Abstract
Hemophilia is a genetic disorder that is caused by mutations in coagulation factor VIII (hemophilia A) or IX (hemophilia B) genes resulting in blood clotting disorders. Despite advances in therapies, such as recombinant proteins and products with extended half-lives, the treatment of hemophilia still faces two major limitations: the short duration of therapeutic effect and production of neutralizing antibodies against clotting factors (inhibitor). To overcome these limitations, new hemophilia treatment strategies have been established such as gene therapy, bispecific antibody, and rebalancing therapy. Although these strategies have shown promising results, it is difficult to achieve a permanent therapeutic effect. Advances in the clustered regularly interspaced short palindromic repeat (CRISPR) technology have allowed sustainable treatment by correcting mutated genes. Since genome editing generates irreversible changes in host genome, safety must be ensured by delivering target organs. Therefore, the delivery tool of the CRISPR system is crucial for safe, accurate, and efficient genome editing. Recently, non-viral vector lipid nanoparticles (LNPs) have emerged as safer tools for delivering CRISPR systems than other viral vectors. Several previous hemophilia pre-clinical studies using LNP-CRISPR showed that sufficient and sustainable therapeutic effects, which means that LNP-CRISPR-mediated genome-editing therapy can be a valid option for the treatment of hemophilia. In this paper, we summarize the latest advancements in the successful treatment of hemophilia and the potential of CRISPR-mediated genome-editing therapy using LNPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The current standard of care for hemophilia is replacement of deficient clotting factors, which has numerous limitations, most significant of which being that it provides only temporary efficacy. |
Genome editing using the clustered regularly interspaced short palindromic repeats (CRISPR) system has the potential to cure genetic diseases long-term by correcting gene variants. |
For in vivo genome editing for therapeutic purposes, a safe and efficient delivery tool is important, and lipid nanoparticles (LNPs) are suitable non-viral vectors for the safe delivery of the CRISPR system, as demonstrated in COVID-19 vaccine applications. |
The LNP-CRISPR system is a combination of two Nobel Prize-winning technologies, from 2020 (CRISPR) and 2023 (LNP as part of the mRNA-LNP platform), respectively, which can be used in future therapy strategies. |
1 Introduction
Hemophilia is a representative loss-of-function inherited disease caused by mutations in coagulation genes that result in the loss of coagulant function and failure of coagulation cascades [1]. Hemophilia is an X-linked recessive disorder due to mutation in factor VIII (hemophilia A) or IX (hemophilia B) genes causing loss of function of these coagulation proteins affecting the generation of thrombin and thus blood clotting ability.
Hemophilia can be classified according to the concentration of coagulation proteins in the blood, mild type > 5–< 40%, moderate type 1–5%, and severe < 1% of normal levels [2]. Severe hemophilia causes persistent internal bleeding as well as blood clotting disorder, and treatment to prevent spontaneous bleeding is important. Approximately 40% of patients with hemophilia A and 30% of patients with hemophilia B have severe hemophilia [3]. The prevalence of hemophilia is 1 in 5,000–10,000 males with hemophilia A, and 1 in 25,000 males with hemophilia B [4]. The World Federation of Hemophilia (WFH) has estimated there are approximately 815,100 cases of hemophilia worldwide. Of these, 347,026 have been confirmed by diagnosis, of which 276,900 cases have been classified as severe hemophilia [5].
Hemophilia has various unmet needs and as yet there is no permanent cure for this disease. The classic treatment for hemophilia symptoms involves the injection of clotting proteins isolated from healthy blood, which has evolved into injections of recombinant proteins synthesized in the laboratory [6]. The development of recombinant factor VIII or IX has increased the life expectancy of patients with concentrates devoid of the risk associated with the use of plasma-derived products before the era of virucidal technologies and blood donor screening for HIV or hepatitis C [6]. Currently, almost all patients with hemophilia are recommended to receive prophylaxis, which involves periodic injections of deficient clotting factors to maintain minimal clotting capacity. Prophylaxis consists of intravenous (IV) injections of recombinant proteins, and because of their short half-lives, averaging 12 and 18 h for FVIII and FIX, respectively, patients require frequent therapy two to three times a week [7]. Such frequent treatments increase the financial burden on patients and reduce their quality of life. Additionally, IV injection at short intervals for babies and children has risks of venous access. Many researchers have attempted to address these limitations by developing recombinant proteins with extended half-lives (EHL) in the human body. Despite a 1.5- to 4.5-fold increase in half-life compared to conventional proteins, EHL products are not long-lasting; therefore, patients still need to be treated once or twice a week [8,9,10]. Another problem is that prophylaxis or treatment can lead to the development of inhibitors. These inhibitors recognize the injected recombinant protein as a foreign protein in their bodies, which greatly decreases or abolishes the efficacy of prophylaxis or treatment [11].
