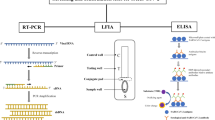
Abstract
The accuracy of diagnostic laboratory tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can impact downstream clinical procedures in managing and controlling the outbreak of coronavirus disease 2019 (COVID-19). To assess the effectiveness of laboratory tools for managing COVID-19 patients in low-income countries (LICs), we systematically searched the PubMed, Embase, Scopus and CINHAL databases for reports published between January 2020 and June 2022. We found that 22 of 1303 articles reported the performance of various SARS-CoV-2 detection tools across 10 LICs. These tools were (1) real-time reverse transcriptase polymerase chain reaction (RT-PCR); (2) reverse transcription loop-mediated isothermal amplification (RT-LAMP); (3) rapid diagnostic tests (RDTs); (4) enzyme-linked immunosorbent assay (ELISA); and (5) dot-blot immunoassay. The detection of COVID-19 is largely divided into two main streams—direct virus (antigen) detection and serology (immunoglobulin)-based detection. Point-of-care testing using antigen-based RDTs is preferred in LICs because of cost effectiveness and simplicity in the test procedures. The nucleic acid amplification technology (RT-PCR and RT-LAMP) has the highest diagnostic performance among the available tests, but it is not broadly used in this context due to costs and shortage of facilities/trained staff. The serology-based test method is affected by antibody interferences and varying amounts of SARS-CoV-2 immunoglobulins expressed at different stages of disease onset. We further discuss the effectiveness and shortcomings of each of these tools in the diagnosis and management of COVID-19. Using the LICs as the study model, our findings highlight ways to improve the quality and turnaround time of COVID-19 testing in resource-constrained settings, notably through local/international collaborative efforts to refine the molecular-based or immunoassay-based testing technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This article describes the effectiveness and challenges of coronavirus disease 2019 (COVID-19) testing strategies in low-income countries where front-end technologies are restricted. Ways to promote efficient and cost-effective testing methods in resource-limited scenarios are discussed. |
1 Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus first detected in December 2019. This virus causes a type of respiratory illness, i.e. coronavirus disease 2019 (COVID-19), which has claimed > 6.6 million lives worldwide as of November 2022, and the death toll is still growing [1]. Several prominent SARS-CoV-2 variants, Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1) and Delta (B1.617.2), were identified [2]. On 26 November 2021, the World Health Organization (WHO) declared a highly mutated variant, Omicron (B.1.1.529), first detected in Bostwana/Hong Kong/South Africa, as a new variant of concern [3, 4]. The Omicron lineage BA.2 was rapidly replaced by two newer lineages with enhanced transmissibility (BA.4 and BA.5) in April 2022, although the impact on severity is mild [5]. The enhanced transmissibility of the newer SARS-CoV-2 variants causes a remarkable increase in the number of people who tested positive for COVID-19, with modelling data demonstrating that the Omicron strain is approximately 10 times more contagious than the original Alpha strain, or 2.8 times more infectious than the Delta variant [2].
1.1 Testing and Management of Suspected and Confirmed Coronavirus Disease 2019 Cases
According to WHO, a suspected case of SARS-CoV-2 infection is defined as an individual who meets the clinical or epidemiological criteria of COVID-19 [6]. The clinical criteria refer to displaying the disease signs/symptoms (e.g. fever, cough and sore throat), while the epidemiological criteria refer to the contact of probable or confirmed cases or linked to the outbreak cluster. While immunoassay-based self-testing is usually the initial diagnostic tool, SARS-CoV-2 infection is confirmed if a person is tested positive by nucleic acid amplification technology (NAAT). WHO published the living guidance for clinical management of COVID-19 on 27 May 2020 [7]. Patients diagnosed with COVID-19 were required to isolate 7–14 days until the NAAT results become negative. At the time of writing, the self-isolation requirements have been alleviated. A study conducted in England between 29 April and 28 July 2021 examined if contacts of confirmed COVID-19 cases may be exempted from the standard 10-day quarantine period [8]. It was demonstrated that exemption from self-isolation for 24 h upon a negative SARS-CoV-2 result was a safe alternative to contain COVID-19, which enabled the non-affected individuals to return to normality. This highlights the importance of accurate diagnostic testing in managing confirmed COVID-19 cases, and to avoid unnecessary quarantine of suspected cases or close contacts.
Large-scale screening and early detection of SARS-CoV-2 may help to contain the spread of COVID-19. NAAT that detects SARS-CoV-2 nucleic acid by real-time reverse transcriptase polymerase chain reaction (RT-PCR) is considered a comparator or reference for evaluating other diagnostic tests of COVID-19. RT-PCR may elicit false-negative and false-positive results as the method can be affected by sample type, timing in sample collection, and nature of the sample preservation. To strengthen this notion, a meta-analysis involving > 18,000 COVID-19-infected subjects demonstrated that the overall false-negative rate for RT-PCR was 0.12 (95% confidence interval 0.10–0.14), equivalent to 12% of the tested population [9]. Due to the rapid evolution of SARS-CoV-2, mutations in the primer hybridisation regions may render RT-PCR ineffective [10]. Data sourced from a Cochrane database systematic review demonstrated that NAAT has an average sensitivity of 95.2% and specificity of 98.9%. A hypothetical cohort of 1000 suspected COVID-19 cases with a prevalence of 10% will pick up 105 positive and 895 negative results. Of these, 10 results will be false positives, giving a positive predictive value (PPV) of 90%, plus 5 false negative results, with a negative predictive value (NPV) of 99% [11]. Each PCR test requires sophisticated equipment, expensive reagents and highly trained personnel. If the turnaround time is not able to meet the needs of early detection, then it may not be suitable for prevention and control of the pandemic.
1.2 Challenges Faced by Low-Income Countries in Coping with the Testing Demands Arising from the COVID-19 Pandemic
In low-income countries (LICs), establishing laboratories equipped with NAAT can be particularly challenging. Here, the LICs are defined based on the key indicator set by the United Nations (UN), where the yearly gross national income per capita on a 3-year average is less than US$1018, also known as the least developed countries [12]. As of 2021, 46 countries were classified as LICs [13]. In LICs, factors such as (1) lack of skilled laboratory scientists, (2) shortage of certified biomedical engineers capable of calibrating/maintaining the biosafety of laboratory equipment, and (3) expensive overhead running costs of RT-PCR have unfortunately discouraged global implementation of NAAT. A report based in Nepal, an LIC, documented that the country lacks trained manpower in conducting viral RNA extraction, which is crucial for the molecular PCR testing protocols [14]. The situation is compounded by insufficient numbers of clinical experts who can correctly interpret the test results, and by the fact that priority is placed for treating other medical emergencies or acute illnesses.
