Abstract
The relationship between Serum Uric Acid (UA) and Cardiovascular (CV) diseases has already been extensively evaluated, and it was found to be an independent predictor of all-cause and cardiovascular mortality but also acute coronary syndrome, stroke and heart failure. Similarly, also many papers have been published on the association between UA and kidney function, while less is known on the role of UA in metabolic derangement and, particularly, in metabolic syndrome. Despite the substantial number of publications on the topic, there are still some elements of doubt: (1) the better cut-off to be used to refine CV risk (also called CV cut-off); (2) the needing for a correction of UA values for kidney function; and (3) the better definition of its role in metabolic syndrome: is UA simply a marker, a bystander or a key pathological element of metabolic dysregulation?. The Uric acid Right for heArt Health (URRAH) project was designed by the Working Group on uric acid and CV risk of the Italian Society of Hypertension to answer the first question. After the first papers that individuates specific cut-off for different CV disease, subsequent articles have been published responding to the other relevant questions. This review will summarise most of the results obtained so far from the URRAH research project.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A dramatically growing body of evidence suggests that serum uric acid (SUA) plays a relevant role in cardiovascular (CV) and metabolic disease incidence and clinical severity, acting either as an independent risk factor or synergistically with other known CV risk factors. Despite the established evidence, the threshold level of SUA able to significantly contribute to cardiovascular risk remains undefined [1]. Moreover, it is not yet clear if SUA is the best marker of purine metabolism and if purine dysmetabolism is per se a cardiometabolic risk factor or if SUA is an indirect marker of oxidative stress that is per se a cardiometabolic risk factor [2].
Anyway, the association of SUA and CV disease risk has also been largely investigated in Mendelian randomization analyses. It has been suggested that every 1-SD increase in genetically predicted SUA was associated with an increased risk of coronary heart disease (odds ratio 1.19 [95% CI 1.10–1.30]; P = 4 × 10−5), peripheral artery disease (1.12 [95%CI 1.03–1.21]; P = 9 × 10−3), and stroke (1.11 [95% CI 1.05–1.18]; P = 2 × 10−4), where elevated blood pressure was estimated to mediate approximately one-third of the effect of urate on CV disease risk [3]. In a very large genome-wide study (N. 457,690 individuals), the Mendelian randomization analysis showed that elevated genetically determined serum urate levels were associated with increased risks of coronary heart disease in men but not in women [4].
Furthermore, recently, Moshkovits et al. have evaluated the impact of SUA on the risk of developing a composite of death, acute coronary syndrome, or stroke in 19,769 asymptomatic self-referred adults aged 40–79 years free of cardiovascular disease and diabetes (mean age 50 ± 8 years, 31% women) annually screened in a preventive healthcare setting. During a median follow-up of 6 years, 1658 (8%) subjects reached the study endpoint. Continuous net reclassification improvement analysis showed a 13% improvement in the accuracy of classification when high SUA was added to either pooled cohort equations (PCE) model or Systematic COronary Risk Evaluation 2 (SCORE2) model (P < 0.001 for both). In particular, Subgroup analyses showed a significant 16–20% improvement in the model performance among normal-weight and low-risk subjects (P < 0.001 for PCE; P = 0.026 and P < 0.001 for SCORE2, respectively) [5].
In this context, the Uric acid Right for heArt Health (URRAH) project has been designed to define, as primary objective, the SUA level above which the independent risk of cardiovascular disease increases in a significant manner in a large and well-characterized Mediterranean population sample [6]. However, after answering this first question, we find that such a database will be able to provide important information on many other still unanswered questions about SUA, such as modifying factors of the relationship with CV events and a better explanation of its role in kidney disease and metabolic derangements. This review describes the main results of the URRAH research project.
2 The URRAH Project
The protocol of this study has been described extensively in previous publications [7]. Briefly, this is a multicentre retrospective, observational cohort study which has involved the collection of data on (mainly hypertensive outpatients and subjects from general population with a median follow-up period of 10 years (up to 31 July 2017).
Data from participant’s centre were collected and has been included into a general database. The various cohorts included comes from Italian Centres of Hypertension, distributed in almost all the Italian regions and recognised by the Italian Society of Hypertension.
Inclusion criteria were the availability of at least one or serum UA levels determination and of complete information’s about demographics, CV risk factor (known history of arterial hypertension, diabetes mellitus, smoking habit, overweight/obesity defined through body mass index and waist circumference), previous CV events, CV drug therapies, blood pressure values, biochemical data (total and fractioned cholesterol, triglycerides and renal function estimated). From some centre also data on cardiac (Left Ventricular Hypertrophy – LVH) and renal (urinary albumin excretion) hypertension-mediated organ damage were reported.
At the end of the follow-up, the following hard endpoints (based on the International Classification of Diseases, Tenth Revision - ICD-10) has been evaluated: all-cause mortality, CV mortality, fatal and non-fatal acute myocardial infarction, coronary revascularization, fatal and non-fatal stroke and heart failure.
Depending on the aim and on the variable needed for the specific analysis total number of the subjects used could vary between the different sub-studies but, in general, the whole population is composed by 23,475 subjects that presents a mean age of 57 ± 15 years and of which 51% were males. The main characteristics of study population cohorts and the main results are summarized in Table 1.
