Abstract
Background
Prostate cancer patients with pathological prognostic factors have a poor prognosis, but it is unclear whether pathological prognostic factors are associated with prognosis limited to low-risk patients with good prognosis according to NCCN guidelines. The present study examined whether prognosis is influenced by pathological prognostic factors using radical prostatectomy (RP) specimens from low-risk patients.
Methods
We evaluated diagnostic accuracy by examining biochemical recurrence (BCR)-free survival with respect to clinical and pathological prognostic factors in 419 all-risk patients who underwent RP. Clinical prognostic factors included age, prostate-specific antigen (PSA) levels, PSA density, and risk stratification, while pathological prognostic factors included grade group, lymphovascular space invasion, extraprostatic extension, surgical margins, seminal vesicle invasion, intraductal carcinoma of the prostate (IDCP), and pT. In a subsequent analysis restricted to 104 low-risk patients, survival curves were estimated for pathological prognostic factors using the Kaplan–Meier method and compared using log-rank and generalized Wilcoxon tests.
Results
In the overall risk analysis, the presence of pathological prognostic factors significantly shortened BCR-free survival (p < 0.05). Univariable analysis revealed that PSA density, risk categories, and pathological prognostic factors were significantly associated with BCR-free survival, although age and PSA were not. In multivariable analysis, age, risk categories, grade group, IDCP, and pT significantly predicted BCR-free survival (p < 0.05). Conversely, no statistically significant differences were found for any pathological prognostic factors in low-risk patients.
Conclusions
In low-risk patients, pathological prognostic factors did not affect BCR-free survival, which suggests that additional treatment may be unnecessary even if pathological prognostic factors are observed in low-risk patients with RP.
Similar content being viewed by others
Background
Prostate cancer (PCa) is a clinically heterogeneous disease, ranging from non-life-threatening “insignificant cancers” to poor-prognosis cancers that metastasize and cause death. Therefore, pre-treatment risk stratification is important to determine the appropriate treatment. The NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines) classify PCa into very low-, low-, favorable/unfavorable intermediate-, high-, and very high-risk categories based on clinical stage, grade group (GG), prostate-specific antigen (PSA) levels, PSA density (PSAD), number of positive cores at biopsy, and occupancy rates [1]. A decision tree for treatment options is organized according to expected patient survival and risk category. For very low- and low-risk patients, treatment options include active surveillance (AS), external beam radiotherapy (EBRT), brachytherapy (BT), or radical prostatectomy (RP).
Prognostic factors for biochemical recurrence (BCR) of clinically or pathologically localized PCa include clinical prognostic factors such as age [2], PSA [3], and PSAD [4], and pathological prognostic factors such as GG [5], lymphovascular invasion (LVI) [6], extraprostatic extensions (EPE) [7], surgical margins (SM) [8], seminal vesicle invasion (SVI) [9], and pT [10]. Additionally, intraductal carcinoma of the prostate (IDCP) has recently been considered an independent and strong prognostic factor [11]. IDCP is morphologically similar to the cribriform pattern, but IDCP proliferates within pre-existing ducts. Both IDCP and cribriform pattern are poor prognostic factors, but the distinction is sometimes difficult. The presence of these pathological prognostic factors results in a worse prognosis, even in the short-term.
Low-risk patients have significantly higher BCR-free survival rates than intermediate- and high-risk patients [12], but existing studies of low-risk patients with RP showed that EPE was found in 8–36%, SVI in 1.5%, and positive SM in 19–33% of RP specimens [13,14,15]. The prevalence of IDCP in low-risk patients has been reported to be 2.1% [16]. These treatises did not examine whether pathological prognostic factors in low-risk patients were associated with prognosis. Thus, it remains unclear whether the presence of pathological prognostic factors in low-risk patients who are considered to have a good prognosis is associated with a poor prognosis. Here, we report the results of a study attempting to elucidate this.
Materials and methods
Patient selection
A flowchart of patient selection is shown in Fig. 1. We performed a retrospective review of data collected from the database which included 419 patients that underwent RP at Mie University Hospital between 2015 and 2021. Of the 419 patients identified, 46 patients who received preoperative treatment and 1 whose cancer was not detected postoperatively were excluded, leaving 372 patients in the overall risk analysis.
For the analysis of the low-risk group, patients from the Japanese Red Cross Ise Hospital were included to increase the sample size for statistical analysis. Of the 513 patients who underwent RP at the Japanese Red Cross Ise Hospital between 2012 and 2021, 76 low-risk patients were enrolled in our study after excluding those who received preoperative hormone therapy. There were no very low-risk patients among those who underwent RP at the above two hospitals.