Considering this, diagnostic tests that do not rely on RT-PCR were developed as an alternative mechanism to detect SARS-CoV-2. Such examples include the rapid diagnostic test (RDT) and enzyme-linked immunosorbent assay (ELISA). These immunoassay-based tests can detect either the viral protein fragments (antigen) in throat swab and sputum or immunoglobulin (Ig) G and IgM in blood produced upon exposure to SARS-CoV-2 infection. Using the STANDARD™ Q COVID-19 Ag Test (SD Biosensor, Suwon-si, South Korea) as an example, the test detects the presence of SARS-CoV-2 antigen in the specimen. The antigen will bind to the anti-SARS-CoV-2 antibody conjugated with chromogenic agent on the test cassette. The antigen-antibody complex will migrate on the nitrocellulose membrane via capillary action until it is captured by a secondary anti-SARS-CoV-2 antibody pre-coated on the membrane, which eventually develops a coloured test line [15]. Some ELISA tests can detect IgG and IgM in infected samples since the solid surface is coated with the recombinant SARS-CoV-2 proteins (spike and nucleocapsid) [16]. Results from these immunoassay-based methods can typically be obtained within 30 min after testing; however, their performance varies significantly, with an average sensitivity of only 56.2% (compared with 95.2% in NAAT) [11].
1.3 Objective of this Review
In this review, we asked the question “What is the effectiveness of testing strategies for COVID-19 in LICs?” Our review identified knowledge gaps on the types of diagnostic tests available in LICs in detecting SARS-CoV-2. We aimed at mapping ways to improve the quality and turnaround time of COVID-19 testing in LICs, and how resource-rich countries may play a role in achieving these goals.
2 Methods
2.1 Data Sources and Search Strategy
Using the readily available data from the literature, the focus of our research was to integrate meaningful information to elucidate what tests are being used to detect SARS-CoV-2 in LICs, as well as their effectiveness. Our search methodology is confined exclusively to the ‘least developed countries’ with a low gross national income per capita as determined by the UN [12]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for scoping review were followed to conduct a systematic literature search in four databases (PubMed, Embase, Scopus and CINHAL) to identify relevant observational studies, randomised control trials and systematic reviews published from January 2020 to June 2022. The PICO (Population, Intervention, Comparison and Outcomes) model was adopted to formulate the search eligibility criteria (Table 1). We used search terms including ‘SARS-CoV-2 AND low-resource AND testing’, ‘COVID-19 AND resource-limited AND nucleic acid test’, ‘Novel coronavirus AND limited resource AND serological rapid test’ to capture the relevant literature. The search strategy was adopted from the paper by Ouma et al. [17]. New keywords including ‘prognosis’, ‘molecular test’ and ‘nucleic acid test’ were added to our search. Articles with keywords associated with ‘cancer’, ‘cancer treatment’, ‘malignancy’ and ‘malignancy treatment’ were excluded. A complete list of search terms used is outlined in electronic supplementary Table 1. The search process was further refined by applying the following inclusion/exclusion criteria:
Inclusion criteria:
-
Peer-reviewed articles published in English from January 2020 to June 2022.
-
Content addresses SARS-CoV-2 testing strategies in LICs.
Exclusion criteria:
-
Grey literature (narrative reviews, web-based guidelines and editorials).
-
Studies conducted outside LICs.
-
No evidence of SARS-CoV-2 molecular/immunoassay-based diagnostic tests.
-
No evaluation on the diagnostic test performance.
2.2 Data Extraction Strategy
All relevant articles were exported to EndNote™ 20 (Thomson Reuters, New York, NY, USA). After removing duplicates, the title/abstract of each article was independently screened by two authors (YPC and CXL) to determine if they met the inclusion/exclusion criteria. Discrepancy in opinions was resolved by discussion until consensus was achieved. Where opinions remained unresolved, a third colleague (KWC) was invited to provide additional insights. Articles that did not fulfil the selection criteria were removed. The data extracted for each article comprised country, COVID-19 diagnostic test, manufacturer, research sample size/type, impact of testing strategy and challenge encountered.
3 Findings and Discussion
3.1 Description of the Search Results
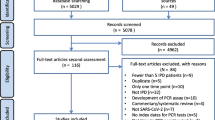
We began with a total of 1303 eligible articles extracted from four databases; 177 duplicates were removed. The remaining 1126 publications were screened via reading the title/abstract and 1069 articles were removed based on the exclusion criteria. The full text of 57 articles was individually examined and a total of 35 articles were excluded, largely due to not fulfilling the content criterion (see Sect. 2.1). Of the 35 excluded articles, one was not peer-reviewed; 17 studies were conducted outside LICs; 7 studies were not related to SARS-CoV2 molecular/immunoassay-based diagnostic tests; and 10 studies did not evaluate the performance of the diagnostic tests. Finally, a total of 22 articles were eligible for analysis of our primary research question. The workflow is summarised in Fig. 1.
Our findings are thus based on 22 reports pertaining to 10 LICs: Bangladesh (4), Benin (2), Central African Republic (1), Ethiopia (3), Madagascar (3), Mali (1), Mozambique (1), Nepal (1), Uganda (5) and Zambia (1). Of these, five studies were conducted in collaboration with researchers outside LICs. Our findings reveal a series of COVID-19 testing protocols available in these LICs (Table 2).
Only two countries (Central African Republic, Mali) evaluated the serology-based detection method; three countries (Mozambique, Nepal, Zambia) examined the utility of direct virus detection; and the remaining five countries (Bangladesh, Benin, Ethiopia, Madagascar, Uganda) explored the use of both viral and serological detection methods. Our search results uncover the performance of the individual COVID-19 test methods (Table 3), together with the impact and pitfalls of each diagnostic tool in managing COVID-19 (Table 4). We elaborate below on the effectiveness of these testing strategies and how challenges may be overcome to promote efficient and cost-effective SARS-CoV-2 detection.