2.1 Hyperuricemia and Cardiovascular Diseases
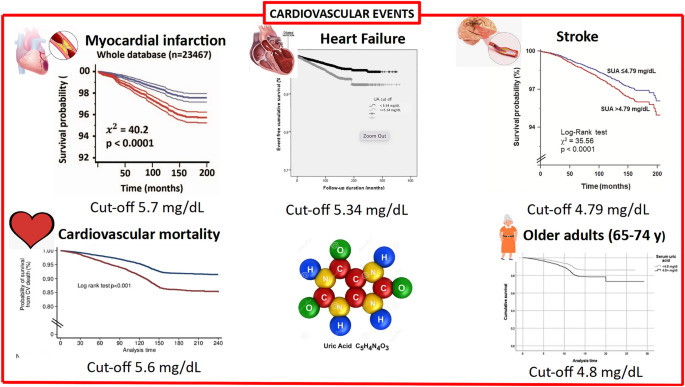
In the URRAH study, whether SUA could be related to CV diseases has been extensively investigated with a particular interest in founding specific cut-offs. The first published work identified the cut-off most suitable for predicting total and CV mortality in the entire study population [7]. During a median follow-up time of 134 months (interquartile range 74–164), 1571 CV deaths (over 3279 all-cause death, 47.9%) were identified, and SUA significantly correlates in univariate analysis (Hazard Ratio – HR: 1.28; 95% CI 1.24–1.33, p < 0.001). In multivariable analysis with all the classic CV risk factors inserted as covariates, the associations were consistently significant; even better, the strength of the association increased (HR 2.08; 95% CI 1.46–2.97, p < 0.001).
As expected in the hypothesis of the project, a lower cut-off was founded when compared to the classic one (6 mg/dL for females and 7 mg/dL for males). From ROC curve analysis, the optimal cut point for CV mortality was 5.6 mg/dL. Furthermore, the addition of SUA values increases the predictivity of the Heart Score (Harrell’s C 0.780 vs. 0.754, p < 0.001) and correctly reclassified 40.06% of subjects without events over the Heart Score at the cost of a false negative association of 12.28%, providing a significant Net Reclassification Improvement of 0.27 (p < 0.001).
The second paper published [8] regards the possible cut-off values for fatal Myocardial Infarction (MI) prediction. 445 subjects experienced the endpoint of the present analysis with a significant association with SUA (HR: 1.381; 95% CI 1.096–1.758, p = 0.006). At ROC analysis, the best cut-off for fatal MI was 5.70 mg/dL. When gender-specific analyses were performed, the significant association was confirmed in women (HR: 0.154; 95% CI 1.105–2.075, p < 0.001) but not in men (HR 1.294, 95% CI 0.924–1.813, p = 0.1). The role of gender in the relationship between SUA and CV disease has been extensively discussed in a previous review of the URRAH study [9].
A further paper on the URRAH project focused on Heart Failure (HF) [10]. SUA was found to be a significant predictor of incident HF (HR: 1.29; 95% CI 1.23–1.359, p < 0.001) and of fatal HF (HR: 1.268; 95% CI 1.121–1.35, p < 0.001), as well. In this case, the most discriminant cut-offs for SUA were 5.34 mg/dL and 4.89 mg/dL, respectively. Our results are in accordance with other population studies and meta-analyses regarding the ability of SUA to predict HF development [11, 12]. However, published paper results are more heterogeneous when SUA is evaluated in patients with HF. In these patients, SUA could act as a detrimental factor on left ventricular function and metabolism, but the opposite could also be true, i.e. the worst peripheral tissue vascularisation could increase SUA levels. In fact, SUA could result from increased purine degradation determined by hypoxia and tissue catabolism, which also determines an increased lactate release that reduces renal UA excretion [13]. Furthermore, HF is frequently associated with kidney impairment (and again a lower SUA clearance), and an increase in xanthine oxidase activity has been found in acute decompensated HF [14].
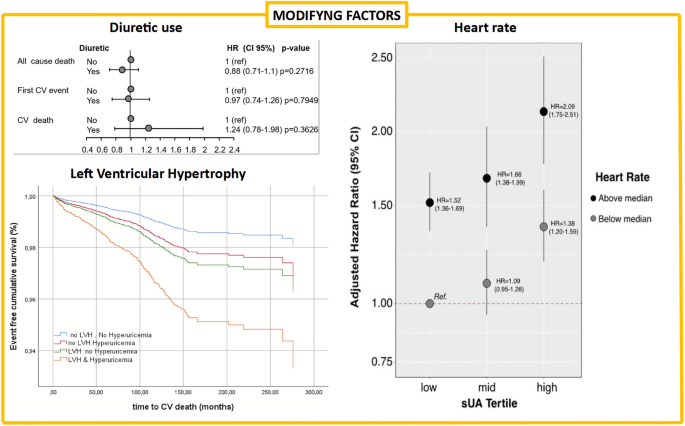
A problem interpreting the relationship between SUA and HF is the issue related to diuretic use. Diuretic treatments (especially thiazides) are able to determine an increased urate renal reabsorption that leads to hyperuricemia development. Diuretics are quite common in hypertensive patients and represent the principal therapeutic option to reduce congestion in HF patients. These secondary effects of diuretics were considered a benign problem, but data from the URRAH study argues against this hypothesis. In a specific analysis of the URRAH database, we found that 17.2% of individuals take diuretics, of whom 58% had SUA higher than the median value (4.8 mg/dL) [15]. Subjects with hyperuricemia using diuretic seems to present a higher prevalence of death for any cause (21.9 vs. 19.0%, p < 0.001) and first CV events (11.3 vs. 8.1%, p < 0.001) when compared with subjects with hyperuricemia without diuretic use while no differences were seen regarding CV deaths (5.1 vs. 4.1%, p = 0.013). However, at multivariable analysis (covariates: age, sex, systolic blood pressure, body mass index, glycemia, total and HDL cholesterol, smoking, CV therapies and estimated glomerular filtration rate), the above-mentioned findings were not confirmed for all three evaluated outcomes.
Since the central hypothesis linking UA to CV events is the role of xanthine oxidase as a trigger for oxidative stress, diuretic-induced reduction of UA renal excretion was thought to be not related to an increase in CV events. Although this study presents some limitations, it was the first to confirm that diuretic-related hyperuricemia was associated with a significant increase in CV diseases and mortality. The two main limitations were that we could not discriminate between patients with reduced uric acid excretion and those with uric acid overproduction (in fact, the presence of hyperuricemia during diuretic use does not automatically exclude the coexistence of increased production) but also that all the types of diuretic drugs were included in the present analysis. However, the effect on SUA level is more commonly observed with thiazides. Unfortunately, data on diuretic type were available only for a fraction of patients too small for a sub-group analysis.