Patients were treated according to the NCCN guidelines. For low-risk patients, treatment options included AS, EBRT, BT, or RP. Patients could choose the treatment of their choice. This study, in collaboration with the Japanese Red Cross Ise Hospital, was approved by the Mie University Clinical Research Review Committee (approval number H2020-110), and the requirement for obtaining informed consent from the participants was waived owing to the retrospective and observational nature of the study.
Medical record data and pathological evaluation
Preoperative clinical prognostic variables (age, PSA, PSAD) and biopsy results were retrieved from patient records. Risk classification was based on the NCCN guidelines (version 4, 2022) [1]. Low-risk patients were defined as cT1 ~ cT2a and GG1 and PSA < 10 ng/mL.
RP specimens were fixed in 10% neutral buffered formalin, and hematoxylin and eosin-stained (HE-stained) slides were prepared. The pathological prognostic factors in RP specimens for BCR, GG, LVI, EPE, SM, SVI, IDCP, and pT were independently re-evaluated by two experienced genitourinary pathologists (RS and KU). GG and IDCP were evaluated according to the 2016 WHO classification [17]. We diagnosed IDCP with only HE-stained slides and did not use immunohistochemistry. EPE is diagnosed when cancer bulges beyond the boundaries of the prostate and does not include bladder neck invasion. SM was defined as positive if cancer cells were in contact with the specimen surface. pT was evaluated based on the TNM Classification of Malignant Tumors, 8th edition [18].
Follow up
Serum PSA was measured after RP every 3 months for the first 3 years and every 6 months thereafter. Imaging studies (including computed tomography and bone scintigraphy) were performed as needed.
BCR was defined as a rise in PSA to 0.2 ng/mL and a confirmatory value of 0.2 ng/ml. The first PSA above or equal to 0.2 ng/ml was used to define the time to BCR. Even if EPE, positive SM, or SVI were observed in RP specimens, no additional treatment was given until BCR was confirmed.
Statistical analyses
The primary endpoint of this study was BCR-free survival, defined as the time from RP to BCR. For the overall risk analysis, survival curves were estimated for risk categories and pathological prognostic factors using the Kaplan–Meier method and compared using log-rank and generalized Wilcoxon tests. For clinical and pathological prognostic factors, Cox proportional hazards regression models were used to estimate relative risks and 95% confidence intervals for unadjusted and adjusted comparisons of BCR. Univariable and multivariable Cox proportional hazards models were built with BCR-free survival as the outcome and clinical and pathological prognostic factors as the covariates. The multivariable model was adjusted for age, risk categories, GG, IDCP, and pT. PSA and PSAD were excluded from the multivariable analysis because they were associated with risk classification, and EPE, SM [19], and SVI were also excluded because of their association with pT.
For the analysis of low-risk patients, survival curves were estimated for pathological prognostic factors using the Kaplan–Meier method and compared using log-rank and generalized Wilcoxon tests. Nominal variables were calculated using Fisher's exact test, and continuous variables were calculated using the t-test and Mann–Whitney U test.
P value < 0.05 was considered statistically significant. All statistical analyses were performed using EZR version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R version 4.1.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical characteristics of the study participants
The clinicopathological characteristics of the 372 patients included in the overall risk analysis are summarized in Table 1. The median age of patients was 68 years (range: 43–76 years), median PSA was 7.8 ng/ml (range: 2.78–90.5 ng/ml), and median PSAD was 0.3 ng/mL/cc (range: 0.06–5.36 ng/mL/cc). Risk stratification of patients based on the NCCN guidelines was as follows: low-risk, 12% (n = 46); favorable intermediate-risk, 26% (n = 96); unfavorable intermediate-risk, 22% (n = 80); high-risk, 34% (n = 127); and very high-risk, 6% (n = 23). The GGs based on the 2016 WHO classification in patients were GG1 in 41(11%), GG2 in 141 (38%), GG3 in 90 (24%), GG4 in 36 (10%), and GG5 in 64 (17%). In addition, LVI, EPE, positive SM, SVI, and IDCP were found in 37 (10%), 105 (28%), 111 (30%), 30 (8%), and 66 (18%) patients, respectively. pT was classified as pT2 in 265 (71%), pT3a in 77 (21%), and pT3b in 30 (8%) patients. The median follow-up duration was 18 months (range: 0–74 months), and no patient died of PCa during follow-up. Forty-seven patients (13%) had BCR, and the median time to BCR was 16 months (range: 0–72 months).
