, which is characterized by central obesity, have been identified, mainly in genes of lipid metabolism pathways [27]. However, there is little evidence for susceptibility loci to the syndrome as an entity [27].
4.4 Imaging Aspects
We used a single transversal slice obtained at the level of the coronary sinus to evaluate EAT and MAT [28]. This measurement level was selected because it was easy to identify in all study subjects and because the imaging plane was perpendicular to the cardiac and mediastinal structures and, accordingly, methodologically suitable for planimetry with minimal partial volume effect.
4.5 Strengths and Limitations
Strengths: The strength of the study is that the study subjects were selected from a very large, well-characterized population-based study, allowing the ruling out of generally obese and hypertensive subjects, as well as those with abnormal glucose tolerance. Consequently, we could investigate the independent effects of central obesity on LV and IAT in a unique study design.
Limitations: While this deliberate exclusion enhances the internal validity of our findings by reducing the confounding effects of these comorbidities, it is essential to recognize that this sampling strategy may limit the generalizability of our results to the broader population of individuals with central obesity. Also, the relatively small sample size of the study is a limitation; however, it has been substantiated as adequate through the power analysis. In this study we excluded hypertension patients a) by excluding patients with hypertensive medication and b) by measuring blood pressure twice at rest in the office. This approach presents a limitation when compared to the more comprehensive 24-hour blood pressure monitoring method. Unfortunately, due to constraints within our research setting, implementing the 24-hour monitoring method was not feasible. Although a very large number of subjects of the METSIM study were screened to find subjects with isolated central obesity without exclusion criteria, the study design was not a case-control study, but all consecutive subjects fulfilling the inclusion criteria were recruited. Consequently, the control and central obesity groups were not well matched for BMI and age. We have, however, taken this into account by adjusting for BMI and age in all pivotal analyses. Also, the absence of female study subjects can be viewed as a limitation. Finally, it's worth noting that myocardial triglyceride content was not evaluated with spectroscopy [29], and the importance of lipid accumulation in the myocardium as the cause of LVH associated with central obesity could not be evaluated.
4.6 Clinical Implications
This study demonstrated that a considerable number of subjects with central obesity but without general obesity defined as BMI over 30 kg/m2, hypertension, or glucose intolerance, have LVH characterized by increased maximal LV wall thickness. LVH is a major risk factor for cardiac death [30] and consequently, may contribute to increased cardiovascular morbidity and mortality in subjects with central obesity. Our findings contribute to the present concept of the harmfulness of central obesity to cardiovascular health [3, 31].
Our findings suggest that central obesity may contribute to the development of LVH. In central obesity, LVH appears to be common, of relatively mild severity, predominantly affecting the interventricular septum, and typically associated with nondilated LV. In our study, over two-thirds of the centrally obese subjects had an LVMWT exceeding the normal upper limit of 12 mm, with values up to 15 mm. Furthermore, we found out that WHR was an independent predictor of maximal thickness of the LV wall, explaining 36% of its variation among centrally obese males. Notably, BMI had no significant association with LVMWT. In our study, the optimal cut-off (using Youden’s index) for LVH (LVMWT ≥12mm) was WHR 0.98, with 85% sensitivity and 89% specificity. The diagnostic accuracy was better than what Rodrigues et al. have previously reported among a more heterogenic male population (n = 277 of which 56% had diagnosed hypertension and 15% had BMI ≥ 30 kg/m2) [32]. In their study, the identified cut-off for WHR to detect LVH (measured with echocardiography as left ventricular mass indexed to height ≥ 47.7 gm-2.7), was 0.94, exhibiting a sensitivity of 65% and specificity of 66%. However, it's notable that both thresholds are somewhat higher than the World Health Organization states to have an association with a higher risk for metabolic complications (WHR ≥ 0.90) in men. [33]. While further large-scale studies are needed to establish the precise contribution of central obesity to LVH, the results of the present study imply that central obesity is an independent risk factor for LVH and should be considered in the differential diagnosis of borderline LVH. Interestingly, our findings also suggest that the most sensitive indicator of LVH in central obesity—as in hypertrophic cardiomyopathy [34]—appears to be increased LVMWT rather than LV mass.
5 Conclusion
Central obesity is associated with LV hypertrophy, characterized by increased LV wall thickness, and with an increased amount of IAT. In centrally obese subjects, IAT is associated with increased LV maximal wall thickness and decreased LV end-diastolic and stroke volumes, suggesting that IAT plays a significant role in LV remodeling.
Data availability
The data that support the findings of this study are not publicly available. However, the data, or a portion of it, can be made available from the corresponding author upon reasonable request.
References
Wong MCS, Huang J, Wang J, Chan PSF, Lok V, Chen X, Leung C, Wang HHX, Lao XQ, Zheng ZJ. Global, regional and time-trend prevalence of central obesity: a systematic review and meta-analysis of 132 million subjects. Eur J Epidemiol. 2020;35(7):673–83.
