Abstract
Aortic stenosis (AS) is the most common degenerative valvular disease in western word. In patients with severe AS, small changes in aortic valve area can lead to large changes in hemodynamics. The correct understanding of cardiac hemodynamics and its interaction with vascular function is of paramount importance for correct identification of severe AS and to plan effective strategies for its treatment. In the current review with highlight the importance of pressure recovery phenomenon and valvular arterial impedance as novel tools in the evaluation of patients with aortic stenosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pathologic thickening, degeneration or fusion of the aortic valve leaflets results in restricted leaflet mobility and a decrease in the effective aortic valve area, leading to aortic valve stenosis (AS) [1]. The etiology of AS in developed countries has changed dramatically in the last few decades [2]. With the aging of the population and concurrent decrease in rheumatic fever, degenerative calcific AS and bicuspid AS are now by far the most common causes of AS [3]. In this review paper we focus on degenerative calcific AS.
As the AS progresses, the valve orifice decreases, and the obstruction to blood flow increases. Subsequent a pressure gradient develops between the LV and the aorta. This leads to increased LV load and wall stress which induces compensatory LV hypertrophy [4]. This adaptation allows the LV to generate the necessary pressure to maintain cardiac output, but can lead to abnormalities in diastolic LV function, coronary perfusion, and eventually LV systolic dysfunction [5]. In mild AS, intracardiac pressures and cardiac output will appear normal. As the valve becomes more stenotic, the patient may have normal hemodynamic findings at rest, but may be unable to increase cardiac output during exercise. In severe AS, decreased cardiac output is present even at rest. In moderate to severe AS, patients may develop elevated filling pressures to compensate for the increase in LV end-diastolic pressure. In a minority of patients LV systolic failure also occurs, which may lead to further elevation in intracardiac pressures. However, most patients with severe AS and reduced LV systolic function have concomitant coronary artery disease. It is important to remember that the pressure gradient across the aortic valve increases exponentially (not linearly) with decreasing aortic valve area. Thus, in patients with severe AS, small changes in aortic valve area can lead to large changes in hemodynamics. The correct understanding of cardiac hemodynamics and its interaction with vascular function is of paramount importance for correct identification of severe AS and to plan effective strategies for these patients.
2 Overview of Cardiac Hemodynamic in in AS
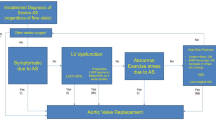
Flow through a stenotic aortic valve is well approximated by flow through a convergent orifice (e.g., a nozzle), which causes acceleration of blood velocity as it passes from the LV outflow tract (LVOT) through the stenotic valve. The point of maximum velocity is termed the vena contracta (VC) of the jet and the area of the flow jet at the VC is known as the effective orifice area (EOA). Doppler evaluation enables the noninvasive measurement of blood flow velocity with estimation of aortic valve gradient and valve area [6]. The following parameters are commonly measured: (a) maximum systolic velocity across the aortic valve; (b) mean aortic valve pressure gradient; and (c) aortic valve area (Fig. 1).
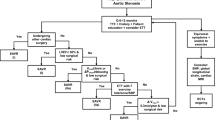
Mean trans-aortic pressure gradient is the average difference in pressure between the LV and aorta during systole. Peak velocity and mean gradient provide independent information regarding AS severity, with the relationship depending on the shape of the velocity curve. The mean gradient is calculated by averaging the instantaneous gradients over the ejection period followed by calculation of pressure from velocity using a simplification of the Bernoulli equation: ∆P=4v2. All these parameters have been historically used to assess the AS severity using the continuity equation, but many technical pitfalls have been highlighted [7]. In clinical practice, AS may be graded discordantly or concordantly when velocity/gradient and aortic valve area are combined. Current ESC guidelines on management of valvular heart disease therefore recommend to assess stroke volume indexed to body surface area (SVi) and LV ejection fraction in patients with discordantly graded AS. Following this approach, three different types of severe AS may be identified among patients with normal LV ejection fraction: normal flow-high gradient AS, low flow-low gradient AS and normal flow-low gradient AS [1].