3.2 Real-Time Reverse Transcriptase Polymerase Chain Reaction
3.2.1 Sampling Methods
Our study demonstrated that 6 of the 10 LICs (Bangladesh, Benin, Ethiopia, Madagascar, Uganda and Zambia) utilise commercial RT-PCR for the diagnosis of COVID-19 [18,19,20,21,22,23]. Nasopharyngeal swab is perceived as the gold standard to confirm COVID-19 as this sampling method gives the highest diagnostic yield for respiratory viruses [24]. Due to a lack of qualified healthcare professionals in collecting nasopharyngeal swabs, and discomfort of the procedure to patients, other less invasive sample collection techniques, including pooled nasal and throat swabs, nasal swabs, throat swabs and saliva were subsequently introduced [25]. In LICs, the RT-PCR was primarily performed on nasopharyngeal and oropharyngeal specimens [19,20,21, 23]. More recently, in Bangladesh, the diagnostic performance of self-collected saliva was assessed against the nasopharyngeal swab by RT-PCR [26]. It was observed that the overall sensitivity of saliva specimens was 80.35% relative to the nasopharyngeal swabs; the sensitivity could be improved if specimens were collected within 5 days post onset of symptoms. Given the salivary sampling method is low cost, the effectiveness of self-collected saliva for COVID-19 diagnosis could be further explored to favour a more economical testing strategy in low-resource settings.
3.2.2 Pooled Testing
Pooled testing was introduced in the 1940s to detect syphilis [27]. Negative results were reported for all individuals in the same pool if the combined serum was tested negative for the bacteria causing syphilis. If positive, all samples making up the pool must be retested individually to ascertain which member(s) is (are) infected. Our findings show that Uganda adopted the pooled testing procedure for SARS-CoV-2 detection, where a total of 1280 respiratory swabs were tested in batches of 10 samples per pool by RT-PCR [18]. This approach reduces the running cost by fourfold in comparison with the single testing method [18]. Nyazika and colleagues commented that pooled testing offers great benefit compared with single-test RT-PCR [28]. Pooled testing not only conserves resources but also speeds up analytical run times, thus favouring mass testing and, in turn, minimises the risk of viral transmission in the wider population. To substantiate this, researchers in Kenya, a sub-Saharan African country outside LICs found that analysing pooled respiratory swabs (combinations of 6 individual samples) by RT-PCR remarkably improves the testing capacity by approximately 100%, and conserves resources by saving up to 50% of the reagents for RNA extraction and RT-PCR [29].
However, there are known limitations of pooled testing. A pitfall is that the threshold cycle (ΔCt) was estimated at 1.59 times higher for samples tested in pools compared with those tested individually, which may compromise diagnostic sensitivity [29]. The viral loads are inversely correlated with the threshold cycle of RT-PCR [29, 30]. Pooled testing is only feasible when the incidence rate of SARS-CoV-2 infection within a population is low, typically < 15%. If the infection prevalence reaches 30%, the chance of most or all combined serum tested positive will be higher, and this strategy will then not benefit laboratory outputs and turnaround times.
3.2.3 Portable RT-PCR
Prior to the SARS-COV-2 pandemic, the portable PCR GeneXpert (Xpert; Cepheid, Sunnyvale, CA, USA) had already been employed as a diagnostic tool for tuberculosis in Madagascar [31]. The utility of GeneXpert was upscaled for COVID-19 diagnosis in the same country in response to the pandemic [19]. The GeneXpert platform uses Xpert Xpress SARS-CoV-2 assay, and the system combines sample processing, nucleic acid extraction and amplification, and detection of SARS-CoV-2 target sequence in a disposable single-use cartridge with a turnaround time of approximately 45 min per sample. The diagnostic performance of Xpert Xpress SARS-CoV-2 was evaluated in Madagascar before implementing it nationwide [19]. When comparing its performance with another NAAT assay, 2019 Novel Coronavirus (2019-nCoV) RNA (DaAn Gene, Guangzhou, China), on 40 nasopharyngeal samples, it was observed that Xpert Xpress provided a sensitivity and specificity of 100% and 80%, respectively. It appeared challenging to accurately determine the numbers of true positive samples, partly due to the electronic result interpretation by the software overestimating false positives, leading to unnecessary overload of the healthcare system. The widespread use of portable PCR is limited by the suboptimal availability of stable power supply in LICs, where power interruptions tend to occur regularly. Although the average cost of a portable PCR unit is under US$800, large-scale employment of portable PCRs still requires a significant initial cost investment by local governments. The ongoing maintenance cost is also a potential issue as portable PCRs require routine maintenance to ensure operating efficacy [32]. Nevertheless, the deployment of portable PCR in LICs is relatively cost effective since its operation involves minimal trained staff and does not require expensive thermal cycler equipment compared with conventional RT-PCR. Other similar portable RT-PCR platforms that have been evaluated elsewhere include the Roche cobas® Liat® and Roche cobas® 6800/8800 [33]. The results show that the detection performance of both platforms together with GeneXpert was robust and unaffected by multiple mutations in the SARS-CoV-2 genome.
3.3 Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP)
An attractive feature of LAMP is that the amplification of DNA does not involve heat denaturation of double-stranded DNAs. Baba and co-workers evaluated the performance of SARS-CoV-2 colorimetric RT-LAMP in sub-Saharan Africa [34]. This method produces a very good specificity (98%) and comparable sensitivity (87%) for medium–high viral loads, but the primer design for effective RT-LAMP can be challenging. The technical difficulty stems in optimising the number of primers involved in amplifying a limited fragment of DNA to avoid self-hybridisation of these primers [35]. The sensitivity of RT-LAMP decreases when the viral RNA abundance is low. Of note, an article by Huang et al. describes the development of new primer features to boost the sensitivity and specificity of RT-LAMP [36]. The authors claimed that SARS-CoV-2 in salivary samples can be directly detected by their new primers without the need to isolate RNA, making the diagnostic process more cost effective and beneficial for point-of-care testing.
3.4 Rapid Diagnostic Tests (RDTs)
3.4.1 Antigen-Based RDTs
Our search findings further reveal the broad utility of antigen-based RDTs in several LICs [37,38,39,40,41,42,43]. The performance of the STANDARD™ Q COVID-19 Ag Test was examined in four countries: Uganda, Madagascar, Bangladesh and Mozambique [38, 40, 41, 43]. The results show that the test kit provides comparable diagnostic specificity ranging from 92% to 100%, but the diagnostic sensitivity varies significantly from 34.6% to 85%. An RDT named OnSite® COVID-19 Ag Rapid Test (CTK Biotech Inc., Poway, CA, USA) that recognises the nucleocapsid protein of SARS-CoV-2 was evaluated in Bangladesh [41]. This test kit provides an overall sensitivity and specificity of 91% and 99.2%, respectively, and demonstrates the capacity to detect patients even after 6 days post onset of COVID-19 symptoms. In Uganda, field evaluation was undertaken to investigate the performance of seven antigen-based RDTs that were claimed to have high sensitivity (60–100%) and specificity (97.8–100%) by the manufacturers [37]. Paradoxically, it was reported that none of the kit tested had a sensitivity of ≥80% at Ct ≤ 33 and ≤ 39. Four kits, i.e. (1) BIOCREDIT COVID -19 Ag (RapiGEN, INC, Suwon-si, South Korea), (2) COVID-19 Ag Respi-Strip (Coris BioConcept, Gembloux, Belgium), (3) MEDsan® SARS-CoV-2 Antigen Rapid test (MEDsan®, Hamburg, Germany), and (4) Panbio™ COVID-19 Ag Rapid Test (Abbott Rapid Diagnostics, Jena, Germany), showed specificity of ≥ 97%, which conforms to the manufacturers’ statements. The field evaluation has identified Panbio™ COVID-19 Ag Rapid Test as the best-performing diagnostic kit that meets the requirement recommended by WHO, with a sensitivity of ≥ 80% at Ct ≤ 29 and specificity ≥ 97%. The test kit could potentially be implemented for large-scale COVID-19 testing in Uganda.
