Abstract
Introduction
Patients with arterial hypertension frequently present with comorbidities that are associated with increased cardiorenal risk, such as metabolic dysfunction-associated fatty liver disease (MAFLD).
Aims
Our study aimed to assess the prevalence and the association of MAFLD with cardiorenal risk markers in newly diagnosed, treatment-naïve hypertensive patients.
Methods
We recruited 281 individuals with new-onset hypertension who were not prescribed any medication. Medical history, clinical examination findings, and laboratory test results were recorded. Liver steatosis was assessed through fatty liver index (FLI) calculation. Patients with FLI ≥ 60 together with one main metabolic abnormality (type 2 diabetes mellitus or overweight/obesity) or at least two metabolic risk abnormalities (increased waist circumference, blood pressure, plasma triglycerides, presence of prediabetes or insulin resistance, decreased plasma high-density lipoprotein) fulfilled the diagnostic criteria for MAFLD.
Results
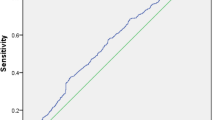
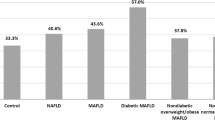
The prevalence of MAFLD in our study population was 28.7%. Individuals with MAFLD were more frequently male and had increased body mass index. Systolic, diastolic, and pulse pressure values were significantly higher in this group of patients. Moreover, lipid, renal, glucose, and inflammatory markers were considerably deranged in patients with MAFLD. After multivariate regression analysis, uric acid, ferritin, and apoE emerged as independent predictors of MAFLD. Area under receiver operating characteristics curve revealed that uric acid had the greatest diagnostic accuracy, with the ideal cutoff being ≥ 5.2 mg/dl (sensitivity: 77.6%, specificity: 76.3%).
Conclusion
MAFLD represents a common comorbidity in hypertensive patients and is associated with markers of cardiorenal risk. Uric acid may be indicative of MAFLD in particular.



Similar content being viewed by others
References
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37. https://doi.org/10.1038/s41581-019-0244-2.
Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the association of the 2017 and 2014 hypertension guidelines with cardiovascular events and deaths in US adults: an analysis of national data. JAMA Cardiol. 2018;3:572–81. https://doi.org/10.1001/jamacardio.2018.1240.
Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, Shao L, Tian Y, Dong Y, Zheng C, Wang J, Zhu M, Weintraub WS, Gao R, China Hypertension Survey I. Status of hypertension in China: results from the China hypertension survey, 2012–2015. Circulation. 2018;137:2344–56. https://doi.org/10.1161/CIRCULATIONAHA.117.032380.
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Jarvinen H, Fan JG, Gronbaek H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9. https://doi.org/10.1016/j.jhep.2020.03.039.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. https://doi.org/10.1136/bmj.39335.541782.AD.
Stergiou GS, Palatini P, Parati G, O’Brien E, Januszewicz A, Lurbe E, Persu A, Mancia G, Kreutz R, European Society of Hypertension C, the European Society of Hypertension Working Group on Blood Pressure M, Cardiovascular V. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39:1293–302. https://doi.org/10.1097/HJH.0000000000002843.
Williams B, Mancia G, Spiering W, AgabitiRosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Group ESCSD. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–104. https://doi.org/10.1093/eurheartj/ehy339.
Niiranen TJ, Kalesan B, Mitchell GF, Vasan RS. Relative contributions of pulse pressure and arterial stiffness to cardiovascular disease. Hypertension. 2019;73:712–7. https://doi.org/10.1161/HYPERTENSIONAHA.118.12289.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. https://doi.org/10.1007/BF00280883.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. https://doi.org/10.1210/jcem.85.7.6661.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–97. https://doi.org/10.1001/jama.285.19.2486
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. https://doi.org/10.1186/1471-230X-6-33.
Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–72. https://doi.org/10.1002/bimj.200410135.
Theofilis P, Vordoni A, Kalaitzidis RG. Metabolic dysfunction-associated fatty liver disease in the National Health and Nutrition Examination Survey 2017–2020: epidemiology, clinical correlates, and the role of diagnostic scores. Metabolites. 2022;12:1070.
Theofilis P, Vordoni A, Kalaitzidis RG. Interplay between metabolic dysfunction-associated fatty liver disease and chronic kidney disease: epidemiology, pathophysiologic mechanisms, and treatment considerations. World J Gastroenterol. 2022;28:5691–706.
Mantovani A, Csermely A, Tilg H, Byrne CD, Targher G. Comparative effects of non-alcoholic fatty liver disease and metabolic dysfunction-associated fatty liver disease on risk of incident cardiovascular events: a meta-analysis of about 13 million individuals. Gut. 2022. https://doi.org/10.1136/gutjnl-2022-328224.