Concomitant arterial hypertension complicates the assessment of AS severity. Contributing factors include increased aortic stiffness, remodelling of the proximal aorta, increased global LV load, more abnormal LV geometry and function [8]. Both hypertension and arterial stiffness are associated with discordantly graded AS, in particular the low flow-low gradient (LF-LG) severe AS [9, 10]. There is growing interest in the evaluation of stiffness of the arterial tree in the natural history and prognosis of AS, as well as for the elucidation of the effects that surgical and trans-catheter management of AS have on arterial biomarkers [11]. The interplay between AS and functional arterial properties provides significant insights into the pathophysiology of the disease. Arterial stiffness increases after surgical and invasive procedures to treat the stenotic valve due to pressure loading after the relief of the obstruction; however, an inadvertent increase may have an adverse prognostic role. Vascular biomarkers could contribute to more accurate risk stratification and hence, more precise decision-making in patients with AS.
In particular low systemic arterial compliance has been recognized as a main determinant of outcome in patients with aortic stenosis [12, 13]. Recent studies have highlighted that patients with a stiff arterial tree, particularly involving the aorta, have a poorer outcome as the correction of the aortic stenosis by a trans-catheter or surgical approach, simply transfers the load faced by the LV to the stiff aorta, creating in some patients a high afterload, low-output state [14].
3 Pressure Recovery Phenomenon and Its Clinical Utility
The phenomenon of pressure recovery (PR) as a source of discrepancy between Doppler derived and catheter derived gradients across a stenotic aortic valve was first demonstrated in 1989 in vitro [15], followed by animal models [16] and small patient series [17, 18]. Convergence of flow through the AS to the VC leads to conversions of potential energy to kinetic energy and a resulting reduction in pressure at the VC [19]. Reconversion of some kinetic energy to potential energy occurs as streamlines [6, 20, 21] pressure that reflects the load imposed on the LV by the AS rather than the pressure drop at the VC (Fig. 2). The aortic valve area (AVA) calculated by echocardiography using the continuity equation should therefore be adjusted for the pressure recovery for more accurate estimation of the AS severity [19, 22].
In 2000, Garcia et al. derived an equation based on an experimental model on how to calculate the pressure recovery and the pressure recovery adjusted AVA, named energy loss (EL) by Doppler echocardiography [23]. In their study, the EL could be calculated as (AVA×AA)/(AA−AVA), where AVA is the aortic valve area calculated by continuity equation and AA the aortic area [23]. The authors also demonstrated that EL was more closely related to the increase in LV workload than the AVA [23]. Of clinical importance, use of EL rather than AVA is particularly recommended in patients with mild or moderate AS and in patients with a small aortic root, aortic root diameter < 3.0 cm [17, 24, 25]. The prognostic importance of EL was well demonstrated in the large prospective Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) study including 1873 patients with initial mild-moderate asymptomatic AS [26]. AVA index particularly overestimated the AS severity in patients with lower degree of AS and milder degrees of AS [26]. Use of EL index rather than AVA index reduced the prevalence of severe AS in the large Simvastatin Ezetimibe in Aortic Stenosis (SEAS) study by 47.5% [26], this association was also demonstrated including AS patients with initial asymptomatic mild to moderate AS without known atherosclerotic disease or diabetes mellitus [27]. Of note, EL index provided independent and additional prognostic information to that derived from conventional measures of AS severity, including peak aortic jet velocity and mean aortic gradient [27].
Altes et al. tested the use of EL in a study of 379 patients with discordantly graded AS (paradoxical low gradient AS [PLGAS]), identified by a mean aortic gradient < 40 mmHg and an AVA < 1.0 cm2 or < 0.6 cm2/m2. In their study, the use of EL index reclassified 39% of patients from PLGAS to concordantly graded moderate AS [28]. In patients reclassified to moderate AS by EL index, a 50% lower risk of cardiac mortality or need for aortic valve replacement was found compared to those who remained with PLGAS during a median follow-up time of 34 months [28].
As a consequence of these studies, the European Association of Cardiovascular Imaging and American Society of Echocardiography recently recommended to take PR into account in the assessment of AS severity for accurate assessment of AS severity by echocardiography [6]. In particular, PR and EL should be calculated in patients with a diameter of the ascending aorta < 30 mm [6]. In our experience, estimation of pressure recovery should be performed at the sinotubular junction level of the aorta by measuring the inner aortic diameter and using the validated equation for calculation of EL as presented above (Fig. 2) [26, 27]. This method is prognostically validated, and the point of measure is also easily found when serial assessments are necessary during follow-up of individual patients.
