Abstract
Introduction
Proclarix is a CE-marked test that provides the risk of clinically significant prostate cancer (csPCa), ranging from 0% to 100%, based on the serum measurement of Thrombospondin-1, cathepsin D, prostate-specific antigen (PSA), and percentage of free PSA in addition to age. We hypothesize that Proclarix could be correlated with PCa aggressiveness. We analyzed the association of this new biomarker with four surrogates of aggressiveness: grade group (GG) in the biopsy, clinical stage, risk of biochemical recurrence after primary treatment of localized PCa, and pathology in the surgical specimen.
Material and Methods
This is a retrospective study from 606 men with suspicion of PCa [PSA of ≥ 3.0 ng/mL and/or abnormal digital rectal examination (DRE)], in whom Proclarix was assessed (0–100%). The GG was defined by the International Society of Urological Pathology categories. The TNM was used for clinical staging (cT based on DRE, whereas cN and cM were established with computed tomography and 99-technetium bone scintigraphy). The risk of biochemical recurrence of localized PCa after primary treatment was defined by combining PSA, GG, and cT. Finally, an unfavorable pathology in a surgical specimen was defined as GG > 2 or pT ≥ 3.
Results
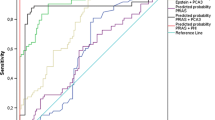
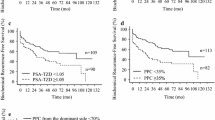
The median age of the cohort was 67 years old, with a median PSA of 7 ng/mL and a rate of abnormal DRE of 23.3%. CsPCa was detected in 254 men (41.9%), with a median Proclarix of 60.1% compared with 37.3% obtained in patients with insignificant PCa and 20.7% in men without PCa. Among patients with GG > 3, Proclarix was significantly higher (58.2%) than in those with GG of 3 or lower (33.1%, p < 0.001). Men with localized tumors exhibited a Proclarix median of 37.3% compared with those with advanced disease (60.1%, p < 0.001). Proclarix levels among 197 patients with low and intermediate risk of biochemical recurrence were 24.9% and 35.0%, respectively, significantly lower compared with patients with high-risk disease (58.7%, p < 0.001). Unfavorable pathology was observed in 35 patients out of the 79 who underwent radical prostatectomy, with a Proclarix median of 35.7% compared with 23.7% obtained in patients with favorable pathology (p = 0.013). Proclarix and magnetic resonance imaging were independent predictors of the four surrogates of aggressiveness analyzed.
Conclusion
There is a correlation between Proclarix and the aggressiveness of PCa.





Similar content being viewed by others
References
Global Cancer Observatory. https://gco.iarc.fr/. Accessed 26 Sept 2022.
Hugosson J, Roobol MJ, Månsson M, et al. A 16-yr follow-up of the European randomized study of screening for prostate cancer HHS Public Access. Eur Urol. 2019;76:43–51.
Delongchamps NB, Peyromaure M, Schull A, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013;189:493–9.
Haider MA, Yao X, Loblaw A, et al. Multiparametric magnetic resonance imaging in the diagnosis of prostate cancer: a systematic review. Clin Oncol (R Coll Radiol). 2016;28:550–67.
van Poppel H, Hogenhout R, Albers P, et al. A European model for an organised risk-stratified early detection programme for prostate cancer. Eur Urol Oncol. 2021;4:731–9.
Sathianathen NJ, Omer A, Harriss E, et al. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the prostate imaging reporting and data system era: a systematic review and meta-analysis. Eur Urol. 2020;78:402–14.
Mazzone E, Stabile A, Pellegrino F, et al. Positive predictive value of prostate imaging reporting and data system version 2 for the detection of clinically significant prostate cancer: a systematic review and meta-analysis. Eur Urol Oncol. 2021;4:697–713.
Osses DF, Roobol MJ, Schoots IG. Molecular sciences prediction medicine: biomarkers, risk calculators and magnetic resonance imaging as risk stratification tools in prostate cancer diagnosis. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20071637.
Schoots IG. MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI-RADS 3 lesions? Transl Androl Urol. 2018;7:70.