Kim H, Lee CJ, Ahn SH, Lee KS, Lee BK, Baik SJ, Kim SU, Lee JI. MAFLD predicts the risk of cardiovascular disease better than NAFLD in asymptomatic subjects with health check-ups. Dig Dis Sci. 2022;67:4919–28. https://doi.org/10.1007/s10620-022-07508-6.
Tanaka M, Mori K, Takahashi S, Higashiura Y, Ohnishi H, Hanawa N, Furuhashi M. Metabolic dysfunction-associated fatty liver disease predicts new onset of chronic kidney disease better than does fatty liver or nonalcoholic fatty liver disease. Nephrol Dial Transplant. 2022. https://doi.org/10.1093/ndt/gfac188.
Quek J, Ng CH, Tang ASP, Chew N, Chan M, Khoo CM, Wei CP, Chin YH, Tay P, Lim G, Tan DJH, Lim WH, Chan KE, Teng M, Tan E, Tamaki N, Huang DQ, Siddiqui MS, Young DY, Noureddin M, Muthiah MD. Metabolic associated fatty liver disease increases the risk of systemic complications and mortality. A Meta-analysis and systematic review of 12,620,736 Individuals. Endocr Pract. 2022;28:667–72. https://doi.org/10.1016/j.eprac.2022.03.016.
Morishita A, Tadokoro T, Fujihara S, Iwama H, Oura K, Fujita K, Tani J, Takuma K, Nakahara M, Shi T, Haba R, Okano K, Nishiyama A, Ono M, Himoto T, Masaki T. Ipragliflozin attenuates non-alcoholic steatohepatitis development in an animal model. PLoS ONE. 2022;17:e0261310. https://doi.org/10.1371/journal.pone.0261310.
Akuta N, Kawamura Y, Fujiyama S, Saito S, Muraishi N, Sezaki H, Hosaka T, Kobayashi M, Kobayashi M, Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kumada H. Favorable impact of long-term SGLT2 inhibitor for NAFLD complicated by diabetes mellitus: a 5-year follow-up study. Hepatol Commun. 2022;6:2286–97. https://doi.org/10.1002/hep4.2005.
Takahashi H, Kessoku T, Kawanaka M, Nonaka M, Hyogo H, Fujii H, Nakajima T, Imajo K, Tanaka K, Kubotsu Y, Isoda H, Oeda S, Kurai O, Yoneda M, Ono M, Kitajima Y, Tajiri R, Takamori A, Kawaguchi A, Aishima S, Kage M, Nakajima A, Eguchi Y, Anzai K. Ipragliflozin improves the hepatic outcomes of patients with diabetes with NAFLD. Hepatol Commun. 2022;6:120–32. https://doi.org/10.1002/hep4.1696.
Li X, Wu X, Jia Y, Fu J, Zhang L, Jiang T, Liu J, Wang G. Liraglutide decreases liver fat content and serum fibroblast growth factor 21 levels in newly diagnosed overweight patients with type 2 diabetes and nonalcoholic fatty liver disease. J Diabetes Res. 2021;2021:3715026. https://doi.org/10.1155/2021/3715026.
Morieri ML, Targher G, Lapolla A, D’Ambrosio M, Tadiotto F, Rigato M, Frison V, Paccagnella A, Simioni N, Avogaro A, Fadini GP. Changes in markers of hepatic steatosis and fibrosis in patients with type 2 diabetes during treatment with glucagon-like peptide-1 receptor agonists. A multicenter retrospective longitudinal study. Nutr Metab Cardiovasc Dis. 2021;31:3474–83. https://doi.org/10.1016/j.numecd.2021.08.049.
Jianping W, Xuelian Z, Anjiang W, Haiying X. Efficacy and safety of glucagon-like peptide-1 receptor agonists in the treatment of metabolic associated fatty liver disease: a systematic review and meta-analysis. J Clin Gastroenterol. 2021;55:586–93. https://doi.org/10.1097/MCG.0000000000001556.
Dutta D, Kumar M, Shivaprasad KS, Kumar A, Sharma M. Impact of semaglutide on biochemical and radiologic measures of metabolic-dysfunction associated fatty liver disease across the spectrum of glycaemia: a meta-analysis. Diabetes Metab Syndr. 2022;16:102539. https://doi.org/10.1016/j.dsx.2022.102539.