Another main source of inaccurate estimation of AS severity is the indexation of AVA to body surface area due to the high proportion of obesity among patients with AS. Rogge et al. demonstrated that indexing AVA and EL for body surface area in patients with obesity may lead to overestimation of the AS severity [29]. Thus, current American and European guidelines on management of valvular heart disease do not recommend indexation of AVA by body surface area due to the increasing prevalence of obesity [30, 31]. In line with this, also EL should be reported without indexation for body surface area [29].
4 Double Hemodynamic Load When AS and Arterial Hypertension Co-exist
AS is typically associated with advanced age, hypertension, and a significantly altered aorta. Although the vascular component is believed to be significant, its specific effect has remained unknown to date [32,33,34]. Arterial stiffness is the consequence of a complicated interaction between endothelial and smooth muscle cell function, extracellular matrix composition, genetics, vasoactive properties, haemodynamic variables and ageing [35]. The central elastic aorta dilates, lengthens, and becomes tortuous with stiffer walls as it ages [36]. Age-related alterations in the ascending aorta's flow velocity, pressure waveform, and vascular load are now well documented. Arterial stiffening accelerates the propagation of the blood pressure wave (measured as the pulse wave velocity) in the aorta, resulting in an earlier return of reflected waves to the proximal aorta. As a result, the reflected wave's time shift raises the peak systolic pressure in the proximal aorta during systole and the central pulse pressure. Increased pulse pressure raises the heart's afterload but decreased diastolic pressure may result in decreased coronary perfusion [37]. The etiology of unfavorable outcomes in individuals with AS is due to an imbalance between global increases in LV load, whether valvular or vascular in origin, and resting and exercise LV reserve. Chronic exposure to a larger amount of afterload ultimately impairs myocardial contractile performance intrinsically [38,39,40]. While the majority of patients with AS retain contractile function via an adaptive LV remodeling process, about one-fifth of patients do not fall neatly into this group and present with low-flow and/or low-ejection conditions [41]. At 5 years, more than 10% of patients who survive TAVI have a worsening of symptoms or re-hospitalization for heart failure [42]. Although the predictors of increasing symptoms and hospitalization for heart failure after TAVI are not well characterized, the subgroup of AS patients with low-flow and/or low-ejection conditions has a significantly lower survival rate when flow or ejection fraction do not improve [43]. This is owing to an unrecognized mismatch in the ventriculo-arterial (VA) connection. While the severity of AS has the greatest influence on structural and functional LV changes, hypertension and increased arterial load both play a significant role in VA coupling mismatch. In a recent series of elderly individuals with AS, hypertension was shown to be more than 75% prevalent [44, 45]. It has been shown that hypertension and increased arterial load have a detrimental effect on LV remodelling, function, and survival [46,47,48,49]. Characterizing the intrinsic features of the arterial tree, on the other hand, remains especially difficult in AS due to the challenges associated with decoupling ventricular-vascular interactions [50]. This is critical because acute treatments in one compartment may result in alterations in the other. For example, after valvular stenosis alleviation by TAVI or surgical aortic valve replacement (AVR), the LV load is often shifted to the stiff arterial tree [51], resulting in clinically significant blood pressure increase. To optimize LV load reduction, it may be necessary to treat hypertension using blood pressure lowering medications. Currently, there are no specialized recommendations for hypertension care in AS, and conventional guidelines for hypertension management in adult patients are used inconsistently. Traditional approaches for assessing the severity of AS include Doppler echocardiography assessment of the flow velocity directly above the valve in the ascending aorta or left heart catheterisation measurement of the pressure differential across the valve. Neither approach is capable of determining the features of the LV afterload that may indicate a bad result. The valvulo-arterial impedance (Zva) is the most often used indicator for Doppler echocardiography-based assessment of global LV stress in patients with AS. Recent research has also employed non-invasive pressure (derived from carotid or radial applanation tonometry [AT]) and flow velocity (derived from the ascending aorta on cardiac magnetic resonance) to assess load in the time or frequency domain [52,53,54].