3.4.2 Immunoglobulin (Ig) G/IgM-Based RDTs
The IgG/IgM-based RDTs were assessed by researchers in three countries: Uganda, Central Africa and Ethiopia [44,45,46,47]. Lutalo et al. evaluated the performance of 25 SARS-CoV-2 RDTs at the Uganda Virus Research Institute [45]. These RDTs were designed to detect anti-SARS-CoV-2 IgG and/or IgM in plasma samples. Of the 25 test kits, only three provide ≥98% sensitivity and specificity. Mboumba Bouassa et al. examined the frequency of false-positives of three antibody-based RDTs: (1) BIOSYNEX® COVID-19 BSS (IgG/IgM) (Biosynex Swiss SA, Freiburg, Switzerland); (2). SIENNA™ COVID-19 IgG/IgM Rapid Test Cassette (Salofa Oy, Salo, Finland); and (3) NG-Test® IgG–IgM COVID-19 (NG Biotech Laboratories, Guipry, France) [46]. Data from this study demonstrated that patients with previous viral (e.g. metapneumovirus, hepatitis C virus, human immunodeficiency virus) or parasitic (e.g. malaria) infections were tested positive for SARS-CoV-2 although they were not diagnosed with COVID-19. The high incidence of false-positives was mainly attributed to the non-specific cross-reactivity of circulating IgM from previous infections. This issue may be resolved by using at least two IgG/IgM-based RDTs simultaneously or in series when performing SARS-CoV-2 testing for patients. Another study by Baker et al. investigated the differential performance of CoronaCHEK (Hangzhou Biotest Biotech Co. Ltd, Hangzhou, China) on populations from two continents—Uganda and Baltimore [44]. They observed that the diagnostic specificity of this test kit was 2.8% lower in the Ugandan samples than in the Baltimore samples, implying that the performance of a test kit may vary in different geographic locations of the specimens tested. The high false positives in the Ugandan samples correlated with antibody cross-reactivity. Hence, it may be inappropriate to extrapolate results from one country to another and assume the same specificity of IgG/IgM-based RDTs across all populations.
3.4.3 Advantages of RDTs
One of the limitations of RDTs is that its performance generally declines when the viral load is low (Ct > 30), which may increase the rate of false negatives [40, 41]. Despite the shortcomings, RDTs can complement NAAT. The RDTs expedite the diagnostic process as the short turnaround time (approximately 30 min) permits large-scale population screening for SARS-CoV-2 in resource-constrained settings [42, 47]. Not only does this help to resolve backlog issues when testing demand is high, but more importantly, patients infected with SARS-CoV-2 can be isolated or treated immediately before severity prevails. In rural areas, the attractiveness of RDTs is even more prominent given the limited access to molecular testing facilities. A study in Madagascar reports that the cost of running the RDTs is significantly cheaper than RT-PCR [40]. The cost of an RT-PCR test is $50, whereas a STANDARD™ Q COVID-19 Ag Test is less than $5. From a low-resource perspective, it is justifiable that RDTs are used more broadly in managing COVID-19 cases. Asymptomatic COVID-19 patients possess viral loads similar to those of symptomatic patients, and can transmit disease to a similar extent as the symptomatic patients [30]. Future work aiming at improving the performance of RDT kits on asymptomatic populations with low viral load may be warranted to promote quality self-testing at home.
3.5 Enzyme-Linked Immunosorbent Assay
Upon exposure to SARS-CoV-2, the immunity reacts by producing a surge in total antibody, IgM and IgG with a median seroconversion time of 9-, 10- and 12-days post onset of symptoms, respectively [48]. The IgM immunoglobulin becomes undetectable by week 7, while IgG persists beyond this period [49]. Therefore, the timing of blood collection is critical. ELISA may not effectively identify SARS-CoV-2 antibodies if blood is collected within 5 days of disease onset (sensitivity < 40%), but may better detect the total antibody, IgM and IgA between 6 and 10 days after onset of symptoms (sensitivity > 80%) [50]. The performance of several ELISA kits was evaluated in Madagascar, Mali and Benin [51,52,53]. In Benin, research was conducted to study the specificity of commercially available ELISA kits by comparing sera of COVID-19-positive patients versus the pre-pandemic controls [53]. It was observed that the IgA-based ELISA gives a higher sensitivity than the IgG-based test, which is consistent with the SARS-CoV-2 antibody profile. However, ELISA is subject to immunoglobulin cross-reactivity. Patients previously infected or co-infected with other acute viral or parasitic diseases, especially malaria, may have a higher risk of obtaining false positive results. In line with this, Emmerich et al. reported that the specificity of four commercial SARS-CoV-2 IgG ELISAs is not consistent across the African countries [51]. The specificity of all four ELISAs was high for the Madagascan samples (93.4–99.4%), but the specificity of the same tests was poor for the Ghanaian and Nigerian samples (Ghana: nucleocapsid antigen-based assays 77.7–89.7%, spike/S1-based assay 94.3%; Nigeria: nucleocapsid antigen-based assays 39.3–82.7%, spike/S1-based assay 90.7%). This may be due to cross-reactivity of immunoglobulins developed from past infections other than COVID-19 across the different populations. Woodford and colleagues also highlighted the issue associated with high background reactivity to SARS-CoV-2 antigens in Mali [52]. The authors suggested that application of a ‘two-immunoassay’ approach with population-specific cut-offs may improve the performance of ELISA. Generally, the costs of running a SARS-CoV-2 IgG ELISA range from US$8–$11 per test [54, 55]; this is significantly cheaper than that of RT-PCR (US$50 per test).