Researchers have developed gene therapies using viral vectors with long-lasting therapeutic effects to overcome the short half-lives for the current hemophilia treatments [12]. Adeno-associated virus (AAV) is one of the most promising viral vectors and is being investigated for the gene therapy of many inherited diseases, including hemophilia [13]. Similar to prophylaxis, AAV gene therapy does not provide a permanent cure [14]. Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) can insert or delete target genes permanently, making them a valuable tool for correcting mutated genes in genetic diseases [15]. The CRISPR system is being explored in both pre-clinical and clinical studies on the treatment of hemophilia. The selection of a delivery tool is critical for efficient and safe delivery of CRISPR systems to target organs, and AAV is preferred for use as a gene carrier because of its high transduction efficiency [16]. Recently, researchers have been working on non-viral vectors, such as lipid nanoparticles (LNPs), to deliver CRISPR systems [17]. LNPs are considered as safer options because they have less immune induction than AAV or other viral vectors [17, 18], many applications are expected as delivery tools of the CRISPR system. Here, we summarize the latest hemophilia drugs and new research trends with a focus on the CRISPR system using LNPs for the development of new hemophilia treatments.
2 Adeno-Associated Virus Gene Therapy for the Treatment of Hemophilia
Gene therapy is a treatment strategy in which a therapeutic gene is delivered to a cell, causing the cell to produce its proteins. In gene therapy for hemophilia, a gene cassette expressing the therapeutic gene is transferred to the patient, enabling the body to produce normal clotting factors and restore the ability of blood to clot [19]. Unlike previous therapies, gene therapy is an attractive option because it allows the patient's body to produce the necessary proteins directly, thereby improving hemophilia severity [20]. Two drugs have been approved by the US Food and Drug Administration (FDA) for gene therapy, both of which are based on a viral vector, AAV [19, 21]. AAV is currently one of the most popular viral vectors and has the advantage of reaching the desired target organ because of its high cell transduction rate and the unique tropism of the serotype [22]. AAVs have safety advantages over other viral vectors such as lentiviruses and adenoviruses because they cannot replicate without helper viruses, rarely integrate randomly into host genes, and induce less of an immune response [13]. In the case of hemophilia B, the DNA-encoding FIX is approximately 1.4 kb; it has been actively investigated since the early phase of AAV gene therapy research [19, 23]. After discovering that the FIX protein variant, Padua (R338L), has a stronger blood clotting ability than the WT protein, pharmaceutical companies have developed gene therapies using it [24]. In hemophilia A, the encoded DNA of FVIII is approximately 7 kb, which exceeds the AAV packaging limit, making the development of gene therapy for hemophilia A challenging. The B domain, the largest FVIII protein domain, is non-functional, and its deletion has been reported to have no significant effect on the physiological function of FVIII [25]. Therefore, much research is underway on gene therapy for hemophilia A, which encodes the F8 B-domain-deletion (F8-BDD) DNA in AAV [26,27,28,29].
Although gene therapy using AAV is a promising approach for supplementing deficient proteins, several issues remain unresolved. Despite having a lower immune response than other viral vectors, three patients injected with AAV at doses higher than 1 × 1014 vg/kg died in clinical trials [30]. The cause of death in deceased patients remains unclear; however, it appears to be related to high doses [30]. AAV has genotoxicity, and can be randomly integrated into the host gene locus. The exact mechanism of the random integration of AAV into the host genome is still unknown, but random integration is known to be dose-dependent [31, 32]. Therefore, it is important to ascertain the lowest dose of AAV to ensure sufficient efficacy with safety. Another limitation is that administration of AAV can induce the production of anti-capsid antibodies [14], resulting in patients having difficulty receiving AAV therapy for another disease of the same serotype. Recently, Mcintosh et al. reported long-term persistence of anti-AAV antibody up to 10 years [33]. No inhibitors have been produced in previous hemophilia gene therapy studies [34,35,36], which is a positive safety result. However, it is not certain whether gene therapy is effective in patients with inhibitors, as patients with pre-existing inhibitors or a high probability of developing an inhibitor were excluded from clinical trials [19]. Studies have shown stable protein expression in AAV gene therapy in clinical trials for the treatment of hemophilia. However, the long-term therapeutic efficacy may not be maintained in all patients. A clinical trial in hemophilia A reported a sharp decline in FVIII expression in some patients by approximately 40% within 2 years [34]. Therefore, although AAV gene therapy can show relatively long-term therapeutic effects compared to prophylaxis, it is difficult to achieve a permanent therapeutic effect.
3 Non-Replacement Therapy for the Treatment of Hemophilia
Replacement therapy showed diminished therapeutic efficacy in patients who develop inhibitors. To overcome this problem, a bispecific antibody mimicking activated FVIII has been developed [37]. The bispecific antibody recognizes both activated FIX (FIXa) and activated factor X (FXa) and promotes FIXa-catalyzed activation of FX in the absence of FVIII [38]. Emicizumab is a remarkable bispecific monoclonal antibody for the treatment of hemophilia A, which received FDA approval in 2018 [39]. Emicizumab prophylaxis significantly reduced the annualized bleeding rate in patients with hemophilia A with or without inhibitors [40]. Next generation FVIII mimetic bispecific antibody designated Mim8 showed 15-fold more potency in correcting hemostasis compared with a sequence identical analogue of Emicizumab in pre-clinical studies [41, 42]. Mim8 is currently in phase 3 clinical trials (NCT05053139). These treatments offer the advantage of preserving therapeutic efficacy and consequently mitigating immune reactions in the presence of FVIII inhibitors. Emicizumab and Mim8 showed a prolonged elimination half-life of approximately 4 weeks to reduce the frequency of treatments, but still necessitate periodic injection [43, 44].