5 Usefulness of Valvulo Arterial Impedance
Mechanical impedance is a measure of how much a structure resists to motion when subjected to a given force. In patients with AS, LV hemodynamic load is composed not only by stenosis severity but also by systemic vascular resistance, volume flow rate and body size [13]. Thus valvulo-arterial impedance (Zva) represent the cost in mmHg for each systemic mL of blood indexed for body size pumped by the LV during systole, taking into account both valvular and arterial load. Zva is calculated by dividing the estimated LV systolic pressure (systolic arterial pressure + mean trans-valvular gradient) by the stroke volume indexed for the body surface area. The valvulo-arterial impedance provides an estimate of the global LV hemodynamic load that results from the summation of the valvular and vascular loads, and the concept is very useful because it incorporates stenosis severity, volume flow rate, body size, and systemic vascular resistance. Moreover, Zva can easily be calculated using Doppler echocardiography from 3 simple measurements, that is, the LV stroke volume indexed for body surface area (SVI), the transvalvular mean gradient, and systolic arterial pressure.
In the SEAS study Cramariuc et al. reported that patients with a markedly increased Zva often have lower LV ejection fraction, mid-wall fractional shortening, and cardiac output [39]. Thus, identification of high Zva may be helpful in identifying patients with impaired intrinsic LV myocardial dysfunction despite normal ejection fraction [39].
Clinical utility of Zva was also demonstrated by its ability to predict occurrence of syncope and adverse outcome in asymptomatic patients with at least moderate AS, even after adjustment for several clinical and echocardiographic confounders [46, 55]. Using more advanced echocardiography, Zito et al. demonstrated that in asymptomatic patients with severe AS, higher global afterload and its consequences on longitudinal LV function contributes to the development of symptoms and indication for aortic valve replacement [56]. The calculation of Zva might be particularly useful in patients with low-flow, low-gradient (LF-LG) severe AS and normal LVEF. Adda et al. reported that such patients had more severe and higher Zva than patients with normal-flow low-gradient AS, suggesting that the main mechanism of paradoxical LF-LG severe AS was elevated global afterload impairing LV function [39, 57].
High Zva is considered present when ≥ 5 mmHg mL−1 m−2. High Zva is associated with poorer prognosis both in pre-operative AS and in patients with severe AS who underwent TAVI [58, 59].
The importance of valvular function assessed by Zva for quality of life and exercise performance was further demonstrate in a cohort of patient with severe AS treated with TAVI [60]. In this study a pre-operative Zva > 5 mmHg·mL−1·m−2 was associated with unfavorable long term quality of life [60]. This demonstrates that in patients with degenerative AS the correction of the valvular load may not be sufficient to completely restore quality of life [61]. Assessment of global arterial-valvular load AS patients candidate for valve replacement may indicate the expected post-operative functional improvement in daily life [61].
6 Conclusions
Understanding cardiovascular hemodynamics in AS is of paramount importance to tailor treatment and surgical approach. Pressure recovery adjustment of the aortic valve area and assessment of valvular arterial impendence are useful in understanding the vascular ventricular interaction in aortic stenosis and contribute to prognostic stratification of patients with combined AS and systemic hypertension.
Change history
19 July 2022
Missing Open Access funding information has been added in the Funding Note.
References
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2022;17(14):e1126–96.
Thaden JJ, Nkomo VT, Enriquez-Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis. 2014;56(6):565–71.
Bakaeen FG, Rosengart TK, Carabello BA. Aortic stenosis. Ann Intern Med. 2017;166(1):ITC1–16.
Cioffi G, de Simone G, Cramariuc D, Mureddu GF, Gerdts E. Inappropriately high left-ventricular mass in asymptomatic mild-moderate aortic stenosis. J Hypertens. 2012;30(2):421–8.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e35–71.
Baumgartner H Chair, Hung J Co-Chair, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017 18(3):254–275.
González-Mansilla A, Martinez-Legazpi P, Prieto A, Gomá E, Haurigot P, Pérez Del Villar C, Cuadrado V, Delgado-Montero A, Prieto R, Mombiela T, Pérez-David E, Rodríguez González E, Benito Y, Yotti R, Pérez-Vallina M, Fernández-Avilés F, Bermejo J. Valve area and the risk of overestimating aortic stenosis. Heart. 2019;105(12):911–9.