Saha M, Manna K, Das SK. Melatonin suppresses NLRP3 inflammasome activation via TLR4/NF-kappaB and P2X7R signaling in high-fat diet-induced murine NASH model. J Inflamm Res. 2022;15:3235–58. https://doi.org/10.2147/JIR.S343236.
Pakravan H, Ahmadian M, Fani A, Aghaee D, Brumanad S, Pakzad B. The effects of melatonin in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Adv Biomed Res. 2017;6:40. https://doi.org/10.4103/2277-9175.204593.
Akhavan Rezayat A, Ghasemi Nour M, Bondarsahebi Y, Hozhabrossadati SA, Amirkhanlou F, Akhavan Rezayat S, Kiani M, Imani B. The effects of melatonin therapy on the treatment of patients with Non-alcoholic steatohepatitis: a systematic review and Meta-analysis on clinical trial studies. Eur J Pharmacol. 2021;905:174154. https://doi.org/10.1016/j.ejphar.2021.174154.
Liu J, Ayada I, Zhang X, Wang L, Li Y, Wen T, Ma Z, Bruno MJ, de Knegt RJ, Cao W, Peppelenbosch MP, Ghanbari M, Li Z, Pan Q. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese adults. Clin Gastroenterol Hepatol. 2022;20:e573–82. https://doi.org/10.1016/j.cgh.2021.02.030.
Ruiz-Manriquez J, Olivas-Martinez A, Chávez-García LC, Fernández-Ramírez A, Moctezuma-Velazquez C, Kauffman-Ortega E, Castro-Narro G, Astudillo-García F, Escalona-Nandez I, Aguilar-Salinas CA, Navarro-Alvarez N, Torre A. Prevalence of metabolic-associated fatty liver disease in Mexico and development of a screening tool: the MAFLD-S score. Gastro Hep Adv. 2022;1:352–8. https://doi.org/10.1016/j.gastha.2021.12.011.
Theofilis P, Vordoni A, Nakas N, Kalaitzidis RG. Endothelial dysfunction in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Life (Basel). 2022;12:718. https://doi.org/10.3390/life12050718.
Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, Federico A, Persico M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613. https://doi.org/10.1155/2018/9547613.
Tan X, Liu Y, Long J, Chen S, Liao G, Wu S, Li C, Wang L, Ling W, Zhu H. Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Mol Nutr Food Res. 2019;63:e1900257. https://doi.org/10.1002/mnfr.201900257.
Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34:431–40. https://doi.org/10.1038/hr.2010.264.
Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis C, Tousoulis D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9:781. https://doi.org/10.3390/biomedicines9070781.
Avery EG, Bartolomaeus H, Maifeld A, Marko L, Wiig H, Wilck N, Rosshart SP, Forslund SK, Muller DN. The gut microbiome in hypertension: recent advances and future perspectives. Circ Res. 2021;128:934–50. https://doi.org/10.1161/CIRCRESAHA.121.318065.
Oikonomou E, Leopoulou M, Theofilis P, Antonopoulos AS, Siasos G, Latsios G, Mystakidi VC, Antoniades C, Tousoulis D. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: clinical and therapeutic implications. Atherosclerosis. 2020;309:16–26. https://doi.org/10.1016/j.atherosclerosis.2020.07.027.
Theofilis P, Sagris M, Antonopoulos AS, Oikonomou E, Tsioufis C, Tousoulis D. Inflammatory mediators of platelet activation: focus on atherosclerosis and COVID-19. Int J Mol Sci. 2021;22:11170. https://doi.org/10.3390/ijms222011170.
Sagris M, Theofilis P, Antonopoulos AS, Oikonomou E, Paschaliori C, Galiatsatos N, Tsioufis K, Tousoulis D. Inflammation in coronary microvascular dysfunction. Int J Mol Sci. 2021;22:13471. https://doi.org/10.3390/ijms222413471.
Sagris M, Theofilis P, Antonopoulos AS, Tsioufis C, Oikonomou E, Antoniades C, Crea F, Kaski JC, Tousoulis D. Inflammatory mechanisms in COVID-19 and atherosclerosis: current pharmaceutical perspectives. Int J Mol Sci. 2021;22:6607. https://doi.org/10.3390/ijms22126607.
Suarez-Ortegon MF, McLachlan S, Fernandez-Real JM, Tuomainen TP, Aregbesola A, Wild SH. Serum ferritin and incident cardiometabolic diseases in Scottish adults. Cardiovasc Diabetol. 2022;21:26. https://doi.org/10.1186/s12933-022-01450-7.
Kang HT, Linton JA, Kwon SK, Park BJ, Lee JH. Ferritin level is positively associated with chronic kidney disease in Korean men, based on the 2010–2012 Korean National Health and Nutrition Examination Survey. Int J Environ Res Public Health. 2016. https://doi.org/10.3390/ijerph13111058.