Steuber T, Heidegger I, Kafka M, et al. PROPOSe: a real-life prospective study of Proclarix, a novel blood-based test to support challenging biopsy decision-making in prostate cancer. Eur Urol Oncol. 2022;5:321–7.
Steuber T, Tennstedt P, Macagno A, et al. Thrombospondin 1 and cathepsin D improve prostate cancer diagnosis by avoiding potentially unnecessary prostate biopsies. BJU Int. 2019;123:826–33.
Cima I, Schiess R, Wild P, et al. Cancer genetics-guided discovery of serum biomarker signatures for diagnosis and prognosis of prostate cancer. Proc Natl Acad Sci USA. 2011;108:3342–7.
Klocker H, Golding B, Weber S, et al. Development and validation of a novel multivariate risk score to guide biopsy decision for the diagnosis of clinically significant prostate cancer. BJUI Compass. 2020;1:15–20.
Morote J, Celma A, Diaz F, et al. Prostatic-specific antigen density behavior according to multiparametric magnetic resonance imaging result. Urol Oncol. 2020;38:410–7.
Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging—reporting and data system: 2015, Version 2. Eur Urol. 2016;69:16–40.
Morote J, Borque-Fernando A, Triquell M, et al. Multiparametric magnetic resonance imaging grades the aggressiveness of prostate cancer. Cancers (Basel). 2022;14:1828.
Boschheidgen M, Schimmöller L, Arsov C, et al. MRI grading for the prediction of prostate cancer aggressiveness. Eur Radiol. 2022;32:2351.
Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–52.
Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol. 2016;69:428.
D’Amico AV, Whittington R, Bruce Malkowicz S, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74.
Brierley J, Gospodarowicz MD, Wittekind CT. TNM classification of malignant tumors international union against cancer. 8th. Oxford: Wiley; 2017, p. 57–62.
Kozminski MA, Tomlins S, Cole A, et al. Standardizing the definition of adverse pathology for lower risk men undergoing radical prostatectomy. Urol Oncol. 2016;34(415):e1-415.e6.
Osses DF, Roobol MJ, Schoots IG. Prediction medicine: biomarkers, risk calculators and magnetic resonance imaging as risk stratification tools in prostate cancer diagnosis. Int J Mol Sci. 2019. https://doi.org/10.3390/IJMS20071637.
Morote J, Campistol M, Celma A, et al. The efficacy of Proclarix to select appropriate candidates for magnetic resonance imaging and derived prostate biopsies in men with suspected prostate cancer. World J Mens Health. 2022. https://doi.org/10.5534/wjmh.210117.
Loeb S, Sanda MG, Broyles DL, et al. The prostate health index selectively identifies clinically significant prostate cancer HHS public access. J Urol. 2015;193:1163–9.
Catalona WJ, Partin AW, Sanda MG, et al. A multi-center study of [−2]Pro-prostate-specific antigen (PSA) in combination with PSA and free PSA for prostate cancer detection in the 2.0 to 10.0 ng/mL PSA range. J Urol. 2011;185:1650.
Bruzzese D, Mazzarella C, Ferro M, et al. Prostate health index vs percent free prostate-specific antigen for prostate cancer detection in men with ‘gray’ prostate-specific antigen levels at first biopsy: systematic review and meta-analysis. Transl Res. 2014;164:444–51.
Ferro M, De Cobelli O, Lucarelli G, et al. Molecular sciences beyond PSA: the role of Prostate Health Index (PHI). Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21041184.
Lazzeri M, Haese A, de La Taille A, et al. Serum isoform [-2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2–10 ng/ml: a multicentric European study. Eur Urol. 2013;63:986–94.
Wang W, Wang M, Wang L, et al. Diagnostic ability of %p2PSA and prostate health index for aggressive prostate cancer: a meta-analysis. Sci Rep. 2014. https://doi.org/10.1038/srep05012.
Dolejsova O, Kucera R, Fuchsova R, et al. The ability of prostate health index (PHI) to predict Gleason score in patients with prostate cancer and discriminate patients between Gleason score 6 and Gleason score higher than 6-a study on 320 patients after radical prostatectomy. Technol Cancer Res Treat. 2018. https://doi.org/10.1177/1533033818787377.