Bruschi G, Maloberti A, Sormani P, Colombo G, Nava S, Vallerio P, Casadei F, Bruno J, Moreo A, Merlanti B, Russo C, Oliva F, Klugmann S, Giannattasio C. Arterial stiffness in aortic stenosis: relationship with severity and echocardiographic procedures response. High Blood Press Cardiovasc Prev. 2017;24(1):19–27.
Mancusi C, de Simone G, Brguljan Hitij J, Sudano I, Mahfoud F, Parati G, Kahan T, Barbato E, Pierard LA, Garbi M, Flachskampf FA, Gerdts E. Management of patients with combined arterial hypertension and aortic valve stenosis: a consensus document from the Council on Hypertension and Council on Valvular Heart Disease of the European Society of Cardiology, the European Association of Cardiovasc. Eur Heart J Cardiovasc Pharmacother. 2021;7(3):242–50.
Bahlmann E, Cramariuc D, Minners J, Lønnebakken MT, Ray S, Gohlke-Baerwolf C, Nienaber CA, Jander N, Seifert R, Chambers JB, Kuck KH, Gerdts E. Small aortic root in aortic valve stenosis: clinical characteristics and prognostic implications. Eur Heart J Cardiovasc Imaging. 2017;18(4):404–12.
Gardikioti V, Terentes-Printzios D, Iliopoulos D, Aznaouridis K, Sigala E, Tsioufis K, Vlachopoulos C. Arterial biomarkers in the evaluation, management and prognosis of aortic stenosis. Atherosclerosis. 2021;332:1–15.
Bahlmann E, Cramariuc D, Saeed S, Chambers JB, Nienaber CA, Kuck KH, Lønnebakken MT, Gerdts E. Low systemic arterial compliance is associated with increased cardiovascular morbidity and mortality in aortic valve stenosis. Heart. 2019;105(19):1507–14.
Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46(2):291–8.
Tanaka T, Asami M, Yahagi K, Ninomiya K, Okuno T, Horiuchi Y, Komiyama K, Tanaka J, Yokozuka M, Miura S, Aoki J, Tanabe K. Prognostic impact of arterial stiffness following transcatheter aortic valve replacement. J Cardiol. 2021;78(1):37–43.
Levine RA, Jimoh A, Cape EG. Pressure recovery distal to a stenosis: potential cause of gradient “overestimation” by Doppler echocardiography. J Am Coll Cardiol. 1989;13:706–15.
Garcia D, Dumesnil J, Durand LG, Kadem L, Pibarot P. Discrepancies between catheter and Doppler estimates of valve effective orifice area can be predicted from the pressure recovery phenomenon. J Am Coll Cardiol. 2003;41:435–42.
Baumgartner H, Stefenelli T, Niederberger J, Schima H, Maurer G. “Overestimation” of catheter gradients by Doppler ultrasound in patients with aortic stenosis: a predictable manifestation of pressure recovery. J Am Coll Cardiol. 1999;33:1655–61.
Schoebel WA, Voelker W, Haase KK, Karsch KR. Extent, determinants and clinical importance of pressure recovery in patients with aortic valve stenosis. Eur Heart J. 1999;20:1355–63.
Clark C. Energy losses in flow through stenosed valves. J Biomech. 1976;12:737–46.
Snell RE, Luchsinger PC. Determination of the external work and power of the left ventricle in intact man. Am Heart J. 1965;69:529–37.
Heinrich RS, Fontaine AA, Grimes RY, Sidhaye A, Yang S, Moore KE, Levine RA, Yoganathan AP. Experimental analysis of fluid mechanical energy losses in aortic valve stenosis: importance of pressure recovery. Ann Biomed Eng. 1996;24:685–94.
Heinrich RS, Marcus RH, Ensley AE, Gibson DE, Yoganathan AP. Valve orifice area alone is an insufficient index of aortic stenosis severity: effects of the proximal and distal geometry on transaortic energy loss. J Heart Valve Dis. 1999;8:509–15.
Garcia D, Pibarot P, Dumesnil J, Sakr F, Durand LG. Assessment of aortic valve stenosis severity: a new Index based on the Energy Loss Concept. Circulation. 2000;101:765–71.