Overall risk analysis of the study participants
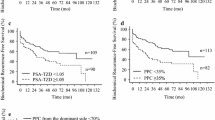
Kaplan–Meier analyses of BCR-free survival stratified according to risk categories, and pathological prognostic factors are shown in Fig. 2a–h. The log-rank and generalized Wilcoxon tests showed statistically significant differences for all factors examined (log-rank test: SM p = 0.00398, the other factors p < 0.001, generalized Wilcoxon test: SM p = 0.00355, the other factors p < 0.001). Univariable analysis revealed that risk category, GG, EPE, positive SM, SVI, IDCP, and pT were all significantly associated with BCR-free survival but not age and PSA (Table 2). In multivariable analysis, age, risk categories, GG, IDCP, and pT significantly predicted BCR-free survival (Table 2).
Biochemical recurrence-free survival for the NCCN risk stratification and pathological prognostic factors in all patients. Kaplan–Meier estimates of biochemical recurrence (BCR)-free survival for all risk category patients according to the NCCN risk stratification (a), grade group (GG) (b), lymphovascular invasion (LVI) (c), extraprostatic extension (EPE) (d), surgical margins (SM) (e), seminal vesicle invasion (SVI) (f), intraductal carcinoma of the prostate (IDCP) (g), and pathological assessment of the primary tumor (pT) (h). The log-rank and generalized Wilcoxon tests showed statistically significant differences for all factors examined (log-rank test: SM p = 0.00398, the other factors p < 0.001, generalized Wilcoxon test: SM p = 0.00355, the other factors p < 0.001)
Clinical characteristics of the low-risk patients
The total number of low-risk patients included in the study was 104; 46 patients from Mie University Hospital and 58 patients from the Japanese Red Cross Ise Hospital. The clinicopathological characteristics of 104 low-risk patients are summarized in Table 3. The median age of the patients was 67.5 years (range: 55–77 years), the median PSA was 5.8 ng/ml (range: 2.78–9.7 ng/ml), and the median PSAD value was 0.16 ng/mL/cc (range: 0.06–0.62 ng/mL/cc). The GGs of low-risk patients were GG1 in 25 (24%), GG2 in 59 (57%), GG3 in 16 (15%), GG4 in 3 (3%), and GG5 in 1 (1%). LVI, EPE, positive SM, and IDCP were found in 11 (11%), 13 (13%), 27 (26%), and 10 (10%) patients, respectively. No patient had SVI. pT was pT2 in 89 (86%) and pT3a in 13 (13%) patients. The median follow-up duration was 35.5 months (range: 0–109 months). During follow-up, no patient died of PCa, 6 patients (6%) had BCR, and the median time to BCR was 19.5 months (range: 0–70 months).
Overall risk analysis of the low-risk patients
Kaplan–Meier analyses of BCR-free survival stratified according to pathological prognostic factors are shown in Fig. 3a–f. The log-rank and generalized Wilcoxon tests did not reveal statistically significant differences for any of the factors examined.
Biochemical recurrence-free survival for pathological prognostic factors in low-risk patients. Kaplan–Meier estimates of biochemical recurrence (BCR)-free survival for low-risk patients according to grade group (GG) (a), lymphovascular invasion (LVI) (b), extraprostatic extension (EPE) (c), surgical margins (SM) (d), intraductal carcinoma of the prostate (IDCP) (e), and pathological assessment of the primary tumor (pT) (f)
Discussion
With the widespread use of PSA screening tests, the incidence rates of prostate cancers have increased, and mortality rates have decreased, while the overdiagnosis and overtreatment of clinically insignificant cancers have also been controversial [20]. Given the heterogeneity of PCa, various risk stratifications and nomograms for the pretreatment of PCa have been created. The NCCN, EAU, and other guidelines classify pretreatment PCa into 3–5 risk categories (very low to very high) [21]. Low-risk patients have significantly lower BCR rates than intermediate-risk and high-risk patients [12]. In this study, we examined BCR-free survival for risk stratification only in patients who underwent RP. The overall difference was statistically significant, supporting the validity of our preoperative diagnoses.