Blood coagulation is essential for preventing excessive blood loss during bleeding. However, excessive coagulation, as with inherited antithrombin deficiency [45], can cause thrombosis [46]. Anticoagulant proteins help maintain the “delicate balance” of blood coagulation and prevent excessive thrombin generation during hemostasis (Fig. 1). The inhibition of anticoagulant proteins increases thrombin generation, which can induce thrombosis [47,48,49,50]. Based on this mechanism, rebalancing therapy is an alternative to replacing deficient coagulation factors by suppressing the expression and function of anticoagulants in the blood to restore the blood coagulation ability [51]. As rebalancing therapy does not directly inject clotting proteins, antibody-antigen reactions with FVIII or FIX inhibitors do not occur. It enables to treatment of patients with pre-existing inhibitors and is applicable to patients with hemophilia A or B [51]. Anticoagulants that can be targeted for hemophilia therapy include tissue factor protein inhibitor (TFPI), activated protein C (APC), and antithrombin (AT) [52]. Two monoclonal antibodies with anti-TFPI activity, concizumab, and marstacacin, are currently undergoing phase 3 clinical studies [53, 54]. SerpinPC is a specific APC inhibitor currently in phase 1/2a clinical trials, and fitusiran is a siRNA-based drug that inhibits AT expression and is currently in ongoing FDA phase 3 trials [55, 56].
Brief overview of the blood coagulation cascade. Recombinant protein VIII (rVIII) or IX (rIX) is used for patients with hemophilia A or B, respectively. Tissue factor protein inhibitor, activated protein C, and antithrombin are representative anticoagulant proteins that can be utilized for rebalancing therapy regardless of anti-FVIII or IX inhibitor
The clinical trial results of this rebalancing therapy showed a significant reduction in all bleeding and spontaneous joint bleeding events. Thus, rebalancing therapy has the advantage of being used as a prophylactic treatment for hemophilia and can be used broadly in patients with hemophilia A or B, with or without inhibitors. However, it requires treatment once a week or even once a month, which is similar to conventional prophylaxis.
4 In vivo Lipid Nanoparticle-Clustered Regularly Interspaced Short Palindromic Repeat Approaches for the Treatment of Hemophilia
Genome editing technology can correct or remove disease-causing variant genes to address the fundamental problems associated with genetic disorders and achieve permanent therapeutic efficacy [57]. Therefore, it has the potential to become one of the best treatment options for genetic diseases in the near future. However, nucleases, such as CRISPR/Cas9, can generate undesirable off-target effects. As genome editing is irreversible, it is necessary to prevent off-target effects and unexpected side effects to ensure safety [58]. To overcome these limitations of the CRISPR system, it is important to develop a delivery tool that can accurately and efficiently deliver the CRISPR system to target organs.
Lipid nanoparticles (LNPs), an improved form of cationic liposomes, are non-viral vectors that are currently receiving significant attention. LNPs typically consist of ionizable cationic lipids, polyethylene glycol (PEG) lipids, zwitterionic phospholipids, and cholesterol, providing ideal vector characteristics [59]. Zwitterionic phospholipids render LNP neutral at physiological pH (pH 7.4), enhancing biocompatibility by reducing interactions with the anionic membranes of blood cells and facilitating the delivery of cargo such as mRNA [60]. Conversely, exposure to an acidic environment during endocytosis makes zwitterionic phospholipids positively charged, promoting membrane destabilization of the endosome and the quick release of cargo into the cytosol [60]. In previous studies by Han et al. and Kenjo et al., high LNP-delivered luciferase mRNA expression was confirmed at 3–4 h after injection [61, 62]. In comparison, PEG lipids decrease opsonin adsorption and prevent nanoparticle (NP) recognition and absorption by macrophages. This leads to low immunogenicity, enabling the repeated administration of LNPs [63]. The low immunogenicity of LNPs makes them an ideal vector for genome editing in the skin or muscle, allowing for multiple injections, as these organs are large and compartmentalized within the fascia. Kenjo et al. demonstrated that in the case of Duchenne muscular dystrophy (DMD) treatment with CRISPR Cas9, unlike AAV, the delivery of Cas9 mRNA to the muscle with LNP is repeatable [62]. Moreover, adjusting the type and ratio of the components allows for target cell-specific delivery. Kim et al. manufactured hepatocyte- or liver sinusoidal endothelial cell (LSEC)-specific LNPs by adjusting the physical size, PEG-lipid content, and inclusion of active targeting ligands [64]. In addition to liver, studies on mRNA and siRNA delivery using lung-, bone-, and spinal-specific LNPs are ongoing [65,66,67,68].
This characteristic makes LNPs suitable delivery vectors for the CRISPR system. In addition to the rapid delivery of cargo, low immunogenicity, and sophisticated target targeting, the use of guide RNA containing various chemical modifications can increase genome-editing efficiency [69]. Yin et al. achieved a significantly high efficiency of over 80% of indels in the liver by delivering chemically modified and in vivo-stabilized sgRNAs, referred to as enhanced sgRNAs, using LNPs [70]. In contrast, using the same sgRNA sequence, unmodified sgRNA delivered by LNPs induced 5% and 24% indels when using AAV [70, 71]. Additionally, LNP-delivered mRNA shows less likelihood for off-target effects compared to the CRISPR-Cas9 gene cassette delivered by viral vectors, because mRNA is expressed in cells for a much shorter duration [72].