3.6 Dot-Blot Immunoassay
This assay is modelled after the sandwich ELISA, where a labelled secondary antibody binds to the immunocomplex, producing colour intensity that can be rated on a 5-point gradient scale by the naked eye [56]. In Bangladesh, the performance of an in-house rapid dot-blot immunoassay was assessed by comparing its ability to detect antibodies against SARS-CoV-2 in four cohorts of COVID-19 patients: (1) symptomatic for 1–7 days; (2) symptomatic for 8–14 days; (3) symptomatic but showed negative results by RT-PCR; and (4) convalescent [57]. The dot-blot immunoassay was less sensitive in picking up patients with symptoms, from 1 to 7 days (sensitivity 11%), but the performance improved as the disease progressed, from 8 to 14 days (sensitivity 41%). Moreover, it detected 69.7% of the convalescent patients who were positive for SARS-CoV-2 confirmed by RT-PCR and asymptomatic for more than 14 days. It was proposed that the dot-blot immunoassay may be applied to screen patients at late-phase infection, especially when the viral load is diminishing and undetectable by RT-PCR. Additionally, the test may assist in confirming whether an individual has been infected by SARS-CoV-2 in the past to enable mapping of the antibody profile of post-vaccinated populations.
A newer discovery indicates that the performance of the dot-blot immunoassay can be optimised by incorporating a flow-through detection system with colloidal gold nanoparticles acting as the assay reporter [58]. The translucent colour produced by colloidal gold nanoparticles is stable and provides high contrast visualisation for easy eye-reading results, which is a desirable feature in LICs. However, the cost of establishing the dot-blot immunoassay was not defined. If nanomaterial labels are available at a low cost, this may place the dot-blot immunoassay a more practical approach for effective management of COVID-19 in LICs.
3.7 Effectiveness of SARS-CoV-2 Testing Strategies in LICs
3.7.1 Point-of-Care Testing
It can be summarised that point-of-care testing (POCT), particularly the antigen-based RDTs, is the most common strategy for SARS-CoV-2 testing in LICs [37,38,39,40,41,42,43]. A major factor driving the wide use of POCT is cost effectiveness. Additionally, POCT can be performed outside of hospitals, does not demand invasive blood sample collection, and self-collected samples can be applied to the test cassette without involvement of laboratory equipment/staff. The fast turnaround time promises rapid testing to effectively treat and/or isolate COVID-19-confirmed cases plus close contacts. The IgG/IgM-based RDTs are also POCT, but they are less preferred as the test outcomes are subject to errors from antibody cross-reactivity [44,45,46,47].
3.7.2 Direct Virus (Antigen) Detection Approach
NAAT with over 95% sensitivity and specificity [11] represents the most accurate diagnostic tool for COVID-19 among the available tests. This molecular-based technology requires laboratory space/staff, consumables/equipment/maintenance and shipping logistics including cold chain that exert extra costs compared with the POCT [19,20,21,22,23]. The operations of portable RT-PCR in underdeveloped countries can be interrupted by recurrent power failure [32]. Alternatively, RT-LAMP that bypasses the use of thermal cycler equipment can technically reduce the costs [34]. Work was done to upgrade the performance of RT-LAMP by incorporating a newly formulated dye to improve assay readout and test accuracy [59]. However, the estimated cost of this new technique is $8 per test, which is still more costly than the antigen-based RDT (STANDARD™ Q COVID-19 Ag test) of $5 per test [40]. Pooled PCR testing is deemed to save costs [18]; this is only strategic provided the prevalence of COVID-19 is low (< 15%) [29].
3.7.3 Serology (Immunoglobulin)-Based Detection Approach
Our findings do not reveal substantial use of commercially available ELISAs for SARS-CoV-2 detection in LICs. The diagnostic performance of ELISAs is highly dependent on the types of immunoglobulins in the blood. Different immunoglobulins (IgA, IgM and IgG) exist in varying amounts at different stages of COVID-19 infection, which may complicate result reporting [53]. Furthermore, serology assays are prone to antibody interferences from past infections or co-infections [51, 52]. Interestingly, our findings uncovered an in-house developed dot-blot immunoassay in Bangladesh [57]. Although this method cannot directly detect the viral antigen, it has the potential to be transformed and utilised in serological surveillance of COVID-19.
3.8 Limitations of this Study
Our literature search is confined to 46 LICs defined by UN [13]. The SARS-CoV-2 testing strategies presented in this review are derived from 22 publications in 10 countries. We acknowledge that the limited number of publications may create a bias, and thus may not holistically reflect the testing strategies across all LICs. Some nations implement combined COVID-19 testing and seroprevalence of SARS-CoV-2 to assess the infected population in the community. In Somalia, for example, a seroprevalence study was carried out in one of the densely populated cities as a surveillance mechanism to postulate the percentage of unreported COVID-19 cases [60]. The data were collected using an immuno-based RDT with test strip coated with a colloidal gold-labelled recombinant SARS-CoV-2 antigen that allows detection of SARS-CoV-2 IgM and IgG simultaneously in whole blood.
4 Summary and Future Directions
In this review, we performed a systematic search on the current literature to ascertain SARS-CoV-2 detection strategies in the diagnosis and management of COVID-19 patients in LICs. We identified 22 articles published by 10 LICs assessing the performance of COVID-19 testing. The test methods included (1) direct virus detection (RT-PCR, RT-LAMP, antigen-based RDTs) and (2) serology-based detection (IgG/IgM-based RDTs, ELISA, dot-blot immunoassay). Our findings reinforce the notion that utility of NAAT is limited in LICs due to high cost, lack of a specifically trained workforce and under-equipped laboratory facilities. In contrast, immunodiagnostic testing is more economical with quicker result output, but its effectiveness is limited by poorer sensitivity and specificity attributed by antibody cross-reactivity. Considering the logistics and cost factors, it can be concluded that the antigen-based RDTs are most preferred for COVID-19 testing in LICs, although their performance is lower than that provided by NAAT.
4.1 Collaboration is Key in Laboratory-Preparedness in Low-Resource Settings
In response to the overwhelming demands for SARS-CoV-2 PCR-based tests, an extensive collaborative network was established in sub-Saharan Africa to improve the molecular diagnostic capacity [61]. The University of Benin Teaching Hospital (UBTH) and Nigeria cooperated with a World Bank-supported institution to establish a SARS-CoV-2 molecular testing platform in UBTH. Priority was also placed to upgrade their current molecular virology laboratory infrastructure. The quality of SARS-CoV-2 testing was validated by an inter-laboratory comparison scheme against a reference laboratory. These combined efforts enabled the laboratory to achieve a turnover of 12,123 tests within 7 months of operation. Future collaborations involving national and international counterparts could be the key in laboratory preparedness to handle the increasing SARS-CoV-2 test demands.