Prognostic factors for BCR of clinically or pathologically localized PCa are as follows: clinical factors, including age [2], PSA [3], and PSAD [4], and pathological factors, including GG [5], LVI[6], EPE [7], SM [8], SVI [9], IDCP [11], and pT [10]. In the overall risk analysis of the study participants, the presence of pathological prognostic factors significantly shortened BCR-free survival, as reported in a previous report. In addition, the hazard ratios for positive SM, SVI, and IDCP were higher in the previous report [22], and similarly in our study, the hazard ratios for pT, reflecting positive SM, SVI, and IDCP, were also higher. These results prove that our postoperative pathological diagnosis is appropriate.
Among the pathological prognostic factors, EPE, positive SM, and SVI are defined as adverse pathologic features according to the NCCN guidelines and influence treatment selection [1]. It is not uncommon to find these features in RP specimens from low-risk patients, which are reported to be 8–36% for EPE, 1.5% for SVI, and 19–33% for positive SM [13,14,15]. In the case of adverse pathologic features, the NCCN guidelines suggest that additional treatment with EBRT or androgen deprivation therapy is an option, regardless of the preoperative risk categories [1]. Though this additional treatment decreases the risk of BCR [23,24,25], it is reported to decrease urinary function, sexual function, and overall quality of life [26] leading to overtreatment. There is no clear criteria on whether this additional treatment should be administered or followed up. To the best of our knowledge, there are no reports examining the correlation between preoperative risk stratification and additional treatment. In this study, we also examined BCR-free survival for each of the pathological prognostic factors in low-risk patients and found no significant difference in any of the factors, which suggests that additional treatment might be unnecessary after RP in low-risk patients even if adverse pathologic features are found. This additional treatment for low-risk patients could actually be overtreatment.
Multiple studies of patients with RP after AS versus those with immediate RP reported no increase in the rate of adverse pathologic features detected between the two groups [27, 28], and no significant difference in BCR-free survival [15], which indicates that AS does not affect patients. In addition, the fact that adverse pathologic features do not affect BCR-free survival, no matter the time point they are detected, suggests that adverse pathologic features in low-risk patients might not be associated with BCR. Adverse pathologic features in low-risk patients with RP could be clinically insignificant.
As the prevalence of IDCP increases, the risk of poor prognosis and BCR increases [11, 16]. However, much is still unknown about IDCP in RP specimens from low-risk patients. PCa with IDCP is more likely to have increased genomic instability, especially somatic MMR gene alterations. The NCCN guidelines state that patients with IDCP at biopsy should undergo germline testing but do not address what should be done if IDCP is found in the RP specimens [1]. Our findings suggest that IDCP in RP specimens from low-risk patients might not affect BCR. Further studies by risk stratification are needed, including investigation of the relations to gene mutations and prognosis.
This study had a few limitations. First, the study was retrospective, and the choice of RP depended on the opinion of the urologist and the patient’s compliance. Second, the overall risk analysis showed no significant difference in BCR-free survival between low-risk and intermediate-risk patients, owing to a small sample size of the low-risk patients in the overall risk analysis. To partially address this limitation, the number of patients included was increased by adding low-risk patients from the Japanese Red Cross Ise Hospital. However, the small sample size was a limitation of this study. Since our study is a short-term observation, additional long-term studies are required. Third, GG was upgraded in 76% of cases of low-risk patients at RP. Upgrading has been reported to occur in 36–71% of cases [29, 30], which in this study was slightly higher than existing reports. Insufficient sampling at the time of the biopsy could be the reason. Since biopsies are often performed at other hospitals, it is difficult to reexamine this issue. Fourth, cribriform carcinoma has a poor prognosis like IDCP, but it is difficult to truly distinguish IDCP from cribriform carcinoma. In this study, we diagnosed IDCP only when it could be reliably identified as IDCP by HE-stained slides alone.
Conclusions
In our study, pathological prognostic factors did not affect BCR-free survival in low-risk patients according to the NCCN guidelines. Thus, additional treatment might be unnecessary even if pathological prognostic factors are observed in low-risk patients with RP.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PCa:
-
Prostate cancer
- GG:
-
Grade group
- PSA:
-
Prostate-specific antigen
- PSAD:
-
PSA density
- AS:
-
Active surveillance
- EBRT:
-
External beam radiotherapy
- BT:
-
Brachytherapy
- RP:
-
Radical prostatectomy
- BCR:
-
Biochemical recurrence
- LVI:
-
Lymphovascular invasion
- EPE:
-
Extraprostatic extension
- SM :
-
Surgical margins
- SVI:
-
Seminal vesicle invasion
- pT:
-
Pathological assessment of the primary tumor
- IDCP:
-
Intraductal carcinoma of the prostate
References
National Comprehensive Cancer Network. Prostate cancer (version 4). 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed 13 July 2022.