To complete the data on the relationship between SUA and CV events, also an analysis on stroke was done [16]. SUA was associated, at multivariable regression analysis adjusted for confounders (age, sex, arterial hypertension, diabetes, chronic kidney disease, smoking habit, ethanol intake, body mass index, low-density lipoprotein cholesterol and use of diuretics) with an HR of 1.249 (95% CI 1.041–1.497, p = 0.016). A prognostic cut-off value of 4.79 mg/dL was identified as the best threshold.
Furthermore, an age-stratified survival analysis was performed in the older adults (≥ 65 years; n = 8000) of the URRAH study participants. While no independent association was found between patients older than 75 years and mortality (all-cause and CV), patients in the 65–74 year age group showed optimal discrimination using a dedicated cut-off of 4.8 mg/dL, which is valid for both All-Cause Mortality (ACM) and CV mortality (CVM): HR 1.45, (95% CI 1.23–1.68) and 1.46 (95% CI 1.20–1.79). Peculiarly, in participants older than 75, the relationship between SUA and mortality was described by J-shaped curves [17].
Two URRAH papers focused on factors that could modulate the SUA relationship with CV mortality. The first focused on heart rate and the possibility that SUA action on CV events is by sympathetic activity [18]. Heart rate is a well-known CV risk factor that directly damages the heart and the arterial wall, but it can also represent a marker of a sympathetic nervous system overdrive. At multivariable analysis, each unit increase in SUA determined an increase in the HR for CV mortality of 9.4%, while the increase was 5.3% for each heart rate unit increment. In categorical analysis, we have seen that hyperuricemia (SUA > 5.5 mg/dL) exerts a higher increase in HR for CV mortality in subjects with elevated heart rate. The HR for the relationship between SUA and CV mortality in subjects with a heart rate under the median value (71.3 bpm) was 1.38 (95% CI 1.20–1.59), while this increase to an HR of 2.09 (95% CI 1.75–2.51) in those over the median value. So, one could hypothesize that the sympathetic nervous system overactivity facilitates uric acid action on CV events.
Finally, the most recently published URRAH paper focused on the relationship of SUA with LVH and the possibility that this target organ damage modifies its relation with CV mortality [19]. In multiple regression analysis, SUA was significantly associated with Left Ventricular Mass Index in men and women (beta = 0.095 and 0.069, respectively, p < 0.001 for both analyses). Both SUA and LVH were, as expected, associated with CV death in multivariable models (hyperuricemia HR 1.751; 95% CI 1.394–2199, p = 0.001; LVH HR 2.050; 95% CI 1.576–2.668, p = 0.001). The combined presence of hyperuricemia (SUA > 5.1 mg/dL for females and > 5.6 mg/dL for males [7]) and LVH (LVMI > 95 g/m2 for females and > 115 g/m2 for males) significantly increase the HR for CV mortality when compared to those factors taken individually to a total HR of 3785 (95% CI 1.789–8.008) for women and 5.273 (95% CI 3.044–9.135) for men.
In conclusion, our data suggest that other factors could modulate the contribution of SUA to CV mortality. This is undoubtedly modulated by the presence of tachycardia and LVH, both of which increase the ability of SUA to detect CV disease.
Figure 1 resumes the results of the URRAH study in terms of the different cut-offs established for CV events while Fig. 2 summarize data on factors able to modifying that relationship.
2.2 Hyperuricemia and Kidney Disease in Predicting Mortality
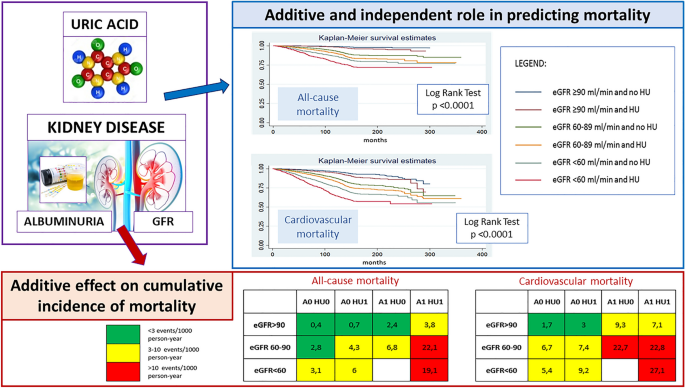
Glomerular filtration rate (GFR) is unquestionably one of the main determinants of SUA levels, thus hyperuricemia is a typical finding in chronic kidney disease (CKD) patients. Consistent evidence indicates that the relationship between hyperuricemia and CKD is bidirectional. As well a reduction in GFR can precede and lead to the development of hyperuricemia, increased SUA levels per se can adversely impact renal function [20, 21]. However, how GFR and SUA levels are related and the appropriate threshold of SUA for defining asymptomatic hyperuricemia in the contest of CKD remain unclear. The URRAH database, with its large baseline cohort and relatively long-term follow-up [22], provided an ideal opportunity to investigate this interaction between SUA levels and CKD components (eGFR and albuminuria) and their impact in determining mortality.
A recent cross-sectional analysis [23] including 26,971 individuals from the URRAH database provided new insights into these complex relationships. The study showed the more significant the severity of the CKD stage, the higher the occurrence of hyperuricemia. Prevalence of hyperuricemia defined based on previously validated URRAH cut-offs specific for CVM and ACM was 32 and 57%, respectively, and increased significantly from 20 to 33% in subjects with eGFR > 90 ml/min to 60, and 80% in CKD 3b patients. Multiple logistic regression analyses indicated that the main covariates associated with hyperuricemia defined with CVM threshold were CKD stage, male gender, history of hypertension, and triglycerides (TG) levels.