Fossati N, Buffi NM, Haese A, et al. Preoperative prostate-specific antigen isoform p2PSA and its derivatives, %p2PSA and prostate health index, predict pathologic outcomes in patients undergoing radical prostatectomy for prostate cancer: results from a multicentric European prospective study. Eur Urol. 2015;68:132–8.
Ferro M, Crocetto F, Bruzzese D, et al. Prostate health index and multiparametric MRI: partners in crime fighting overdiagnosis and overtreatment in prostate cancer. Cancers. 2021;13:4723.
Gentile F, La Civita E, Della Ventura B, et al. A combinatorial neural network analysis reveals a synergistic behaviour of multiparametric magnetic resonance and prostate health index in the identification of clinically significant prostate cancer. Clin Genitourin Cancer. 2022;20:e406–10.
Parekh DJ, Punnen S, Sjoberg DD, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol. 2015;68:464–70.
Bryant RJ, Sjoberg DD, Vickers AJ, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. JNCI J Natl Cancer Inst. 2015;107:95.
Carlsson S, Maschino A, Schröder F, et al. Predictive value of four kallikrein markers for pathologically insignificant compared with aggressive prostate cancer in radical prostatectomy specimens: results from the European Randomized Study of Screening for Prostate Cancer section Rotterdam. Eur Urol. 2013;64:693–9.
Margolis E, Brown G, Partin A, et al. Predicting high-grade prostate cancer at initial biopsy: clinical performance of the ExoDx (EPI) Prostate Intelliscore test in three independent prospective studies. Clin Res. 2021. https://doi.org/10.1038/s41391-021-00456-8.
Kretschmer A, Tutrone R, Alter J, et al. Pre-diagnosis urine exosomal RNA (ExoDx EPI score) is associated with post-prostatectomy pathology outcome. World J Urol. 2022;40:983–9.
van Neste L, Hendriks RJ, Dijkstra S, et al. Detection of high-grade prostate cancer using a urinary molecular biomarker-based risk score. Eur Urol. 2016;70:740–8.
Haese A, Trooskens G, Steyaert S, et al. Multicenter optimization and validation of a 2-gene mRNA urine test for detection of clinically significant prostate cancer before initial prostate biopsy. J Urol. 2019;202:256–62.
Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 1979;2005(310):644–8.
Fine SW, Gopalan A, Leversha MA, et al. TMPRSS2-ERG gene fusion is associated with low Gleason scores and not with high grade morphologic features. Mod Pathol. 2010;23:1325–33.
Pettersson A, Graff RE, Bauer SR, et al. The TMPRSS2:ERG rearrangement, erg expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomark Prev. 2012. https://doi.org/10.1158/1055-9965.EPI-12-0042.
Tomlins SA, Day JR, Lonigro RJ, et al. Urine TMPRSS2:ERG plus PCA3 for individualized prostate cancer risk assessment HHS public access. Eur Urol. 2016;70:45–53.
Leyten GHJM, Hessels D, Jannink SA, et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol. 2014;65:534–42.
Ferro M, Bruzzese D, Perdonà S, et al. Prostate health index (Phi) and prostate cancer antigen 3 (PCA3) significantly improve prostate cancer detection at initial biopsy in a total PSA range of 2–10 ng/ml. PLoS ONE. 2013;8:67687.
Cullen J, Rosner IL, Brand TC, et al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate cancer. Eur Urol. 2015;68:123–31.
van den Eeden SK, Lu R, Zhang N, et al. A biopsy-based 17-gene genomic prostate score as a predictor of metastases and prostate cancer death in surgically treated men with clinically localized disease. Eur Urol. 2018;73:129–38.
Shore ND, Kella N, Moran B, et al. Impact of the cell cycle progression test on physician and patient treatment selection for localized prostate cancer. J Urol. 2016;195:612–8.
Klein EA, Haddad Z, Yousefi K, et al. Decipher genomic classifier measured on prostate biopsy predicts metastasis risk. Urology. 2016;90:148–52.