As a delivery tool, LNPs are known to exhibit low immunogenicity. However, even though the frequency of severe anaphylaxis observed with COVID-19 vaccines is very low (approximately five cases per million), LNPs have been shown to have a potential risk [73,74,75]. The major components of mRNA-encapsulated LNPs that trigger an immune response are RNA, empty LNPs, and PEG lipids [76]. RNA activates TLR3, TLR7, and TLR8 to secrete IL1β, which again secretes the proinflammation cytokines IL-6 and TNF [77]. Empty LNP can act as adjuvants by activating follicular helper T cells, and the anti-PEG lipid antibodies generated by repeated injections can cause anaphylaxis [76]. Anti-PEG lipid antibodies accelerate blood clearance of LNP, rapidly reducing their therapeutic efficacy, and these side effects adversely affect not only the safety of LNPs but also their therapeutic efficacy.
Currently, CRISPR-mediated clinical trials using AAV or LNPs as vectors are actively underway (Table 1). Among the drugs currently under development at Intellia Therapeutics, NTLA-2001 and NTLA-2002 are LNP-CRISPR-based drugs designed to target the TTR gene (encoding transthyretin) for transthyretin amyloidosis and KLKB1 gene (encoding kallikrein B1) for hereditary angioedema, respectively, to prevent the accumulation of misfolded proteins and improve the symptoms of genetic diseases. Each trial was an FDA phase 1 and phase 1/2 clinical trial [78, 86, 87]. Qiu et al. demonstrated a gene-editing efficiency of 38.5% using a highly liver-specific LNP-CRISPR targeting Angptl3, which regulated plasma protein levels and effectively decreased blood triglyceride and LDL-C levels [88]. Additionally, Rosenblum et al. used cancer-specific LNP to deliver PKL1 gene-targeting sgRNA and Cas9 mRNA, achieving up to 80% editing efficiency in tumor cells in vivo, suppressing tumor growth, and increasing survival rates by 80% [89].
The ultimate therapy for loss-of-function diseases, such as hemophilia, is the restoration of deficient proteins. Therefore, a knockout (KO) strategy using CRISPR/Cas9 is inadequate to restore the function of a gene. However, as in the case of rebalancing, the knockout of an anticoagulant gene via CRISPR/Cas9 is expected to have long-term therapeutic effects. Han et al. performed in vivo genome editing with a strategy to knockout SerpinC1 (gene encoding antithrombin III) using LNP-CRISPR, which encapsulates Cas9 mRNA and sgRNA into 246C10 LNP [61]. This study successfully generated liver-specific genome editing and stably suppressed AT protein expression for up to 45 weeks by a single IV injection, demonstrating that LNP-CRISPR is sustainable and efficient for in vivo genome editing. Furthermore, after three repeated injections of LNP-CRISPR, no anti-Cas9 antibody production was found. This suggests that the number of injections administered in clinical trials should be adjusted to achieve the desired level of downregulation. While LNP-CRISPR-mediated rebalancing therapy enhanced thrombin generation, it showed insufficient efficacy against external injuries due to the absence of clotting factors. Therefore, therapeutic gene knock-in should be considered a more effective treatment option.
5 In vivo Gene Knock-In Strategies for the Treatment of Hemophilia
To perform gene knock-in in vivo, the ribonucleoprotein (RNP) complex and the donor template must be simultaneously located in the nucleus [72, 90]. The endocytosis of LNPs proceeds in the following order: endosome escape, LNP degradation, and mRNA release into the cytosol [91]. Since it is physically difficult for negatively charged nucleic acids to pass through the nucleus, genetic materials such as donor templates in the cytosol are unlikely to be transmitted into the nucleus unless the cell divides after mitosis. While the normal condition of many human tissues, such as the brain and liver cells, remains in the G0 phase, the delivery of nucleic acids to the cell nucleus using LNPs remains challenging [92, 93].
Currently, viral vectors are the most efficient delivery tools for transferring genetic material into the nucleus. Previous studies have used AAV as an in vivo gene KI for hemophilia treatment. Even when AAV is used for gene delivery, the low in vivo efficiency of KI is a major hurdle. To overcome this limitation, researchers have selected the target loci for KI to be those with high protein expression. Two different types of AAV were used, AAV-CRISPR and AAV-donor, to induce the therapeutic gene KI at the Albumin or ApoC3 locus in hemophilia A or B mouse models [28, 94, 95]. Despite low genome-editing efficiency, successful therapeutic effects have been demonstrated and maintained for a long time; however, several limitations have been identified. The simultaneous injection of multiple AAVs showed interference effects between viruses, which may be due to the competitive inhibitory effect between AAVs, as AAVs are transduced into cells using a common receptor, regardless of serotype [95]. Therefore, larger amounts of AAV may be injected for KI efficiency when using more than one type of AAV than when using a single AAV. Administration of high-dose AAV increases the risk of immune reactions and random integration [31, 96]. In addition, long-term expression of AAV allows the CRISPR system to function continuously in the body. Ironically, long-term expression, one of the greatest advantages of AAV, increases the risk of off-target events and unpredictable side effects [72].