Moreover, this was the largest population study in which the relationship between SUA levels and the presence of micro and macro-albuminuria have been investigated. Hyperuricemia was more likely present in patients with albuminuria (50.5%, 54.9%, and 57.1% in patients with normoalbuminuria, microalbuminuria and macroalbuminuria, respectively, p = 0.0062). Those with albuminuria showed higher SUA levels (5.18 ± 1.40 vs. 5.45 ± 1.56, p < 0.0001), more frequent use of allopurinol and a history of gout compared to those without albuminuria. Unexpectedly, in patients with GFR below 45 ml/min, the prevalence of hyperuricemia was lower in the presence of macroalbuminuria. While this finding is unforeseen, it may be related to a higher prevalence of individuals with diabetes among these patients with more severe kidney impairment. This data could be explained by an increase in glycosuria and a greater loss of uric acid in the urine reported in patients with decompensated diabetes, resulting in decreased SUA levels [24].
Despite the known great impact of CKD components (both reduced GFR and the presence of albuminuria) on CV and mortality risk [25, 26], the interplay between SUA, GFR and albuminuria in causing mortality and the independent role of each one of these conditions on CV and mortality risk is a matter of research. The URRAH Study Group [27] tried to answer these questions with a longitudinal study. The cohort was composed of 21,963 patients who were followed for 9.8 years. A ROC curve analysis yielded plausible GFR stage-specific cut-off values of SUA for CVM (cut-off points of SUA = 4.1, 5.8 and 6.9 mg/dL in subjects with GFR > 90, 60–90 and < 60 ml/min/m2, respectively), and for ACM (cut-off points of SUA = 5.1, 4.8 and 6.8 mg/dL in subjects with GFR > 90, 60–90 and < 60 ml/min/1.73 m2, respectively). Results of interaction effect regression analysis indicated that SUA and GFR interplay in determining mortality. More interestingly, it was described for the first time as the independent predictive power of SUA tends to decrease along with the severity of renal impairment. This study concluded that high SUA levels are a risk factor for CV and all-cause mortality independently and additively to reduced GFR and the presence of albuminuria in patients at cardiovascular risk (Fig. 3).
Altogether, these data suggest that in the context of greater global risk, as is the case when GFR is even slightly reduced, SUA becomes a significant correlate of unfavourable outcome only at serum concentration greater than what is observed in subjects with normal renal function. From a pathophysiological point of view, these data sustain the hypothesis that while hyperuricemia may result from reduced kidney clearance, uric acid perpetuates renal injury, leading to a progressive vicious cycle of CKD progression and after that to an increased CV and mortality risk. Several pathogenetic mechanisms explaining uric acid-mediated kidney and vascular damage have been hypothesized, including inflammation with cytokine release and oxidative stress [28], promotion of endothelial, vascular [29], and interstitial damage, upregulation of the renin–angiotensin aldosterone system, and changes in glomerular hemodynamics leading to glomerulosclerosis and fibrosis [30].
The findings of specific SUA levels predicting mortality in different CKD strata could also explain the relatively conflicting data previously reported in the literature on the relationship between hyperuricemia and unfavourable outcomes in the presence of CKD at different stages [31]. In fact, renal function may have acted as a confounder in the relationship between hyperuricemia and CV risk when data were analyzed in aggregate without adjusting for GFR values.
Because SUA is so strongly dependent on renal function, the URRAH Study Group determined the prognostic cut-off values for the ratio between SUAmg/dl and serum creatinine (sCrmg/dl), assessing sCr as an adequate indicator of the kidney function (the addiction of GFR to the Cox models did not change the results significantly) [32]. This study identified a SUA/sCr cut-off predictive of CV risk for the first time. The SUA/sCr cut-off of 5.35, by indexing for renal function, proved to be a functional tool for identifying those patients with the highest risk of developing CV events during follow-up, with no significant differences between men and women. Therefore, this a-dimensional, purely numerical variable was proposed as a novel element that can be treated in the epidemiological field as a new variable for screening individuals at increased risk.
In summary, there is consistent evidence from URRAH data that SUA levels and CKD stages are closely related. For this reason, there is a need for a specific cut-off defining asymptomatic hyperuricemia in the presence of CKD. In particular, the proposal derived from the URRAH Project is to use a cut-off of SUA > 7.0 mg/dl for patients with CKD 3 or a cut-off of SUA adjusted for creatinine of 5.35. The second major point emerging from these studies is the independent and additive role of each condition (hyperuricemia, GFR reduction and albuminuria) in increasing mortality risk. This suggests that despite CKD being a major determinant of the presence and degree of hyperuricemia, these two conditions may have, at least in part, different pathogenetic mechanisms by which they both contribute to the excess of CV morbidity and mortality. Nevertheless, further translational research is needed.
2.3 Serum Uric Acid in the Context of Metabolic Dysregulation
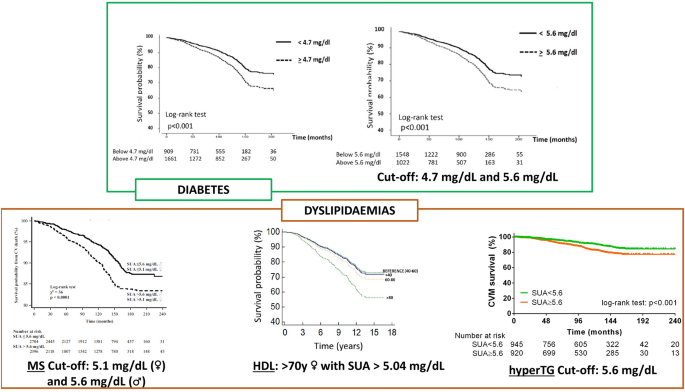
The definition of a high-risk CV phenotype cannot ignore the individual metabolic profile. Impaired metabolic homeostasis defines ageing and cardiometabolic diseases such as dyslipidemia, obesity, type 2 diabetes (T2D) and arterial hypertension and drives CV risk. Moreover, it is involved in a deleterious interplay with other actors of detrimental CV evolution [33]. Uric acid is not exempt from this harmful connection, as it has a double-edged relationship with metabolic disorders. Elevated SUA is a common finding in metabolic diseases [34], and pathophysiological and clinical evidence supports a potential interaction between SUA, glucose and lipid metabolism [35, 36]. Hyperglycaemia and altered levels of circulating lipoproteins have common molecular pathways of damage that are also shared with SUA. One of these is the activation of the NLRP3-inflammasome [33], which leads to the persistent low-grade systemic inflammatory response that is a hallmark of cardiometabolic disease [37]. The pro-oxidant activity that SUA acquires at higher concentrations can further disrupt the homeostatic balance, altering HDL metabolism and function [38]. Given the dramatic importance that metabolic diseases are gaining in the global health landscape [39], the URRAH study investigated, in metabolic subgroups among URRAH study participants, the relationship between uric acid and cardiometabolic diseases from a clinical perspective [40,41,42,43] (Fig. 4).