Balkau B, Deanfield JE, Després JP, Bassand JP, Fox KAA, Smith SC, Barter P, Tan CE, Van Gaal L, Wittchen HU, Massien C, Haffner SM. International day for the evaluation of abdominal obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168 000 primary care patients in 63 countries. Circulation. 2007;116(17):1942–51.
Jayedi A, Soltani S, Zargar MS, Khan TA, Shab-Bidar S. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies, vol. 370. BMJ Publishing Group; 2020.
Shah RV, Anderson A, Ding J, Budoff M, Rider O, Petersen SE, Jensen MK, Koch M, Allison M, Kawel-Boehm N, Wisocky J, Jerosch-Herold M, Mukamal K, Lima JAC, Murthy VL. Pericardial, but not hepatic, fat by CT Is associated with CV outcomes and structure: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2017;10(9):1016–27.
Kim JS, Kim SW, Lee JS, Lee SK, Abbott R, Lee KY, Lim HE, Sung KC, Cho GY, Koh KK, Kim SH, Shin C, Kim SH. Association of pericardial adipose tissue with left ventricular structure and function: a region-specific effect? Cardiovasc Diabetol. 2021. https://doi.org/10.1186/s12933-021-01219-4.
Khan I, Chong M, Le A, Mohammadi-Shemirani P, Morton R, Brinza C, Kiflen M, Narula S, Akhabir L, Mao S, Morrison K, Pigeyre M, Paré G, American Medical Association. Surrogate adiposity markers and mortality. JAMA Network Open. 2023;6:2334836.
Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19:593–606.
Ojha S, Fainberg HP, Wilson V, Pelella G, Castellanos M, May ST, Lotto AA, Sacks H, Symonds ME, Budge H. Gene pathway development in human epicardial adipose tissue during early life. JCI Insight. 2016. https://doi.org/10.1172/jci.insight.87460.
Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O’Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30(7):850–6.
Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, Montgomery MK, Binny S, Watts T, Joshi SB, Lui E, Sim CB, Larobina M, O’Keefe M, Goldblatt J, Royse A, Lee G, Porrello ER, Watt MJ, Kistler PM, Sanders P, Delbridge LMD, Kalman JM. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol. 2020;76(10):1197–211.
Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, Levy D, Larson MG, Agostino RBD, O’onnell CJ, Manning WJ. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function. Circulation. 2009;119(12):1586–91.
Nyman K, Granér M, Pentikäinen MO, Lundbom J, Hakkarainen A, Sirén R, Nieminen MS, Taskinen MR, Lundbom N, Lauerma K. Cardiac steatosis and left ventricular function in men with metabolic syndrome [Internet]. 2013. Available from: http://jcmr-online.com/content/15/1/103. Accessed 5 Jan 2024.
Laakso M, Kuusisto J, Stančáková A, Kuulasmaa T, Pajukanta P, Lusis AJ, Collins FS, Mohlke KL, Boehnke M. The metabolic syndrome in men study: a resource for studies of metabolic & cardiovascular diseases. J Lipid Res. 2017;58(3):481–93.
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WPT, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–5.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation. 2002;105(4):539–42. https://doi.org/10.1161/hc0402.102975.
Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. 2008; Available from: www.prv.org. Accessed 5 Jan 2024.
Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity. 2008;16(7):1693–7.
Akasheh A, Wu Y, Li Y, Dustin LD, Wong ND, Gardin JM, Azen SP, Detrano RC. Association of blood pressure with left ventricular mass in untreated hypertensives in rural yunnan province. Am J Hypertens. 2009;22(7):730–4.
Grajewski KG, Stojanovska J, Ibrahim ESH, Sayyouh M, Attili A. Left Ventricular Hypertrophy: Evaluation With Cardiac MRI. Curr Probl Diagn Radiol [Internet]. 2020;49(6):460–75. Available from: https://www.sciencedirect.com/science/article/pii/S0363018819302075. Accessed 5 Jan 2024.
Mazurek T, Zhang LF, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6.
Rider OJ, Lewandowski A, Nethononda R, Petersen SE, Francis JM, Pitcher A, Holloway CJ, Dass S, Banerjee R, Byrne JP, Leeson P, Neubauer S. Gender-specific differences in left ventricular remodelling in obesity: Insights from cardiovascular magnetic resonance imaging. Eur Heart J. 2013;34(4):292–9.
Granér M, Siren R, Nyman K, Lundbom J, Hakkarainen A, Pentikäinen MO, Lauerma K, Lundbom N, Adiels M, Nieminen MS, Taskinen MR. Cardiac steatosis associates with visceral obesity in nondiabetic obese men. J Clin Endocrinol Metab. 2013;98(3):1189–97.
Anumonwo JMB, Herron T. Fatty infiltration of the myocardium and arrhythmogenesis: potential cellular and molecular mechanisms. Front Physiol. 2018. https://doi.org/10.3389/fphys.2018.00002.
Kianu Phanzu B, Nkodila Natuhoyila A, Kintoki Vita E, M’Buyamba Kabangu JR, Longo-Mbenza B. Association between insulin resistance and left ventricular hypertrophy in asymptomatic, Black, sub-Saharan African, hypertensive patients: a case–control study. BMC Cardiovasc Disord. 2021. https://doi.org/10.1186/s12872-020-01829-y.