4.2 Examples of Improved Molecular-Based COVID-19 Testing
4.2.1 Extraction-Free RT-LAMP
An extraction-free RT-LAMP designed to save on processing time and cost was developed in the UK [62]. The nasopharyngeal and throat samples were heat inactivated (95 °C, 5 min) then tested directly without prior RNA extraction. The assay generated results within 30 min after sample collection, similar to the RDTs. It yielded 86.7% sensitivity and 98.4% specificity. A similar diagnostic performance was reproduced when the assay was validated in Malawi, an LIC. A significant advantage of an extraction-free LAMP assay is that the reaction can resist temperature fluctuations in the event of power interruptions. The reagents for RT-LAMP are much cheaper (£3, against £30 for RT-PCR); they can be shipped or stored lyophilised at room temperature to mitigate issues around cold chain transport in underdeveloped regions.
4.2.2 Automated Mobile Laboratory for On-Site COVID-19 Testing
A van-based mobile laboratory deployed in China adopts a six-axis robotic arm to collect oropharyngeal swabs [63]. Virus in the sample is inactivated by an infrared heating module, while nucleic acid amplification and readouts are completed through a ‘sample in, answer out’ model using a self-contained microfluidic analyser driven by RT-LAMP. The mobile laboratory contains eight microfluidic nucleic acid analysers that collectively increase testing outputs to 150 samples in 8 h. Patient results are electronically reported by an onboard laboratory information system. This strategy promises fast deployment of on-site COVID-19 testing in remote or hotspot areas to rapidly contain disease outbreak. Future work aiming at sourcing funds from non-governmental organisations or philanthropists to implement the mobile laboratory campaigns in these areas may lead to more strategic COVID-19 testing in low-income settings.
5 Concluding Remarks
The accuracy of diagnostic laboratory tests for SARS-CoV-2 can impact downstream clinical procedures in managing and controlling the outbreak of COVID-19. Our review maps the types of diagnostic tools available for COVID-19 in resource-limited scenarios. PCR-based testing is the reference method and RDTs complement NAAT. Given mutations in SARS-CoV-2 generate variants with extremely high transmission rates, the increased demand for RT-PCR could impose extra burden to the already stressed healthcare and economy systems. Although our search findings are limited to the LICs defined by the UN, the diagnostic strategies described in this review may shed light on ways to maximise COVID-19 testing outputs, especially when test demands exceed resources.
Change history
06 March 2023
Missing Open Access funding information has been added in the Funding Note.
References
World Health Organization. WHO Coronavirus (COVID-19) Dashboard 2022 [cited 18 Nov 2022]. https://covid19.who.int/. Accessed 23 Jan 2023.
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). Treasure Island: StatPearls; 2022.
GISAID. Tracking of hCoV-19 Variants 2022 [cited 18 Nov 2022]. https://gisaid.org/hcov19-variants/. Accessed 23 Jan 2023.
World Health Organization. Update on Omicron 2021 [cited 18 Nov 2022]. Available at: https://www.who.int/news/item/28-11-2021-update-on-omicron. Accessed 23 Jan 2023.
Tegally H, Moir M, Everatt J, Giovanetti M, Scheepers C, Wilkinson E, et al. Emergence of SARS-CoV-2 Omicron lineages BA4 and BA.5 in South Africa. Nat Med. 2022;28(9):1785–90. https://doi.org/10.1038/s41591-022-01911-2.
World Health Organization. WHO COVID-19 Case Definition 2020 [cited 18 Nov 2022]. https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2022.1. Accessed 23 Jan 2023.
World Health Organization. Clinical Management of COVID-19 Interim Guidance 27 May 2020 2020 [cited 18 Nov 2022]. https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf. Accessed 23 Jan 2023.
Love NK, Ready DR, Turner C, Verlander NQ, French CE, Martin AF, et al. Daily use of lateral flow devices by contacts of confirmed COVID-19 cases to enable exemption from isolation compared with standard self-isolation to reduce onward transmission of SARS-CoV-2 in England: a randomised, controlled, non-inferiority trial. Lancet Respir Med. 2022;10(11):1074–85. https://doi.org/10.1016/S2213-2600(22)00267-3.
Pecoraro V, Negro A, Pirotti T, Trenti T. Estimate false-negative RT-PCR rates for SARS-CoV-2. A systematic review and meta-analysis. Eur J Clin Invest. 2022;52(2):e13706. https://doi.org/10.1111/eci.13706.
Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453–4. https://doi.org/10.1080/14737159.2020.1757437.
Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8(8):CD013705. https://doi.org/10.1002/14651858.CD013705.
United Nations. Least Devloped Countries Identification Criteria and Indicators 2021 [cited 18 Nov 2022]. https://www.un.org/development/desa/dpad/least-developed-country-category/ldc-criteria.html. Accessed 23 Jan 2023.
United Nations. Least Developed Countries (LDC) list 2021 [cited 18 Nov 2022]. https://www.un.org/development/desa/dpad/least-developed-country-category/ldcs-at-a-glance.html. Accessed 23 Jan 2023.
Giri AK, Rana DR. Charting the challenges behind the testing of COVID-19 in developing countries: Nepal as a case study. Biosafety and Health. 2020;2(2):53–6. https://doi.org/10.1016/j.bsheal.2020.05.002.
SD Biosensor. STANDARD Q COVID-19 Ag Test Principle 2020 [cited 18 Nov 2022]. https://cdn.who.int/media/docs/default-source/in-vitro-diagnostics/eul-0563-117-00-standard-q-covid19-ag-ifu.pdf?sfvrsn=f9e40e76_2&download=true. Accessed 23 Jan 2023.
Liu W, Liu L, Kou G, Zheng Y, Ding Y, Ni W, et al. Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J Clin Microbiol. 2020. https://doi.org/10.1128/JCM.00461-20.
Ouma OK, Ephraim K, Loyce N, Namisango E, Nalugoda F, Ndagire R, et al. Role and utility of COVID-19 laboratory testing in low-income and middle-income countries: protocol for rapid evidence synthesis. BMJ Open. 2021;11(10):e050296. https://doi.org/10.1136/bmjopen-2021-050296.