In another study in URRAH patients with diabetes (n = 2,570), the established thresholds of SUA identified for ACM (SUA ≥ 4.7 mg/dL) and CV mortality (SUA ≥ 5.6 mg/dL) [7] confirmed their predictive power [40]: HR 1.23, (95% CI 1.04–1.47) and 1.31 (95% CI, 1.03–1.66), respectively. Similar results were seen in a subpopulation (n = 8124) with different degrees of cardiometabolic damage (patients with obesity, arterial hypertension, T2D, either alone or combined) but without established cardiovascular disease (subjects with previous CV events, history of heart failure, systolic/diastolic blood pressure > 240/149 mmHg, fasting blood glucose > 350 mg/dl, or serum creatinine > 4 mg/dL were excluded). A total of 8,124 patients were included in the final analysis : HR 1.25, (95% CI 1.12–1.40) for ACM and 1.31 (95% CI 1.11–1.74) for CV mortality [42]. The association with CV mortality was also examined in a sub-cohort of patients selected based on the data availability for the metabolic syndrome (MS) parameters (n = 9589; n = 5100 met MS criteria), confirming that SUA ≥ 5.6 mg/dL was strongly associated with CV death: HR 1.79 (95% CI 1.15–2.79). In this context, it was also verified how including sex-specific SUA cut-offs in the MS definition improved CV mortality reclassification in patients with and without MS (net reclassification improvement of 7.1%) [43].
The URRAH study group also investigated the relationship between the different parameters of the metabolic profile and the clinical relevance of their interplay. SUA was found to be inversely correlated with HDL (r = −0.206) [41], with plasma glucose in patients with diabetes (r = −0.09) [40], and positively correlated with TG in patients without established CV disease (r = 0.332) [42] In female patients with high HDL (> 80 mg/dL), a specific SUA cut-off (4.96 mg/dL) was able to identify a higher mortality risk (HR 1.61; 95% CI 1.08–2.39). Notably, the inclusion of the BMI in the model reduced the strength of the association (HR 1.51; 95% CI, 1.00–2.27) [41]. Concerning TG and using the acknowledged SUA cut-offs [7], the interaction with SUA was significant regarding ACM and CV mortality, and no gender-specific effect was observed [42]. Although with the limitation of reduced sample size due to further stratification into distinct disease subgroups, an exploratory analysis supports the predictive power of SUA since the initial cardiometabolic derangement [42].
In conclusion, although limited by the observational nature of the URRAH study, all these results confirm the relevant association between SUA and impaired metabolic states. More importantly, they suggest that SUA retains its predictive power across the cardiometabolic spectrum and highlight a relevant adverse interplay with part of the lipid profile. Finally, the J-shaped curves found in patients over 75 years of age call for specific follow-up studies [39]. Given the longer life expectancy of the population and the dramatic increase in the prevalence of metabolic diseases [38], SUA, which is inexpensive and widely available in plasma, seems particularly relevant in the context of early risk stratification and possible therapeutic intervention.
3 Conclusions
Despite the availability of always more refined algorithms to predict incident CV diseases [44], a relatively large number of events remain unexpected. This could be due to different reasons, among them the lack of knowledge of some risk factors and the lack of attention to clearly emerging risk factors. Among them, SUA seems to be of particular interest. Increasing epidemiological evidence supports the need for further investigation of the determinants of cardiometabolic and renal risk associated with SUA and other purine metabolism biomarkers. In fact, from one side, suboptimal SUA levels are associated with several risk factors, organ damage and pathological conditions per se associated with an increased CV and metabolic risk, while on the other side, SUA reduction has not been yet clearly associated with a reduction of CV risk [45].
Thus, when interpreting epidemiological data on SUA as a risk factor, we have to consider that widely prevalent (and often underestimated) conditions like wrong lifestyle [46], overweight/obesity and insulin resistance [47] impair SUA levels at the same time increasing cardiometabolic risk. Conversely, some commonly used drugs reduced SUA levels and CV risk, such as losartan, fenofibrate and Sodium-glucose cotransporter-2 (SGLT2) inhibitors [48].
A recent network meta-analysis including 23 double-blind, placebo-controlled, randomized clinical trials carried out with different SUA lowering drugs concluded that allopurinol and febuxostat had significantly lower composite renal events (deterioration of renal function, end-stage renal disease, and initiation of renal replacement therapy) than placebo (Relative Risk - RR 0.39, 95% CI 0.23–0.66, and RR 0.68, 95% CI 0.46–0.99, respectively), but no apparent effect on major CV events [49]. A larger meta-analysis including 30 trials (N = 18,585) concluded that xanthine oxidase inhibitors produced a 6.0% reduction in relative risk for major adverse cardiovascular events, mainly because of the benefit attributed to allopurinol (RR: 0.61, 95% CI 0.46–0.80, I2 = 21.0%), while febuxostat would have a more neutral effect (RR: 1.09, 95% CI 0.998–1.19, I2 = 0.0%) [50]. These data should be interpreted cautiously since women and racial and ethnical minorities are underrepresented in controlled clinical trials testing SUA-lowering drugs [51], and it is well known that CV risk, SUA levels and the impact of SUA on CV risk are different in men and women, and in white versus other ethnicities. Besides, at least one part of the cardiovascular event preventive effects of the SGLT2 inhibitors have been attributed to their ability to improve SUA levels significantly [52].