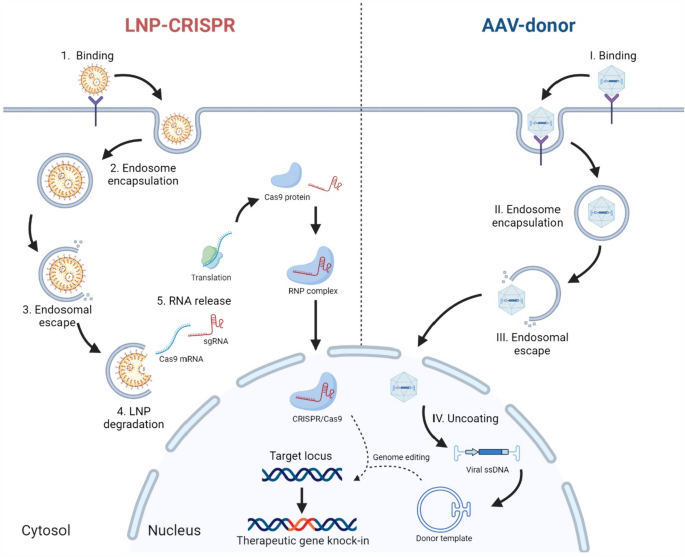
Intellia Therapeutics reported the concept of utilizing the advantages of both LNPs and AAV. Cas9 mRNA and sgRNA were encapsulated in LNP, and donor template was encoded in AAV to achieve therapeutic efficacy by in vivo KI of the hF9 gene to the Albumin locus in a mouse model (Fig. 2) [97]. The strategy of using AAV and LNP in a hybrid form (AAV-LNP hybrid) has several advantages. First, AAV transduction interference is decreased, resulting in a decreased dose requirement of AAV. Secondly, LNP-CRISPR minimizes off-target events because the CRISPR system acts and disappears rapidly, within a few days. Finally, genome editing can only occur in organs which the AAV tropism and LNP targets are matched. Lee et al. used a CRISPR system targeting the SerpinC1 gene locus and the hF9 AAV-donor to simultaneously achieve rebalancing therapy and therapeutic gene KI to maximize therapeutic efficacy [98]. The results of this study confirmed the therapeutic effect of aPTT, as well as an increase in thrombin generation, hFIX concentration measured approximately 1000 ng/mL in blood. Interestingly, the results showed that the liver forced accelerated proliferation by hepatectomy (PHx), and the indel and KI rates in edited cells did not change significantly before and after PHx. This suggests that cells genetically edited using CRISPR can be maintained by dividing, even with turnover, and may sustain therapeutic effects. However, strong therapeutic effects were observed in the AAV-donor control group, using a viral dose of 2 × 1013 vg/kg without the CRISPR system. In the present study, whole-genome sequencing confirmed that random integration occurred in the liver. In a follow-up study, the same strategy was used to KI the human F8-BDD sequence at the SerpinC1 locus in a hemophilia A mouse model [99]. In this study, the viral dose was lowered to 1 × 1012 vg/kg, 1/20 of the previous study, to minimize the random integration of AAV. However, this study also showed slight therapeutic effects in the AAV-donor group, indicating that a viral dose of 1 × 1012 vg/kg may be unsafe for random integration.
Overview of in vivo gene knock-in strategy using AAV-LNP hybrid. Delivery orders of LNPs are described using Arabic numerals, and AAV using Roman numerals. 1. Cargo-loaded LNPs bind to receptor. 2. LNPs are internalized via lipid raft-mediated endocytosis pathways. 3. A low pH of endosomes induces endosomal escape. 4. LNP degradation. 5. Cargos are released into the cytosol and translated to Cas9 protein. Cas9 protein forms an RNP complex with sgRNA and goes to the nucleus via a Cas9-tagged-nucleus localizing signal. I. AAV binds to receptors on the surface of target cells. II. AAV is internalized via endocytosis. III. AAV escapes from endosomes and goes to the nucleus. IV. The AAV capsid is uncoated in the nucleus, and single-strand DNA is released to the nucleus and becomes donor templates for genome editing. AAV adeno-associated virus, LNP lipid nanoparticle, sgRNA single-guide RNA, RNP ribonucleoproteins, ssDNA single-strand DNA
6 Future Directions
The CRISPR/Cas9 system has developed remarkably over the last 20 years since it was first discovered as a nuclease. Recently, the world’s first CRISPR therapy, Casgevy for the treatment of sickle cell disease, has been approved in the UK and the USA [100]. Due to the limitation of low genome-editing efficiency, many research teams have chosen the strategy of KI at loci such as Albumin, ApoC3, and SerpinC1, which have higher gene expression than F8 or F9, for hemophilia therapy studies [28, 94, 95, 98, 99]. However, these loci do not follow the physiological signals of the coagulation mechanisms of the body when a bleeding event occurs. This can lead to side effects such as thrombosis if excessive amounts of therapeutic proteins are unnecessarily produced. Therefore, the best therapeutic strategy for hemophilia is to restore the original gene by inserting it into the defective locus of the clotting protein.