Bogere N, Bongomin F, Katende A, Ssebambulidde K, Ssengooba W, Ssenfuka H, et al. Performance and cost-effectiveness of a pooled testing strategy for SARS-CoV-2 using real-time polymerase chain reaction in Uganda. Int J Infect Dis. 2021;113:355–8. https://doi.org/10.1016/j.ijid.2021.10.038.
Rakotosamimanana N, Randrianirina F, Randremanana R, Raherison MS, Rasolofo V, Solofomalala GD, et al. GeneXpert for the diagnosis of COVID-19 in LMICs. Lancet Glob Health. 2020;8(12):e1457–8. https://doi.org/10.1016/S2214-109X(20)30428-9.
Roy S, Paul SK, Barman TK, Ahmed S, Haque N, Mazid R, et al. SARS-CoV-2 detection using real time PCR by a commercial diagnostic kit. Mymensingh Med J. 2020;29(3):596–600.
Sander AL, Yadouleton A, Moreira-Soto A, Tchibozo C, Hounkanrin G, Badou Y, et al. An observational laboratory-based assessment of SARS-CoV-2 molecular diagnostics in Benin, Western Africa. mSphere. 2021;6(1):1–8. https://doi.org/10.1128/mSphere.00979-20.
Sisay A, Abera A, Dufera B, Endrias T, Tasew G, Tesfaye A, et al. Diagnostic accuracy of three commercially available one step RT-PCR assays for the detection of SARS-CoV-2 in resource limited settings. PLoS ONE. 2022;17(1):e0262178. https://doi.org/10.1371/journal.pone.0262178.
Tembo J, Egbe NF, Maluzi K, Mulonga K, Chilufya M, Kapata N, et al. Evaluation of SARS-CoV-2 diagnostics and risk factors associated with SARS-CoV-2 infection in Zambia. Int J Infect Dis. 2022;120:150–7. https://doi.org/10.1016/j.ijid.2022.04.017.
Lee RA, Herigon JC, Benedetti A, Pollock NR, Denkinger CM. Performance of saliva, oropharyngeal swabs, and nasal swabs for SARS-CoV-2 molecular detection: a systematic review and meta-analysis. J Clin Microbiol. 2021. https://doi.org/10.1128/JCM.02881-20.
Tsang NNY, So HC, Ng KY, Cowling BJ, Leung GM, Ip DKM. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21(9):1233–45. https://doi.org/10.1016/S1473-3099(21)00146-8.
Uddin MKM, Shirin T, Hossain ME, Alam AN, Ami JQ, Hasan R, et al. Diagnostic performance of self-collected saliva versus nasopharyngeal swab for the molecular detection of SARS-CoV-2 in the clinical setting. Microbiol Spectr. 2021;9(3):e0046821. https://doi.org/10.1128/Spectrum.00468-21.
Dorfman R. The detection of defective members of large populations. Ann Math Stat. 1943;14:436–40.
Nyazika TK, Kaela R, Mugoni M, Musomekwa K, Kyei-Baafour E, Chiwanda S, et al. Implementation of antibody rapid diagnostic testing versus real-time reverse transcription-PCR sample pooling in the screening of COVID-19: a case of different testing strategies in Africa. mSphere. 2020. https://doi.org/10.1128/mSphere.00524-20.
Agoti CN, Mutunga M, Lambisia AW, Kimani D, Cheruiyot R, Kiyuka P, et al. Pooled testing conserves SARS-CoV-2 laboratory resources and improves test turn-around time: experience on the Kenyan Coast. Wellcome Open Res. 2020;5:186. https://doi.org/10.12688/wellcomeopenres.16113.2.
Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–9. https://doi.org/10.1056/NEJMc2001737.
Knoblauch AM, Grandjean Lapierre S, Randriamanana D, Raherison MS, Rakotoson A, Raholijaona BS, et al. Multidrug-resistant tuberculosis surveillance and cascade of care in Madagascar: a five-year (2012–2017) retrospective study. BMC Med. 2020;18(1):173. https://doi.org/10.1186/s12916-020-01626-6.
Gonzalez-Gonzalez E, Trujillo-de Santiago G, Lara-Mayorga IM, Martinez-Chapa SO, Alvarez MM. Portable and accurate diagnostics for COVID-19: Combined use of the miniPCR thermocycler and a well-plate reader for SARS-CoV-2 virus detection. PLoS ONE. 2020;15(8):e0237418. https://doi.org/10.1371/journal.pone.0237418.
Sit BHM, Po KHL, Cheung YY, Tsang AKL, Leung PKL, Zheng J, et al. Detection of SARS-CoV-2 VOC-Omicron using commercial sample-to-answer real-time RT-PCR platforms and melting curve-based SNP assays. J Clin Virol Plus. 2022;2(3):100091. https://doi.org/10.1016/j.jcvp.2022.100091.
Baba MM, Bitew M, Fokam J, Lelo EA, Ahidjo A, Asmamaw K, et al. Diagnostic performance of a colorimetric RT-LAMP for the identification of SARS-CoV-2: a multicenter prospective clinical evaluation in sub-Saharan Africa. EClinicalMedicine. 2021;40:101101. https://doi.org/10.1016/j.eclinm.2021.101101.
Mora-Cardenas E, Marcello A. Switch-on the LAMP to spot Zika. Ann Transl Med. 2017;5(24):500. https://doi.org/10.21037/atm.2017.10.19.
Huang X, Tang G, Ismail N, Wang X. Developing RT-LAMP assays for rapid diagnosis of SARS-CoV-2 in saliva. EBioMedicine. 2021;75:103736. https://doi.org/10.1016/j.ebiom.2021.103736.
Bwogi J, Lutalo T, Tushabe P, Bukenya H, Eliku JP, Ssewanyana I, et al. Field evaluation of the performance of seven Antigen Rapid diagnostic tests for the diagnosis of SARs-CoV-2 virus infection in Uganda. PLoS ONE. 2022;17(5):e0265334. https://doi.org/10.1371/journal.pone.0265334.
Nalumansi A, Lutalo T, Kayiwa J, Watera C, Balinandi S, Kiconco J, et al. Field evaluation of the performance of a SARS-CoV-2 antigen rapid diagnostic test in Uganda using nasopharyngeal samples. Int J Infect Dis. 2021;104:282–6. https://doi.org/10.1016/j.ijid.2020.10.073.
Rahman MM, Hoque AF, Karim Y, Kawser Z, Siddik AB, Sumiya MK, et al. Clinical evaluation of SARS-CoV-2 antigen-based rapid diagnostic test kit for detection of COVID-19 cases in Bangladesh. Heliyon. 2021;7(11):e08455. https://doi.org/10.1016/j.heliyon.2021.e08455.