Obek C, Lai S, Sadek S, Civantos F, Soloway MS. Age as a prognostic factor for disease recurrence after radical prostatectomy. Urology. 1999;54:533–8. https://doi.org/10.1016/s0090-4295(99)00168-5.
Tilki D, Mandel P, Karakiewicz PI, Heinze A, Huland H, Graefen M, et al. The impact of very high initial PSA on oncological outcomes after radical prostatectomy for clinically localized prostate cancer. Urol Oncol. 2020;38:379–85. https://doi.org/10.1016/j.urolonc.2019.12.027.
Hamada R, Nakashima J, Ohori M, Ohno Y, Komori O, Yoshioka K, et al. Preoperative predictive factors and further risk stratification of biochemical recurrence in clinically localized high-risk prostate cancer. Int J Clin Oncol. 2016;21:595–600. https://doi.org/10.1007/s10147-015-0923-3.
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason Score. Eur Urol. 2016;69:428–35. https://doi.org/10.1016/j.eururo.2015.06.046.
May M, Kaufmann O, Hammermann F, Loy V, Siegsmund M. Prognostic impact of lymphovascular invasion in radical prostatectomy specimens. BJU Int. 2007;99:539–44. https://doi.org/10.1111/j.1464-410X.2006.06650.x.
Wheeler TM, Dillioglugil O, Kattan MW, Arakawa A, Soh S, Suyama K, et al. Clinical and pathological significance of the level and extent of capsular invasion in clinical stage T1–2 prostate cancer. Hum Pathol. 1998;29:856–62. https://doi.org/10.1016/s0046-8177(98)90457-9.
Kato D, Ebara S, Tatenuma T, Sasaki T, Ikehata Y, Nakayama A, et al. Short-term oncological and surgical outcomes of robot-assisted radical prostatectomy: A retrospective multicenter cohort study in Japan (the MSUG94 group). Asian J Endosc Surg. 2022;15:745–52. https://doi.org/10.1111/ases.13074.
Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. https://doi.org/10.1016/s0094-0143(05)70386-4
Chun FK, Graefen M, Zacharias M, Haese A, Steuber T, Schlomm T, et al. Anatomic radical retropubic prostatectomy-long-term recurrence-free survival rates for localized prostate cancer. World J Urol. 2006;24:273–80. https://doi.org/10.1007/s00345-006-0058-2.
Kimura K, Tsuzuki T, Kato M, Saito AM, Sassa N, Ishida R, et al. Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate. 2014;74:680–7. https://doi.org/10.1002/pros.22786.
Reese AC, Pierorazio PM, Han M, Partin AW. Contemporary evaluation of the National Comprehensive Cancer Network prostate cancer risk classification system. Urology. 2012;80:1075–9. https://doi.org/10.1016/j.urology.2012.07.040.
Park JW, Koh DH, Jang WS, Cho KS, Ham WS, Rha KH, et al. Predictors of adverse pathologic features after radical prostatectomy in low-risk prostate cancer. BMC Cancer. 2018;18:545. https://doi.org/10.1186/s12885-018-4416-4.
Mitsuzuka K, Narita S, Koie T, Kaiho Y, Tsuchiya N, Yoneyama T, et al. Pathological and biochemical outcomes after radical prostatectomy in men with low-risk prostate cancer meeting the Prostate Cancer International: Active Surveillance criteria. BJU Int. 2013;111:914–20. https://doi.org/10.1111/j.1464-410X.2012.11658.x.
van den Bergh RC, Steyerberg EW, Khatami A, Aus G, Pihl CG, Wolters T, et al. Swedish and Dutch sections of the European Randomized Study of Screening for Prostate Cancer. Is delayed radical prostatectomy in men with low-risk screen-detected prostate cancer associated with a higher risk of unfavorable outcomes? Cancer. 2010;116:1281–90. https://doi.org/10.1002/cncr.24882
Porter LH, Lawrence MG, Ilic D, Clouston D, Bolton DM, Frydenberg M, et al. Systematic review links the prevalence of intraductal carcinoma of the prostate to prostate cancer risk categories. Eur Urol. 2017;72:492–5. https://doi.org/10.1016/j.eururo.2017.03.013.
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016;70:106–19. https://doi.org/10.1016/j.eururo.2016.02.028.
Brierley JD, Gospodarowicz MK, Wittekind C. The TNM classification of malignant tumours. 8th ed. Oxford: Wiley Blackwell; 2017. editors.