Wu YH, Wang SY, Li MX, He H, Yin WJ, Guo YH, Zhang HQ, Sun ZM, Zhang D, Wang X, Sun SY, Tang SX, Du R, Zhang CH. Serum ferritin independently predicts the incidence of chronic kidney disease in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2020;13:99–105. https://doi.org/10.2147/DMSO.S228335.
Fibrinogen Studies C, Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D’Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha-Pinango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, Diez-Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssonen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Despres JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben-Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Lewington S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–809. https://doi.org/10.1001/jama.294.14.1799.
Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett-Connor E, Benjamin EJ, Bjorkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D’Agostino RB Sr, Dankner R, Davey-Smith G, Deeg D, Dekker JM, Engstrom G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jorgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil-Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. https://doi.org/10.1056/NEJMoa1107477.
Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. https://doi.org/10.1161/01.cir.0000042700.48769.59.
Goicoechea M, de Vinuesa SG, Gomez-Campdera F, Aragoncillo I, Verdalles U, Mosse A, Luno J. Serum fibrinogen levels are an independent predictor of mortality in patients with chronic kidney disease (CKD) stages 3 and 4. Kidney Int Suppl. 2008. https://doi.org/10.1038/ki.2008.519.
Huang MJ, Wei RB, Wang Y, Su TY, Di P, Li QP, Yang X, Li P, Chen XM. Blood coagulation system in patients with chronic kidney disease: a prospective observational study. BMJ Open. 2017;7:e014294. https://doi.org/10.1136/bmjopen-2016-014294.
Hagstrom E, Steg PG, Szarek M, Bhatt DL, Bittner VA, Danchin N, Diaz R, Goodman SG, Harrington RA, Jukema JW, Liberopoulos E, Marx N, McGinniss J, Manvelian G, Pordy R, Scemama M, White HD, Zeiher AM, Schwartz GG, Investigators OO. Apolipoprotein B, residual cardiovascular risk after acute coronary syndrome, and effects of alirocumab. Circulation. 2022;146:657–72. https://doi.org/10.1161/CIRCULATIONAHA.121.057807.
Walldius G, de Faire U, Alfredsson L, Leander K, Westerholm P, Malmstrom H, Ivert T, Hammar N. Long-term risk of a major cardiovascular event by apoB, apoA-1, and the apoB/apoA-1 ratio-Experience from the Swedish AMORIS cohort: a cohort study. PLoS Med. 2021;18:e1003853. https://doi.org/10.1371/journal.pmed.1003853.
Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, Dweck MR, Koschinsky M, Lambert G, Mach F, McNeal CJ, Moriarty PM, Natarajan P, Nordestgaard BG, Parhofer KG, Virani SS, von Eckardstein A, Watts GF, Stock JK, Ray KK, Tokgozoglu LS, Catapano AL. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022. https://doi.org/10.1093/eurheartj/ehac361.
Goek ON, Kottgen A, Hoogeveen RC, Ballantyne CM, Coresh J, Astor BC. Association of apolipoprotein A1 and B with kidney function and chronic kidney disease in two multiethnic population samples. Nephrol Dial Transplant. 2012;27:2839–47. https://doi.org/10.1093/ndt/gfr795.
Hopewell JC, Haynes R, Baigent C. The role of lipoprotein (a) in chronic kidney disease. J Lipid Res. 2018;59:577–85. https://doi.org/10.1194/jlr.R083626.
Sofat R, Cooper JA, Kumari M, Casas JP, Mitchell JP, Acharya J, Thom S, Hughes AD, Humphries SE, Hingorani AD. Circulating apolipoprotein E concentration and cardiovascular disease risk: meta-analysis of results from three studies. PLoS Med. 2016;13:e1002146. https://doi.org/10.1371/journal.pmed.1002146.
Cicero AFG, Fogacci F, Giovannini M, Grandi E, Rosticci M, D’Addato S, Borghi C. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci Rep. 2018;8:11529. https://doi.org/10.1038/s41598-018-29955-w.
Pugliese NR, Mengozzi A, Virdis A, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, Bombelli M, Dell’Oro R, Bruno B, Lippa L, D’Elia L, Verdecchia P, Mallamaci F, Cirillo M, Rattazzi M, Cirillo P, Gesualdo L, Mazza A, Giannattasio C, Maloberti A, Volpe M, Tocci G, Georgiopoulos G, Iaccarino G, Nazzaro P, Parati G, Palatini P, Galletti F, Ferri C, Desideri G, Viazzi F, Pontremoli R, Muiesan ML, Grassi G, Masi S, Borghi C, Working Group on Uric A, Cardiovascular Risk of the Italian Society of H. The importance of including uric acid in the definition of metabolic syndrome when assessing the mortality risk. Clin Res Cardiol. 2021;110:1073–82. https://doi.org/10.1007/s00392-021-01815-0.