Porter CR, Kodama K, Gibbons RP, et al. 25-year prostate cancer control and survival outcomes: a 40-year radical prostatectomy single institution series. J Urol. 2006;176:569–74.
Hull GW, Rabbani F, Abbas F, et al. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528–34.
Pinto F, Prayer-Galetti T, Gardiman M, et al. Clinical and pathological characteristics of patients presenting with biochemical progression after radical retropubic prostatectomy for pathologically organ-confined prostate cancer. Urol Int. 2006;76:202–8.
Lepor A, Catalona WJ, Loeb S. The prostate health index: its utility in prostate cancer detection. Urol Clin. 2016. https://doi.org/10.1016/j.ucl.2015.08.001.
Lughezzani G, Lazzeri M, Buffi NM, et al. Preoperative prostate health index is an independent predictor of early biochemical recurrence after radical prostatectomy: results from a prospective single-center study. Urol Oncol Semin Orig Investig. 2015;33(337):e7-337.e14.
Haese A, Tin AL, Carlsson SV, et al. A pre-specified model based on four kallikrein markers in blood improves predictions of adverse pathology and biochemical recurrence after radical prostatectomy. Br J Cancer. 2020;123:604–9.
Ross AE, Feng FY, Ghadessi M, et al. A genomic classifier predicting metastatic disease progression in men with biochemical recurrence after prostatectomy. Prostate Cancer Prostatic Dis. 2014;17:64–9.
Kim H, Jung G, Hyuck Kim J, et al. Role of prostate health index to predict Gleason score upgrading and high-risk prostate cancer in radical prostatectomy specimens. Sci Rep. 2021;11:17447.
Foj L, Filella X. Development and internal validation of a novel PHI-nomogram to identify aggressive prostate cancer. Clin Chim Acta. 2020;501:174–8.
Ferro M, Lucarelli G, De CO, et al. The emerging landscape of tumor marker panels for the identification of aggressive prostate cancer: the perspective through bibliometric analysis of an Italian translational working group in uro-oncology. Minerva Urol Nephrol. 2021;73:442–51.
Punnen S, Nahar B, Prakash NS, et al. The 4Kscore predicts the grade and stage of prostate cancer in the radical prostatectomy specimen: results from a multi-institutional prospective trial. Eur Urol Focus. 2017;3:94–9.
Eggener S, Karsh LI, Richardson T, et al. A 17-gene panel for prediction of adverse prostate cancer pathologic features: prospective clinical validation and utility. Urology. 2019;126:76–82.
Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66:550–60.
Stattin P, Vickers AJ, Sjoberg DD, et al. Improving the specificity of screening for lethal prostate cancer using prostate-specific antigen and a panel of kallikrein markers: a nested case-control study HHS public access. Eur Urol. 2015;68:207–13.
Terracciano D, La Civita E, Athanasiou A, et al. New strategy for the identification of prostate cancer: the combination of Proclarix and the prostate health index. Prostate. 2022;82:1469–76.
Abreu-Gomez J, Wu M, McInnes MDF, et al. Shape analysis of peripheral zone observations on prostate DWI: Correlation to histopathology outcomes after radical prostatectomy. Am J Roentgenol. 2020. https://doi.org/10.2214/AJR.19.22318.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific support from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors (MC, MT, LR, AC, IdT, MES, RM, OM, JP, ET & JM) have no conflicts of interest to declare.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Consent to participate
Not applicable.
Statement of ethics
Written informed consent was obtained from all subjects involved in the study.
Code availability
Not applicable.
Author contributions
All authors contributed to the study conception and design. Miriam Campistol, Juan Morote, and Enrique Trilla performed the study selection. Miriam Campistol drafted the manuscript and all authors commented on previous versions of the manuscript. Juan Morote, Enrique Trilla, Marina Triquell, Ana Celma, Lucas Regis, Jacques Planas, Inés de Torres, María E. Semidey, Richard Mast, and Olga Mendez critically revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Campistol, M., Triquell, M., Regis, L. et al. Relationship between Proclarix and the Aggressiveness of Prostate Cancer. Mol Diagn Ther 27, 487–498 (2023). https://doi.org/10.1007/s40291-023-00649-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-023-00649-y