Yang CW, Wang HH, Hassouna MF, Chand M, Huang WJS, Chung HJ. Prediction of a positive surgical margin and biochemical recurrence after robot-assisted radical prostatectomy. Sci Rep. 2021;11:14329. https://doi.org/10.1038/s41598-021-93860-y.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. https://doi.org/10.1016/S0140-6736(14)60525-0.
Zelic R, Garmo H, Zugna D, Stattin P, Richiardi L, Akre O, et al. Predicting prostate cancer death with different pretreatment risk stratification tools: a head-to-head comparison in a nationwide cohort study. Eur Urol. 2020;77:180–8. https://doi.org/10.1016/j.eururo.2019.09.027.
O’Brien BA, Cohen RJ, Wheeler TM, Moorin RE. A post-radical-prostatectomy nomogram incorporating new pathological variables and interaction terms for improved prognosis. BJU Int. 2011;107:389–95. https://doi.org/10.1111/j.1464-410X.2010.09539.x.
Swanson GP, Goldman B, Tangen CM, Chin J, Messing E, Canby-Hagino E, et al. The prognostic impact of seminal vesicle involvement found at prostatectomy and the effects of adjuvant radiation: data from Southwest Oncology Group 8794. J Urol. 2008;180:2453–8. https://doi.org/10.1016/j.juro.2008.08.037.
Van der Kwast TH, Bolla M, Van Poppel H, Van Cangh P, Vekemans K, Da Pozzo L, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25:4178–86. https://doi.org/10.1200/JCO.2006.10.4067.
Hackman G, Taari K, Tammela TL, Matikainen M, Kouri M, Joensuu T, et al. Randomised trial of adjuvant radiotherapy following radical prostatectomy versus radical prostatectomy alone in prostate cancer patients with positive margins or extracapsular extension. Eur Urol. 2019;76:586–95. https://doi.org/10.1016/j.eururo.2019.07.001.
Adam M, Tennstedt P, Lanwehr D, Tilki D, Steuber T, Beyer B, et al. Functional outcomes and quality of life after radical prostatectomy only versus a combination of prostatectomy with radiation and hormonal therapy. Eur Urol. 2017;71:330–6. https://doi.org/10.1016/j.eururo.2016.11.01.
Dall’Era MA, Cowan JE, Simko J, Shinohara K, Davies B, Konety BR, et al. Surgical management after active surveillance for low-risk prostate cancer: pathological outcomes compared with men undergoing immediate treatment. BJU Int. 2011;107:1232–7. https://doi.org/10.1111/j.1464-410X.2010.09589.x.
Filippou P, Welty CJ, Cowan JE, Perez N, Shinohara K, Carroll PR. Immediate versus delayed radical prostatectomy: updated outcomes following active surveillance of prostate cancer. Eur Urol. 2015;68:458–63. https://doi.org/10.1016/j.eururo.2015.06.011.
Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012;61:1019–24. https://doi.org/10.1016/j.eururo.2012.01.050.
Maruyama Y, Sadahira T, Araki M, Mitsui Y, Wada K, Rodrigo AGH, et al. Factors predicting pathological upgrading after prostatectomy in patients with Gleason grade group 1 prostate cancer based on opinion-matched biopsy specimens. Mol Clin Oncol. 2020;12:384–9. https://doi.org/10.3892/mco.2020.1996.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All of them have also participated in the analysis and interpretation of the data, as well as drafting the work. While each author was involved in all the aspects above, specific authors carried a greater burden of responsibility as follows:As principal investigator, KU had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.RS and YH developed the study concept and design of the work.TO, TS and TI recruited patients and acquired data.RS produced the figures, statistically interpreted the data, drafted the work.TS, TI and MW revised the work critically for important intellectual content.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol for this research project has been approved by a suitably constituted Ethics Committee of each institution, and it conforms to the provisions of the Declaration of Helsinki (Mie University Hospital Clinical Research Ethics Committee, Approval No. H2020-110 and institutional review boards). As the study design was retrospective and observational, the requirement for obtaining informed consent from the participants was waived by the Mie University Clinical Research Review Committee (approval number H2020-110).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Semba, R., Uchida, K., Hirokawa, Y. et al. Short-term prognosis of low-risk prostate cancer patients is favorable despite the presence of pathological prognostic factors: a retrospective study. BMC Urol 23, 174 (2023). https://doi.org/10.1186/s12894-023-01345-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-023-01345-z