Sarnecki J, Obrycki Ł, Feber J, Chełstowska S, Jurkiewicz E, Litwin M. Isolated systolic hypertension is associated with increased left ventricular mass index and aortic stiffness in adolescents: a cardiac magnetic resonance study. J Hypertens. 2022;40(5):985–95.
El-Saiedi SA, Hafez MH, Sedky YM, Sharaf SA, Kamel MS, Abdelmassih AF. Novel biomarkers for subtle myocardial involvement in type I diabetes mellitus. Cardiovasc Endocrinol Metab. 2021;10(3):175–81.
Kristiansson K, Perola M, Tikkanen E, Kettunen J, Surakka I, Havulinna AS, Stančáková A, Barnes C, Widen E, Kajantie E, Eriksson JG, Viikari J, Kähönen M, Lehtimäki T, Raitakari OT, Hartikainen AL, Ruokonen A, Pouta A, Jula A, Kangas AJ, Soininen P, Ala-Korpela M, Männistö S, Jousilahti P, Bonnycastle LL, Järvelin MR, Kuusisto J, Collins FS, Laakso M, Hurles ME, Palotie A, Peltonen L, Ripatti S, Salomaa V. Genome-wide screen for metabolic syndrome susceptibility loci reveals strong lipid gene contribution but no evidence for common genetic basis for clustering of metabolic syndrome traits. Circ Cardiovas Genet. 2012. https://doi.org/10.1161/CIRCGENETICS.111.961482/-/DC1.
Oyama N, Goto D, Ito YM, Ishimori N, Mimura R, Furumoto T, Kato F, Tsutsui H, Tamaki N, Terae S, Shirato H. Single-slice epicardial fat area measurement: do we need to measure the total epicardial fat volume? Jpn J Radiol. 2011;29(2):104–9.
Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W, Unger R, Victor RG. Myocardial triglycerides and systolic function in humans. In vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49(3):417–23.
Haukilahti MAE, Kenttä TV, Tikkanen JT, Anttonen O, Aro AL, Kerola T, Eranti A, Holkeri A, Rissanen H, Heliövaara M, Knekt P, Junttila MJ, Huikuri HV. Electrocardiographic risk markers of cardiac death: gender differences in the general population. Front Physiol. 2021;5:11.
Xu C, Guo Y, Zhang S, Lai Y, Huang M, Zhan R, Liu M, Xiong Z, Huang Y, Huang R, Liao X, Zhuang X, Cai Z. Visceral adiposity index and the risk of heart failure, late-life cardiac structure, and function in ARIC study. Eur J Prev Cardiol. 2023. https://doi.org/10.1093/eurjpc/zwad099/7111614.
Rodrigues SL, Baldo MP, Sá Cunha R, Angelo LC, Pereira AC, Krieger JE, Mill JG. Anthropometric measures of increased central and overall adiposity in association with echocardiographic left ventricular hypertrophy. Hypertens Res. 2010;33(1):83–7.
World Health Organization. Waist circumference and waist-hip ratio : report of a WHO expert consultation, Geneva, 8-11 December 2008. World Health Organization; 2011.
Zamorano JL, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Piepe PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H, O’Mahony C, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol Ç, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Alfonso F, Basso C, Cardim NM, Gimeno JR, Heymans S, Holm PJ, Keren A, Lionis C, Muneretto C, Priori S, Salvador MJ, Wolpert C. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–79 (Oxford University Press).
Acknowledgments
The authors thank Mr. Sakari Kainulainen and Ms. Satu Nenonen for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contributions
Jarkko Marttila (J.M.): conceptualization, data curation, visualization, writing—original draft, writing—review & editing. Petri Sipola (P.S.): conceptualization, Study design, CMR sequence protocol setup, writing—original draft. Auni Juutilainen: statistical design, data curation, formal analysis. Marja Hedman: writing—review & editing, interpretation of results, investigation. Saara Sillanmäki (S.S.) writing—review & editing, interpretation of results, investigation, funding, statistical analysis. Johanna Kuusisto (J.K.): study design, writing—original draft, writing—review & editing, investigation, funding acquisition, supervision. All authors read and approved the final manuscript.
Funding
Open access funding provided by University of Eastern Finland (including Kuopio University Hospital). J.K. has received grants from the Finnish Heart Research Foundation, the special governmental subsidy for health sciences research of the University Hospital of Kuopio, and the Academy of Finland. S.S. has received the Research Committee of the Kuopio University Hospital Catchment Area for State Research Funding (project 5063586).
Conflict of interest
The authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Marttila, J., Sipola, P., Juutilainen, A. et al. Central Obesity is Associated with Increased Left Ventricular Maximal Wall Thickness and Intrathoracic Adipose Tissue Measured with Cardiac Magnetic Resonance. High Blood Press Cardiovasc Prev (2024). https://doi.org/10.1007/s40292-024-00659-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40292-024-00659-9