Kume T, Okura H, Kawamoto T, Watanabe N, Hayashida A, Neishi Y, Miyamoto Y, Imai K, Yamada R, Yoshida K. Clinical implication of Energy Loss Coefficient in patients with severe aortic stenosis diagnosed by Doppler Echocardiography. Circ J. 2008;72:1265–9.
Spevack D, Almuti K, Ostfeld R, Bello R, Gordon G. Routine adjustment of Doppler echocardiographically derived aortic valve area using a previously derived equation to account for the effect of pressure recovery. J Am Soc Echocardiog. 2008;21:34–7.
Bahlmann E, Cramariuc D, Gerdts E, Gohlke-Baerwolf C, Nienaber CA, Eriksen E, Wachtell K, Chambers J, Kuck KH, Ray S. Impact of pressure recovery on echocardiographic assessment of asymptomatic aortic stenosis: a SEAS substudy. JACC Cardiovasc Imaging. 2010;3:555–62.
Bahlmann E, Gerdts E, Cramariuc D, Gohlke-Baerwolf C, Nienaber CA, Wachtell K, Seifert R, Chambers JB, Kuck KH, Ray S. Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation. 2013;127:1149–56.
Altes A, Ringle A, Bohbot Y, Bouchot O, Appert L, Guerbaai RA, Gun M, Ennezat PV, Tribouilloy C, Marechaux S. Clinical significance of energy loss index in patients with low-gradient severe aortic stenosis and preserved ejection fraction. Eur Heart J Cardiovasc Imaging. 2020;0:1–8.
Rogge BP, Gerdts E, Cramariuc D, Bahlmann E, Jander N, Gohlke-Bärwolf C, Pedersen TR, Lønnebakken MT. Impact of obesity and nonobesity on grading the severity of aortic valve stenosis. Am J Cardiol. 2014;113:1532–5.
Vahanian A, ESC/EACTS Scientific Document Group, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2021;28:ehab395.
Mishimura RA, et al. AHA/ACC Guideline for the management of patients with valvular heart disease: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521-643.
Pibarot JG, Dumesnil P. New concepts in valvular hemodynamics: implications for diagnosis and treatment of aortic stenosis. Can J Cardiol. 2007;23:40–7.
Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–8.
Basile C, Fucile I, Lembo M, Manzi MV, Ilardi F, Franzone A, Mancusi C. Arterial hypertension in aortic valve stenosis: a critical update. J Clin Med. 2021;10(23):5553.
Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–43.
McDonalds, Nichols WWR, O'Rourke MF, O'Rourk N, Hartley C. Blood Flow in Arteries: Theoretical, experimental and clinical principles. Arnold and Oxford University Press Inc. 1997.
Adji A, Kachenoura N, Bollache E, Avolio AP, O’Rourke MF, Mousseaux E. Magnetic resonance and applanation tonometry for noninvasive determination of left ventricular load and ventricular vascular coupling in the time and frequency domain. J Hypertens. 2016;34(6):1099–108.
Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115(22):2856–64.
Cramariuc D, Cioffi G, Rieck AE, Devereux RB, Staal EM, Ray S, Wachtell K, Gerdts E. Low-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS Substudy. JACC Cardiovasc Imaging. 2009;2(4):390–9.
Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation. 2003;107(25):3170–5.
Iung B, Baron G, Tornos P, Gohlke-Bärwolf C, Butchart EG, Vahanian A. Valvular heart disease in the community: a European experience. Curr Probl Cardiol. 2007;32(11):609–61.
Makkar RR, Thourani VH, Mack MJ, et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. 2020;382(9):799–809.
Saybolt MD, Fiorilli PN, Gertz ZM, Herrmann HC. Low-flow severe aortic stenosis: evolving role of transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2017;10(8):e004838.
Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790–8.
Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55(11):1080–90.
Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54(11):1003–11.
Rieck ÅE, Cramariuc D, Boman K, et al. Hypertension in aortic stenosis: implications for left ventricular structure and cardiovascular events. Hypertension. 2012;60(1):90–7.
Nielsen OW, Sajadieh A, Sabbah M, et al. Assessing optimal blood pressure in patients with asymptomatic aortic valve stenosis: the simvastatin ezetimibe in aortic stenosis study (SEAS). Circulation. 2016;134(6):455–68.