The AAV-LNP hybrid gene delivery strategy is expected to be safer and more efficient for genome editing than dual or triple AAVs, with a lower chance of exposure to anti-Cas9 antibodies, off-targets effects, or random integration. However, AAV is not completely free of capsid immunogenicity and the risk of random integration. For the safe use of AAV, it is necessary to develop a chimeric AAV serotype that can more effectively transmit DNA to the target organ, so that even low doses are sufficient to achieve the desired effect. In addition, it is necessary to elucidate the causes of random integration of AAV and to study methods to suppress the occurrence of random integration. LNPs developed to date are not suitable for KI because it is hard to deliver cargoes into the nucleus. Non-viral vectors should be considered for safer in vivo gene KI that are rapidly cleared from the body. Therefore, the development of next-generation LNPs or novel non-viral vectors research is needed.
References
Garagiola I, et al. X Chromosome inactivation: a modifier of factor VIII and IX plasma levels and bleeding phenotype in Haemophilia carriers. Eur J Hum Genet. 2021;29(2):241–9.
Castaman G, et al. Mild and moderate hemophilia a: neglected conditions, still with unmet needs. MDPI; 2023. p. 1368.
Iorio A, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta-analytic approach using national registries. Ann Intern Med. 2019;171(8):540–6.
Thorat T, Neumann PJ, Chambers JD. Hemophilia burden of disease: a systematic review of the cost-utility literature for hemophilia. J Manag Care Spec Pharm. 2018;24(7):632–42.
Ndoumba-Mintya A, et al. Optimizing haemophilia care in resource-limited countries: current challenges and future prospects. J Blood Med. 2023;14:141–6.
Castro HE, et al. The history and evolution of the clinical effectiveness of haemophilia type a treatment: a systematic review. Indian J Hematol Blood Transfus. 2014;30:1–11.
Roy S, De AK. Effect of prophylactic management of hemophilia on bleeding episodes. Indian J Hematol Blood Transfus. 2019;35(3):496–501.
Hodgson J. Drug pipeline 1Q23—everything everywhere all over the place. Nat Biotechnol. 2023;41(5):591–3.
Administration, U.F.a.D. ALPROLIX® coagulation factor IX (recombinant), Fc fusion protein. 2014.
Administration, U.F.a.D. ELOCTATE® [antihemophilic factor (recombinant), Fc fusion protein] lyophilized powder for solution for intravenous injection. 2014.
Meeks SL, Batsuli G. Hemophilia and inhibitors: current treatment options and potential new therapeutic approaches. In: Hematology 2014, the American Society of Hematology Education Program Book, vol 2016, no 1. 2016. p. 657–662.
Batty P, Lillicrap D. Advances and challenges for hemophilia gene therapy. Hum Mol Genet. 2019;28(R1):R95–101.
Naso MF, et al. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31(4):317–34.
Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol Ther. 2020;28(3):723–46.
Asmamaw M, Zawdie B. Mechanism and applications of CRISPR/Cas-9-mediated genome editing. Biol Targets Ther. 2021;15:353–61.
Taha EA, Lee J, Hotta A. Delivery of CRISPR-Cas tools for in vivo genome editing therapy: trends and challenges. J Control Release. 2022;342:345–61.
Kazemian P, et al. Lipid-nanoparticle-based delivery of CRISPR/Cas9 genome-editing components. Mol Pharm. 2022;19(6):1669–86.
Zhao Y, Huang L. Lipid nanoparticles for gene delivery. Adv Genet. 2014;88:13–36.
Samelson-Jones BJ, George LA. Adeno-associated virus gene therapy for hemophilia. Annu Rev Med. 2023;74:231–47.
Miesbach W, et al. Gene therapy for hemophilia—opportunities and risks. Dtsch Arztebl Int. 2022;119(51–52):887.
Okaygoun D, et al. Advances in the management of haemophilia: emerging treatments and their mechanisms. J Biomed Sci. 2021;28(1):1–13.
Issa SS, et al. Various AAV serotypes and their applications in gene therapy: an overview. Cells. 2023;12(5):785.
Pipe SW, et al. Gene therapy with etranacogene dezaparvovec for hemophilia B. N Engl J Med. 2023;388(8):706–18.
Simioni P, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med. 2009;361(17):1671–5.
Pittman DD, et al. Biochemical, immunological, and in vivo functional characterization of B-domain-deleted factor VIII. 1993.
Chao H, et al. Expression of human factor VIII by splicing between dimerized AAV vectors. Mol Ther. 2002;5(6):716–22.
Nienhuis AW, Nathwani AC, Davidoff AM. Gene therapy for hemophilia. Mol Ther. 2017;25(5):1163–7.
Chen H, et al. Hemophilia A ameliorated in mice by CRISPR-based in vivo genome editing of human Factor VIII. Sci Rep. 2019;9(1):16838.
Mahlangu J, et al. Two-year outcomes of valoctocogene roxaparvovec therapy for hemophilia A. N Engl J Med. 2023;388(8):694–705.
High-dose AAV gene therapy deaths. Nat Biotechnol. 2020;38(8):910.
Zhang M, Liu X, Zhang C. Random integration analysis of recombinant adeno-associated virus 6 packaged in Sf9 insect cells. Mol Biol. 2023;57:1–11.