In this context, data coming from large cohorts of unselected subjects and patients such as the URRAH one helps to more clearly understand the role of SUA as independent CV risk factors and the subcohorts of subjects where suboptimal SUA levels are associated with higher CV risk.
Data availability statement
Original data subsets related to single URRAH published analyses are available upon motivated inquire and approval by the URRAH steering committee.
References
Kuwabara M, Kodama T, Ae R, Kanbay M, Andres-Hernando A, Borghi C, Hisatome I, Lanaspa MA. Update in uric acid, hypertension, and cardiovascular diseases. Hypertens Res. 2023. https://doi.org/10.1038/s41440-023-01273-3. (Epub ahead of print).
Cicero AFG, Fogacci F, Di Micoli V, Angeloni C, Giovannini M, Borghi C. Purine metabolism dysfunctions: experimental methods of detection and diagnostic potential. Int J Mol Sci. 2023;24(8): 7027.
Gill D, Cameron AC, Burgess S, Li X, Doherty DJ, Karhunen V, Abdul-Rahim AH, Taylor-Rowan M, Zuber V, Tsao PS, Klarin D, VA Million Veteran Program, Evangelou E, Elliott P, Damrauer SM, Quinn TJ, Dehghan A, Theodoratou E, Dawson J, Tzoulaki I. Urate, blood pressure, and cardiovascular disease: evidence from mendelian randomization and meta-analysis of clinical trials. Hypertension. 2021;77(2):383–92.
Yang F, Lu Y, Chen S, Wang K, Hu T, Cui H. Sex-specific effect of serum urate levels on coronary heart disease and myocardial infarction prevention: a mendelian randomization study. Nutr Metab Cardiovasc Dis. 2022;32(5):1266–74.
Moshkovits Y, Tiosano S, Kaplan A, Kalstein M, Bayshtok G, Kivity S, Segev S, Grossman E, Segev A, Maor E, Fardman A. Serum uric acid significantly improves the accuracy of cardiovascular risk score models. Eur J Prev Cardiol. 2023;30(7):524–32.
Desideri G, Virdis A, Casiglia E, Borghi C, Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension. Exploration into uric and cardiovascular disease: uric acid right for heArt Health (URRAH) Project, a study protocol for a retrospective observational study. High Blood Press Cardiovasc Prev. 2018;25(2):197–202.
Virdis A, Masi S, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, et al. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension. 2020;75(2):302–8.
Casiglia E, Tikhonoff V, Virdis A, Masi S, Barbagallo CM, Bombelli M, Bruno B, Cicero AFG, Cirillo M, Cirillo P, Desideri G, D’Elia L, Ferri C, Galletti F, Gesualdo L, Giannattasio C, Iaccarino G, Lippa L, Mallamaci F, Maloberti A, Mazza A, Muiesan ML, Nazzaro P, Palatini P, Parati G, Pontremoli R, Quarti-Trevano F, Rattazzi M, Rivasi G, Salvetti M, Tocci G, Ungar A, Verdecchia P, Viazzi F, Volpe M, Grassi G, Borghi C, Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension (SIIA). Serum uric acid and fatal myocardial infarction: detection of prognostic cut-off values: the URRAH (Uric Acid Right for Heart Health) study. J Hypertens. 2020;38(3):412–9.
Maloberti A, Giannattasio C, Bombelli M, Desideri G, Cicero AFG, Muiesan ML, Rosei EA, Salvetti M, Ungar A, Rivasi G, Pontremoli R, Viazzi F, Facchetti R, Ferri C, Bernardino B, Galletti F, D’Elia L, Palatini P, Casiglia E, Tikhonoff V, Barbagallo CM, Verdecchia P, Masi S, Mallamaci F, Cirillo M, Rattazzi M, Pauletto P, Cirillo P, Gesualdo L, Mazza A, Volpe M, Tocci G, Iaccarino G, Nazzaro P, Lippa L, Parati G, Dell’Oro R, Quarti-Trevano F, Grassi G, Virdis A, Borghi C, Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension (SIIA). Hyperuricemia and risk of cardiovascular outcomes: the experience of the URRAH (Uric Acid Right for Heart Health) project. High Blood Press Cardiovasc Prev. 2020;27(2):121–8.
Muiesan ML, Salvetti M, Virdis A, Masi S, Casiglia E, Tikhonoff V, Barbagallo CM, Bombelli M, Cicero AFG, Cirillo M, Cirillo P, Desideri G, D’Eliak L, Ferri C, Galletti F, Gesualdo L, Giannattasio C, Iaccarino G, Mallamaci F, Maloberti A, Mazza A, Nazzaro P, Palatini P, Parati G, Pontremoli R, Rattazzi M, Rivasi G, Tocci G, Ungar A, Verdecchia P, Viazzi F, Volpe M, Grassi G, Borghi C. From the working group on uric acid, cardiovascular risk of the Italian society of hypertension. Serum uric acid, predicts heart failure in a large Italian cohort: search for a cut-off value the URic acid right for heArt health study. J Hypertens. 2021;39(1):62–9.
Huang H, Huang B, Li Y, Huang Y, Li J, Yao H, Jing X, Chen J, Wang J. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail. 2014;16(1):15–24.
Ekundayo OJ, Dell’Italia LJ, Sanders PW, Arnett D, Aban I, Love TE, Filippatos G, Anker SD, Lloyd-Jones DM, Bakris G, Mujib M, Ahmed A. Association between hyperuricemia and incident heart failure among older adults: a propensity-matched study. Int J Cardiol. 2010;142(3):279–87. https://doi.org/10.1016/j.ijcard.2009.01.010.