Musa TA, Uddin A, Fairbairn TA, et al. Assessment of aortic stiffness by cardiovascular magnetic resonance following the treatment of severe aortic stenosis by TAVI and surgical AVR. J Cardiovasc Magn Reson. 2016;18(1):37.
Garcia D, Barenbrug PJ, Pibarot P, et al. A ventricular-vascular coupling model in presence of aortic stenosis. Am J Physiol Heart Circ Physiol. 2005;288(4):H1874–84.
Yotti R, Bermejo J, Gutiérrez-Ibañes E, et al. Systemic vascular load in calcific degenerative aortic valve stenosis: insight from percutaneous valve replacement. J Am Coll Cardiol. 2015;65(5):423–33.
Soulat G, Kachenoura N, Bollache E, et al. New estimate of valvuloarterial impedance in aortic valve stenosis: a cardiac magnetic resonance study. J Magn Reson Imaging. 2017;45(3):795–803.
Hungerford SL, Adji AI, Bart NK, et al. A novel method to assess valvulo-arterial load in patients with aortic valve stenosis. J Hypertens. 2021;39(3):437–46.
Namasivayam M, Adji A, Lin L, et al. Non-invasive quantification of ventricular contractility, arterial elastic function and ventriculo-arterial coupling from a single diagnostic encounter using simultaneous arterial tonometry and magnetic resonance imaging. Cardiovasc Eng Technol. 2020;11(3):283–94.
Harada K, Saitoh T, Tanaka J, Shibayama K, Berdejo J, Shiota T. Valvuloarterial impedance, but not aortic stenosis severity, predicts syncope in patients with aortic stenosis. Circ Cardiovasc Imaging. 2013;6(6):1024–31.
Zito C, Salvia J, Cusmà-Piccione M, Antonini-Canterin F, Lentini S, Oreto G, Di Bella G, Montericcio V, Carerj S. Prognostic significance of valvuloarterial impedance and left ventricular longitudinal function in asymptomatic severe aortic stenosis involving three-cuspid valves. Am J Cardiol. 2011;108(10):1463–9.
Adda J, Mielot C, Giorgi R, Cransac F, Zirphile X, Donal E, Sportouch-Dukhan C, Réant P, Laffitte S, Cade S, Le Dolley Y, Thuny F, Touboul N, Lavoute C, Avierinos JF, Lancellotti P, Habib G. Low-flow, low-gradient severe aortic stenosis despite normal ejection fraction is associated with severe left ventricular dysfunction as assessed by speckle-tracking echocardiography: a multicenter study. Circ Cardiovasc Imaging. 2012;5(1):27–35.
Giannini C, Petronio AS, De Carlo M, Guarracino F, Benedetti G, Delle Donne MG, Dini FL, Marzilli M, Di Bello V. The incremental value of valvuloarterial impedance in evaluating the results of transcatheter aortic valve implantation in symptomatic aortic stenosis. J Am Soc Echocardiogr. 2012;25(4):444–53.
Kobayashi Y, Kim JB, Moneghetti KJ, Kobayashi Y, Zhang R, Brenner DA, O’Malley R, Schnittger I, Fischbein M, Miller DC, Yeung AC, Liang D, Haddad F, Fearon WF. Dynamic changes in aortic impedance after transcatheter aortic valve replacement and its impact on exploratory outcome. Int J Cardiovasc Imaging. 2017;33(11):1693–701.
Nuis RJ, Goudzwaard JA, de Ronde-Tillmans MJAG, Kroon H, Ooms JF, van Wiechen MP, Geleijnse ML, Zijlstra F, Daemen J, Van Mieghem NM, Mattace-Raso FUS, Lenzen MJ, de Jaegere PPT. Impact of valvulo-arterial impedance on long-term quality of life and exercise performance after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2020;13(1):e008372.
Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart. 2010;96(17):1364–71.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mancusi, C., Bahlmann, E., Basile, C. et al. New Evidence About Aortic Valve Stenosis and Cardiovascular Hemodynamics. High Blood Press Cardiovasc Prev 29, 231–237 (2022). https://doi.org/10.1007/s40292-022-00520-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-022-00520-x