Sabatino DE, et al. Evaluating the state of the science for adeno-associated virus (AAV) integration: an integrated perspective. Mol Ther. 2022;30:2646–63.
McIntosh JH, et al. Long-term persistence of antibodies to adeno-associated viral vectors following gene therapy with scAAV8-LP1-Fixco. Blood. 2023;142(Supplement 1):2255–2255.
George LA, et al. Multiyear factor VIII expression after AAV gene transfer for hemophilia A. N Engl J Med. 2021;385(21):1961–73.
Ozelo MC, et al. Valoctocogene roxaparvovec gene therapy for hemophilia A. N Engl J Med. 2022;386(11):1013–25.
Visweshwar N, et al. Updated results of the Alta study, a phase 1/2 study of giroctocogene fitelparvovec (PF-07055480/SB-525) gene therapy in adults with severe hemophilia a. Blood. 2021;138(Supplement 1):564–564.
Uchida N, et al. A first-in-human phase 1 study of ACE910, a novel factor VIII-mimetic bispecific antibody, in healthy subjects. Blood. 2016;127(13):1633–41.
Kitazawa T, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18(10):1570–4.
Scott LJ, Kim ES. Emicizumab-kxwh: First Global Approval. Drugs. 2018;78(2):269–74.
Oldenburg J, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–18.
Ostergaard H, et al. A factor VIIIa-mimetic bispecific antibody, Mim8, ameliorates bleeding upon severe vascular challenge in hemophilia A mice. Blood. 2021;138(14):1258–68.
Lauritzen B, et al. A novel next-generation FVIIIa mimetic, Mim8, has a favorable safety profile and displays potent pharmacodynamic effects: results from safety studies in cynomolgus monkeys. J Thromb Haemost. 2022;20(6):1312–24.
Blair HA. Emicizumab: a review in haemophilia A. Drugs. 2019;79(15):1697–707.
Persson P, et al. Mim8, a novel factor VIIIa mimetic bispecific antibody, shows favorable safety and pharmacokinetics in healthy adults. Res Pract Thromb Haemost. 2023;7(6): 102181.
Patnaik MM, Moll S. Inherited antithrombin deficiency: a review. Haemophilia. 2008;14(6):1229–39.
Kujovich JL. Factor v Leiden thrombophilia. Genet Med. 2011;13(1):1–16.
Alqarni S, Alqarni B, Alsultan A. Antithrombin deficiency is associated with a novel homozygous detrimental mutation in SERPINC1 gene in a Saudi female. Case Rep Med. 2023;2023:1–4.
Wang H-L, et al. Identification and characterization of two SERPINC1 mutations causing congenital antithrombin deficiency. Thromb J. 2023;21(1):1–16.
Xie W, Liu Z, Chen B. Protein C deficiency resulting from two mutations in PROC presenting with recurrent venous thromboembolism. J Vasc Surg Cases Innov Tech. 2017;3(4):254–6.
Kentsis A, et al. Venous thrombosis associated with gene deletion of tissue factor pathway inhibitor. Am J Hematol. 2009;84(11):775.
Ellsworth P, Ma A. Factor-mimetic and rebalancing therapies in hemophilia A and B: the end of factor concentrates? Hematology. 2021;2021(1):219–25.
Zhao Y, Weyand AC, Shavit JA. Novel treatments for hemophilia through rebalancing of the coagulation cascade. Pediatr Blood Cancer. 2021;68(5): e28934.
Matsushita T, et al. Phase 3 trial of concizumab in hemophilia with inhibitors. N Engl J Med. 2023;389(9):783–94.
Mahlangu J, et al. Long-term safety and efficacy of the anti-tissue factor pathway inhibitor marstacimab in participants with severe haemophilia: phase II study results. Br J Haematol. 2023;200(2):240–8.
Polderdijk SG, Baglin TP, Huntington JA. Targeting activated protein C to treat hemophilia. Curr Opin Hematol. 2017;24(5):446.
Kenet G, et al. S303: a phase 3 study (ATLAS-PPX) to evaluate efficacy and safety of fitusiran in people with haemophilia A OR B who have switched from prior clotting factor concentrate or bypassing agent prophylaxis. HemaSphere. 2023;7(S3):e643526e.
Ho BX, et al. In vivo genome editing as a therapeutic approach. Int J Mol Sci. 2018;19(9):2721.
Li H, et al. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther. 2020;5(1):1.
Meng N, Grimm D. Membrane-destabilizing ionizable phospholipids: Novel components for organ-selective mRNA delivery and CRISPR–Cas gene editing. Signal Transduct Target Ther. 2021;6(1):206.
Hou X, et al. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–94.
Han JP, et al. In vivo delivery of CRISPR-Cas9 using lipid nanoparticles enables antithrombin gene editing for sustainable hemophilia A and B therapy. Sci Adv. 2022;8(3):eabj6901.
Kenjo E, et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat Commun. 2021;12(1):7101.
Zelepukin IV, et al. Fast processes of nanoparticle blood clearance: Comprehensive study. J Control Release. 2020;326:181–91.
Kim M, et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci Adv. 2021;7(9):eabf4398.
Qiu M, et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci. 2022;119(8): e2116271119.