Doehner W, Jankowska EA, Springer J, Lainscak M, Anker SD. Uric acid and xanthine oxidase in heart failure - emerging data and therapeutic implications. Int J Cardiol. 2016;213:15–9.
Okazaki H, Shirakabe A, Matsushita M, Shi-bata Y, Sawatani T, Uchiyama S, et al. Plasma xanthine oxidoreductase activity in patients with decompensated acute heart failure re-quiring intensive care. ESC Heart Fail. 2019;6(2):336–43.
Maloberti A, Bombelli M, Facchetti R, Barbagallo CM, Bernardino B, Rosei EA, Casiglia E, Cicero AFG, Cirillo M, Cirillo P, Desideri G, D’elia L, Dell’Oro R, Ferri C, Galletti F, Giannattasio C, Loreto G, Iaccarino G, Lippa L, Mallamaci F, Masi S, Mazza A, Muiesan ML, Nazzaro P, Parati G, Palatini P, Pauletto P, Pontremoli R, Quarti-Trevano F, Rattazzi M, Rivasi G, Salvetti M, Tikhonoff V, Tocci G, Ungar A, Verdecchia P, Viazzi F, Volpe M, Virdis A, Grassi G, Borghi C, Working Group on Uric Acid. Cardiovascular Risk of the Italian society of hypertension (SIIA). Relationships between diuretic-related hyperuricemia and cardiovascular events: data from the URic acid right for heArt health study. J Hypertens. 2021;39(2):333–40.
Tikhonoff V, Casiglia E, Spinella P, Barbagallo CM, Bombelli M, Cicero AFG, Cirillo M, Cirillo P, Desideri G, D’elia L, Ferri C, Galletti F, Gesualdo L, Giannattasio C, Iaccarino G, Mallamaci F, Maloberti A, Masi S, Mazza A, Muiesan ML, Nazzaro P, Palatini P, Parati G, Pontremoli R, Quarti-Trevano F, Rattazzi M, Rivasi G, Salvetti M, Tocci G, Ungar A, Verdecchia P, Viazzi F, Virdis A, Volpe M, Grassi G, Borghi C, Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension (SIIA). Identification of a plausible serum uric acid cut-off value as prognostic marker of stroke: the uric acid right for heart health (URRAH) study. J Hum Hypertens. 2022;36(11):976–82.
Ungar A, Rivasi G, Di Bari M, Virdis A, Casiglia E, Masi S, et al. The association of uric acid with mortality modifies at old age: data from the uric acid right for heart health (URRAH) study. J Hypertens. 2022;40(4):704–11.
Palatini P, Parati G, Virdis A, Reboldi G, Masi S, Mengozzi A, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, Bombelli M, Dell’Oro R, Bruno B, Lippa L, D’Elia L, Verdecchia P, Angeli F, Mallamaci F, Cirillo M, Rattazzi M, Cirillo P, Gesualdo L, Mazza A, Giannattasio C, Maloberti A, Volpe M, Tocci G, Georgiopoulos G, Iaccarino G, Nazzaro P, Galletti F, Ferri C, Desideri G, Viazzi F, Pontremoli R, Muiesan ML, Grassi G, Borghi C. High heart rate amplifies the risk of cardiovascular mortality associated with elevated uric acid. Eur J Prev Cardiol. 2021;14:zwab023. https://doi.org/10.1093/eurjpc/zwab023. (Epub ahead of print).
Muiesan ML, Agabiti Rosei C, Paini A, Casiglia E, Cirillo M, Grassi G, Iaccarino G, Mallamaci F, Maloberti A, Mazza A, Mengozzi A, Palatini P, Parati G, Reboldi G, Rivasi G, Russo E, Salvetti M, Tikhonoff V, Tocci G, Borghi C, Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension (SIIA). Serum uric acid and left ventricular mass index independently predict cardiovascular mortality: The uric acid right for heart health (URRAH) project. Eur J Intern Med. 2023:S0953-6205(23)00123-1. https://doi.org/10.1016/j.ejim.2023.04.010. (Epub ahead of print).
Rincon-Choles H, Jolly SE, Arrigain S, Konig V, Schold JD, Nakhoul G, et al. Impact of uric acid levels on kidney disease progression. Am J Nephrol. 2017;46(4):315–22.
De Cosmo S, Viazzi F, Pacilli A, Giorda C, Ceriello A, Gentile S, et al. Serum uric acid and risk of CKD in type 2 diabetes. Clin J Am Soc Nephrol. 2015;10(11):1921–9.
Del Pinto R, Viazzi F, Pontremoli R, Ferri C, Carubbi F, Russo E. The URRAH study. Panminerva Med. 2021;63(4):416–23.
Russo E, Viazzi F, Pontremoli R, Barbagallo CM, Bombelli M, Casiglia E, et al. Association of uric acid with kidney function and albuminuria: the uric acid right for heArt health (URRAH) project. J Nephrol. 2022;35(1):211–21.
Yan D, Tu Y, Jiang F, Wang J, Zhang R, Sun X, et al. Uric acid is independently associated with diabetic kidney disease: a cross-sectional study in a Chinese population. PLoS One. 2015;10(6): e0129797.
Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–25.
Luo Q, Xia X, Li B, Lin Z, Yu X, Huang F. Serum uric acid and cardiovascular mortality in chronic kidney disease: a meta-analysis. BMC Nephrol. 2019;20(1):18.
Russo E, Viazzi F, Pontremoli R, Barbagallo CM, Bombelli M, Casiglia E, et al. Serum uric acid and kidney disease measures independently predict cardiovascular and total mortality: the uric acid right for heart health (URRAH) project. Front Cardiovasc Med. 2021;8: 713652.
Milanesi S, Verzola D, Cappadona F, Bonino B, Murugavel A, Pontremoli R, et al. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J Cell Physiol. 2019;234(7):10868–76.
Russo E, Bertolotto M, Zanetti V, Picciotto D, Esposito P, Carbone F, et al. Role of uric acid in vascular remodeling: cytoskeleton changes and migration in VSMCs. Int J Mol Sci. 2023;24(3):2960.