Theofilis P, Tsimihodimos V, Vordoni A, Kalaitzidis RG. Serum uric acid levels and cardiometabolic profile in middle-aged, treatment-naive hypertensive patients. High Blood Press Cardiovasc Prev. 2022;29:367–74. https://doi.org/10.1007/s40292-022-00522-9.
Virdis A, Masi S, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, Bombelli M, Dell’Oro R, Bruno B, Lippa L, D’Elia L, Verdecchia P, Mallamaci F, Cirillo M, Rattazzi M, Cirillo P, Gesualdo L, Mazza A, Giannattasio C, Maloberti A, Volpe M, Tocci G, Georgiopoulos G, Iaccarino G, Nazzaro P, Parati G, Palatini P, Galletti F, Ferri C, Desideri G, Viazzi F, Pontremoli R, Muiesan ML, Grassi G, Borghi C, from the Working Group on Uric A, Cardiovascular Risk of the Italian Society of H. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension. 2020;75:302–8. https://doi.org/10.1161/HYPERTENSIONAHA.119.13643.
Son YB, Yang JH, Kim MG, Jo SK, Cho WY, Oh SW. The effect of baseline serum uric acid on chronic kidney disease in normotensive, normoglycemic, and non-obese individuals: a health checkup cohort study. PLoS ONE. 2021;16:e0244106. https://doi.org/10.1371/journal.pone.0244106.
Srivastava A, Kaze AD, McMullan CJ, Isakova T, Waikar SS. Uric Acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis. 2018;71:362–70. https://doi.org/10.1053/j.ajkd.2017.08.017.
Yu H, Zhao L, Liu L, Li Y, Sun J, Liu Y. Relationship between serum uric acid level and nonalcoholic fatty liver disease in type 2 diabetes patients. Medicine (Baltimore). 2021;100:e26946. https://doi.org/10.1097/MD.0000000000026946.
Bao T, Ying Z, Gong L, Du J, Ji G, Li Z, Gao W, Jiang X, Yang H, Huang Y, Tang H. Association between serum uric acid and nonalcoholic fatty liver disease in nonobese postmenopausal women: a cross-sectional study. Sci Rep. 2020;10:10072. https://doi.org/10.1038/s41598-020-66931-9.
Wei F, Li J, Chen C, Zhang K, Cao L, Wang X, Ma J, Feng S, Li WD. Higher serum uric acid level predicts non-alcoholic fatty liver disease: a 4-year prospective cohort study. Front Endocrinol (Lausanne). 2020;11:179. https://doi.org/10.3389/fendo.2020.00179.
Oral A, Sahin T, Turker F, Kocak E. Relationship between serum uric acid levels and nonalcoholic fatty liver disease in non-obese patients. Medicina (Kaunas). 2019. https://doi.org/10.3390/medicina55090600.
Xing Y, Chen J, Liu J, Song G, Ma H. Relationship between serum uric acid-to-creatinine ratio and the risk of metabolic-associated fatty liver disease in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2022;15:257–67. https://doi.org/10.2147/DMSO.S350468.
Theofilis P, Vordoni A, Kalaitzidis RG. Metabolic dysfunction-associated fatty liver disease in the National Health and Nutrition Examination Survey 2017–2020: epidemiology, clinical correlates, and the role of diagnostic scores. Metabolites. 2022. https://doi.org/10.3390/metabo12111070.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Research involving human participants
The study was approved by the Ethics Committee of the Ioannina University Hospital and was carried out according to the Declaration of Helsinki (1989).
Informed consent
All individuals were informed about the aims of the study and provided written informed consent.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
PT interpreted the results and drafted the manuscript. VT conceived and designed the study and revised the manuscript. AV interpreted the results and revised the manuscript. RGK conceived and designed the study, acquired the data, interpreted the results and drafted the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Theofilis, P., Vordoni, A., Tsimihodimos, V. et al. Metabolic Dysfunction-Associated Fatty Liver Disease in Newly Diagnosed, Treatment-Naive Hypertensive Patients and Its Association with Cardiorenal Risk Markers. High Blood Press Cardiovasc Prev 30, 63–72 (2023). https://doi.org/10.1007/s40292-023-00558-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-023-00558-5