Basha G, et al. Lipid nanoparticle delivery of siRNA to osteocytes leads to effective silencing of SOST and inhibition of sclerostin in vivo. Mol Ther Nucleic Acids. 2016;5: e363.
Wang X, et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat Protoc. 2023;18(1):265–91.
Li B, et al. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat Biotechnol. 2023;41(10):1410–5.
Mashima R, Takada S. Lipid nanoparticles: a novel gene delivery technique for clinical application. Curr Issues Mol Biol. 2022;44(10):5013–27.
Yin H, et al. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat Biotechnol. 2017;35(12):1179–87.
Yin H, et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016;34(3):328–33.
Yip BH. Recent advances in CRISPR/Cas9 delivery strategies. Biomolecules. 2020;10(6):839.
Gee J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. Morb Mortal Wkly Rep. 2021;70(8):283.
Ndeupen S, et al. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24(12): 103479.
Moghimi SM, Simberg D. Pro-inflammatory concerns with lipid nanoparticles. Mol Ther. 2022;30(6):2109–10.
Lee Y, et al. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp Mol Med. 2023;55(10):2085–96.
Tahtinen S, et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol. 2022;23(4):532–42.
Gillmore JD, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385(6):493–502.
Cohn D, et al. CRISPR/CAS9 editing of KLKB1 in hereditary angioedema patients: updated results: from a phase 1 study. Ann Allergy Asthma Immunol. 2023;131(5):S32.
Lee RG, et al. Efficacy and safety of an investigational single-course CRISPR base-editing therapy targeting PCSK9 in nonhuman primate and mouse models. Circulation. 2023;147(3):242–53.
H, B. First patient dosed with HIV gene therapy. European Pharmaceutical Review. 2022.
Maeder ML, et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat Med. 2019;25(2):229–33.
Shi L, et al. Development of an RNA targeting based gene therapy product for neovascular age-related macular degeneration (nAMD). Invest Ophthalmol Vis Sci. 2023;64(8):934–934.
Saito M, et al. Recent advances in the understanding of cilia mechanisms and their applications as therapeutic targets. Front Mol Biosci. 2023. https://doi.org/10.3389/fmolb.2023.1232188.
Meloni I. Gene editing as a therapeutic approach for Rett Syndrome (MECPer-3D). 2023. https://classic.clinicaltrials.gov/show/NCT05740761.
Longhurst H, et al. In vivo CRISPR/Cas9 editing of KLKB1 in patients with hereditary angioedema: a first-in-human study. Ann Allergy Asthma Immunol. 2022;129(5):S10–1.
Seitzer J. NTLA-2002: CRISPR/Cas9-mediated gene knockout of KLKB1 to treat hereditary angioedema. J Allergy Clin Immunol. 2021;147(2):AB147.
Qiu M, et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc Natl Acad Sci. 2021;118(10): e2020401118.
Rosenblum D, et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci Adv. 2020;6(47):eabc9450.
Glass Z, et al. Engineering the delivery system for CRISPR-based genome editing. Trends Biotechnol. 2018;36(2):173–85.
Swingle KL, Hamilton AG, Mitchell MJ. Lipid nanoparticle-mediated delivery of mRNA therapeutics and vaccines. Trends Mol Med. 2021;27(6):616–7.
Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39(6):1477–87.
Ohnuma S-I, Harris WA. Neurogenesis and the cell cycle. Neuron. 2003;40(2):199–208.
Wang Q, et al. CRISPR-Cas9-mediated in vivo gene integration at the albumin locus recovers hemostasis in neonatal and adult hemophilia B mice. Mol Ther Methods Clin Dev. 2020;18:520–31.
Lee JH, et al. Genome editing-mediated knock-in of therapeutic genes ameliorates the disease phenotype in a model of hemophilia. Mol Ther Nucl Acids. 2022;29:551–62.
Shieh PB, et al. Re: “Moving forward after two deaths in a gene therapy trial of myotubular myopathy” by Wilson and Flotte. Hum Gene Ther. 2020;31(15–16):787–787.
Huang H-R, et al. CRISPR/Cas9-mediated targeted insertion of human F9 achieves therapeutic circulating protein levels in mice and non-human primates. Mol Ther. 2019;27(4 Suppl 1):7.
Lee JH, et al. In vivo genome editing for hemophilia B therapy by the combination of rebalancing and therapeutic gene knockin using a viral and non-viral vector. Mol Ther Nucl Acids. 2023;32:161–72.
Han JP, et al. In vivo genome editing using 244-cis LNPs and low-dose AAV achieves therapeutic threshold in hemophilia A mice. Mol Ther Nucl Acids. 2023;34: 102050.
Sheridan C. The world’s first CRISPR therapy is approved: who will receive it? Nat Biotechnol. 2023;42:1–3.
Funding
Open Access funding enabled and organized by Seoul National University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable.
Conflict of interest
JHL and JPH declare no conflict of interest.
Availability of data and material
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
JHL and JPH drafted the manuscript. JHL prepared the table. JPH prepared all figures. JHL and JPH revised the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lee, J.H., Han, J.P. In vivo LNP-CRISPR Approaches for the Treatment of Hemophilia. Mol Diagn Ther 28, 239–248 (2024). https://doi.org/10.1007/s40291-024-00705-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-024-00705-1