Russo E, Verzola D, Cappadona F, Leoncini G, Garibotto G, Pontremoli R, et al. The role of uric acid in renal damage - a history of inflammatory pathways and vascular remodeling. Vessel Plus. 2021;5:15.
Russo E, Verzola D, Leoncini G, Cappadona F, Esposito P, Pontremoli R, et al. Treating hyperuricemia: the last word hasn’t been said yet. J Clin Med. 2021;10(4):819.
Casiglia E, Tikhonoff V, Virdis A, Grassi G, Angeli F, Barbagallo CM, et al. Serum uric acid/serum creatinine ratio as a predictor of cardiovascular events. Detection of prognostic cardiovascular cut-off values. J Hypertens. 2023;41(1):180–6.
Schunk SJ, Kleber ME, März W, Pang S, Zewinger S, Triem S, et al. Genetically determined NLRP3 inflammasome activation associates with systemic inflammation and cardiovascular mortality. Eur Heart J. 2021;42(18):1742–56.
Lurbe E, Torro MI, Alvarez-Pitti J, Redon J, Borghi C, Redon P. Uric acid is linked to cardiometabolic risk factors in overweight and obese youths. J Hypertens. 2018;36(9):1840–6.
Li B, Chen L, Hu X, Tan T, Yang J, Bao W, et al. Association of serum uric acid with all-cause and cardiovascular mortality in diabetes. Diabetes Care. 2023;46(2):425–33.
Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018;40:61–73.
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–90.
Barter PJ, Rye KA. HDL cholesterol concentration or HDL function: which matters? Eur Heart J. 2017;38(32):2487–9.
Chew NWS, Ng CH, Tan DJH, Kong G, Lin C, Chin YH, et al. The global burden of metabolic disease: data from 2000 to 2019. Cell Metabol. 2023;35(3):414-428.e413.
Masulli M, D’Elia L, Angeli F, Barbagallo CM, Bilancio G, Bombelli M, et al. Serum uric acid levels threshold for mortality in diabetic individuals: the URic acid right for heArt health (URRAH) project. Nutr Metab Cardiovasc Dis. 2022;32(5):1245–52.
Palatini P, Virdis A, Masi S, Mengozzi A, Casiglia E, Tikhonoff V, et al. Hyperuricemia increases the risk of cardiovascular mortality associated with very high HdL-cholesterol level. Nutr Metab Cardiovasc Dis. 2022;33:323.
Mengozzi A, Pugliese NR, Desideri G, Masi S, Angeli F, Barbagallo CM, et al. Serum uric acid predicts all-cause and cardiovascular mortality independently of hypertriglyceridemia in cardiometabolic patients without established CV disease: a sub-analysis of the URic acid right for heArt health (URRAH) study. Metabolites. 2023;13:2.
Pugliese NR, Mengozzi A, Virdis A, Casiglia E, Tikhonoff V, Cicero AFG, et al. The importance of including uric acid in the definition of metabolic syndrome when assessing the mortality risk. Clin Res Cardiol. 2021;110(7):1073–82.
Graham IM, Di Angelantonio E, Huculeci R, European Society of Cardiology’s Cardiovascular Risk Collaboration (CRC). New way to SCORE risk: updates on the ESC scoring system and incorporation into ESC cardiovascular prevention guidelines. Curr Cardiol Rep. 2022;24(11):1679–84.
Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, Campbell H, Theodoratou E. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomized controlled trials, and mendelian randomization studies. BMJ. 2017;357:j2376.
Yokose C, McCormick N, Choi HK. The role of diet in hyperuricemia and gout. Curr Opin Rheumatol. 2021;33(2):135–44.
Drozdz D, Alvarez-Pitti J, Wójcik M, Borghi C, Gabbianelli R, Mazur A, Herceg-Čavrak V, Lopez-Valcarcel BG, Brzeziński M, Lurbe E, Wühl E. Obesity and cardiometabolic risk factors: from childhood to adulthood. Nutrients. 2021;13(11): 4176.
Cicero AFG, Fogacci F, Kuwabara M, Borghi C. Therapeutic strategies for the treatment of chronic hyperuricemia: an evidence-based update. Medicina. 2021;57(1): 58.
Sapankaew T, Thadanipon K, Ruenroengbun N, Chaiyakittisopon K, Ingsathit A, Numthavaj P, Chaiyakunapruk N, McKay G, Attia J, Thakkinstian A. Efficacy and safety of urate-lowering agents in asymptomatic hyperuricemia: systematic review and network meta-analysis of randomized controlled trials. BMC Nephrol. 2022;23(1):223.
Ying H, Yuan H, Tang X, Guo W, Jiang R, Jiang C. Impact of serum uric acid lowering and contemporary uric acid-lowering therapies on cardiovascular outcomes: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8: 641062.
Fogacci F, Borghi C, Di Micoli A, Degli Esposti D, Cicero AFG. Inequalities in enrollment of women and racial minorities in trials testing uric acid lowering drugs. Nutr Metab Cardiovasc Dis. 2021;31(12):3305–13.
Yip ASY, Leong S, Teo YH, Teo YN, Syn NLX, See RM, Wee CF, Chong EY, Lee CH, Chan MY, Yeo TC, Wong RCC, Chai P, Sia CH. Effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on serum urate levels in patients with and without diabetes: a systematic review and meta-regression of 43 randomized controlled trials. Ther Adv Chronic Dis. 2022;13:20406223221083508.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Conflict of Interest
The Authors declares that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Maloberti, A., Mengozzi, A., Russo, E. et al. The Results of the URRAH (Uric Acid Right for Heart Health) Project: A Focus on Hyperuricemia in Relation to Cardiovascular and Kidney Disease and its Role in Metabolic Dysregulation. High Blood Press Cardiovasc Prev 30, 411–425 (2023). https://doi.org/10.1007/s40292-023-00602-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-023-00602-4