Randriamahazo TR, Andrianarivelo AM, Rakotoarivo AT, Raheritiana TM, Rakotovao LA, Randriamanantany ZA, et al. Evaluation of antigen-based rapid detection test for the diagnosis of SARS CoV-2 in low-income countries. J Virol Methods. 2021;300:114409. https://doi.org/10.1016/j.jviromet.2021.114409.
Sazed SA, Kibria MG, Hossain MS, Zamil MF, Adhikary PC, Hossain ME, et al. Clinical evaluation of a new antigen-based COVID-19 rapid diagnostic test from symptomatic patients. Diagnostics (Basel). 2021. https://doi.org/10.3390/diagnostics11122300.
Shrestha B, Neupane AK, Pant S, Shrestha A, Bastola A, Rajbhandari B, et al. Sensitivity and specificity of lateral flow antigen test kits for COVID-19 in asymptomatic population of quarantine centre of Province 3. Kathmandu Univ Med J (KUMJ). 2021;18(70 2020 COVID-19 SPECIAL ISSUE):36–9.
Sitoe N, Sambo J, Nguenha N, Chilaule J, Chelene I, Loquiha O, et al. Performance evaluation of the STANDARD™ Q COVID-19 and Panbio™ COVID-19 antigen tests in detecting SARS-CoV-2 during high transmission period in mozambique. Diagnostics (Basel). 2022. https://doi.org/10.3390/diagnostics12020475.
Baker OR, Grabowski MK, Galiwango RM, Nalumansi A, Serwanga J, Clarke W, et al. Differential performance of CoronaCHEK SARS-CoV-2 lateral flow antibody assay by geographic origin of samples. J Clin Microbiol. 2021;59(7):e0083721. https://doi.org/10.1128/JCM.00837-21.
Lutalo T, Nalumansi A, Olara D, Kayiwa J, Ogwang B, Odwilo E, et al. Evaluation of the performance of 25 SARS-CoV-2 serological rapid diagnostic tests using a reference panel of plasma specimens at the Uganda Virus Research Institute. Int J Infect Dis. 2021;112:281–7. https://doi.org/10.1016/j.ijid.2021.09.020.
Mboumba Bouassa RS, Pere H, Tonen-Wolyec S, Longo JD, Moussa S, Mbopi-Keou FX, et al. Unexpected high frequency of unspecific reactivities by testing pre-epidemic blood specimens from Europe and Africa with SARS-CoV-2 IgG-IgM antibody rapid tests points to IgM as the Achilles heel. J Med Virol. 2021;93(4):2196–203. https://doi.org/10.1002/jmv.26628.
Sisay A, Tesfaye A, Desale A, Ataro I, Woldesenbet Z, Nigusse B, et al. Diagnostic performance of SARS-CoV-2 IgM/IgG rapid test kits for the detection of the novel coronavirus in Ethiopia. J Multidiscip Healthc. 2021;14:171–80. https://doi.org/10.2147/JMDH.S290711.
Lou B, Li TD, Zheng SF, Su YY, Li ZY, Liu W, et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur Respir J. 2020. https://doi.org/10.1183/13993003.00763-2020.
Xiao AT, Gao C, Zhang S. Profile of specific antibodies to SARS-CoV-2: the first report. J Infect. 2020;81(1):147–78. https://doi.org/10.1016/j.jinf.2020.03.012.
Traugott M, Aberle SW, Aberle JH, Griebler H, Karolyi M, Pawelka E, et al. Performance of severe acute respiratory syndrome coronavirus 2 antibody assays in different stages of infection: comparison of commercial enzyme-linked immunosorbent assays and rapid tests. J Infect Dis. 2020;222(3):362–6. https://doi.org/10.1093/infdis/jiaa305.
Emmerich P, Murawski C, Ehmen C, von Possel R, Pekarek N, Oestereich L, et al. Limited specificity of commercially available SARS-CoV-2 IgG ELISAs in serum samples of African origin. Trop Med Int Health. 2021;26(6):621–31. https://doi.org/10.1111/tmi.13569.
Woodford J, Sagara I, Dicko A, Zeguime A, Doucoure M, Kwan J, et al. SARS-CoV-2 seroassay performance and optimization in a population with high background reactivity in Mali. J Infect Dis. 2021. https://doi.org/10.1093/infdis/jiab498.
Yadouleton A, Sander AL, Moreira-Soto A, Tchibozo C, Hounkanrin G, Badou Y, et al. Limited specificity of serologic tests for SARS-CoV-2 antibody detection. Benin Emerg Infect Dis. 2021;27(1):233–7. https://doi.org/10.3201/eid2701.203281.
Abcam. SARS-CoV-2 IgG ELISA Kit (ab275300) key features and details 2022 [cited 18 Nov 2022]. https://www.abcam.com/sars-cov-2-igg-elisa-kit-ab275300.html?productWallTab=ShowAll. Accessed 23 Jan 2023.
Antibodies-online. SARS-CoV-2 IgG Antibody ELISA Kit Product Details 2022 [cited 18 Nov 2022]. https://www.antibodies-online.com/kit/6952525/SARS-CoV-2+IgG+Antibody+ELISA+Kit/. Accessed 23 Jan 2023.
Ramachandran S, Singhal M, McKenzie KG, Osborn JL, Arjyal A, Dongol S, et al. A rapid, multiplexed, high-throughput flow-through membrane immunoassay: a convenient alternative to ELISA. Diagnostics (Basel). 2013;3(2):244–60. https://doi.org/10.3390/diagnostics3020244.
Haq MA, Jamiruddin M, Khondoker MU, Ahmed MF, Khandker SS, Ali T, et al. Assessment of a rapid pan-antibody dot test for detection of antibodies against SARS-CoV-2. Bangladesh J Med Sci. 2021;20(5):131–9. https://doi.org/10.3329/bjms.v20i5.55407.
Sil BK, Jamiruddin MR, Haq MA, Khondoker MU, Jahan N, Khandker SS, et al. AuNP coupled rapid flow-through dot-blot immuno-assay for enhanced detection of SARS-CoV-2 specific nucleocapsid and receptor binding domain IgG. Int J Nanomedicine. 2021;16:4739–53. https://doi.org/10.2147/IJN.S313140.
Jaroenram W, Chatnuntawech I, Kampeera J, Pengpanich S, Leaungwutiwong P, Tondee B, et al. One-step colorimetric isothermal detection of COVID-19 with AI-assisted automated result analysis: a platform model for future emerging point-of-care RNA/DNA disease diagnosis. Talanta. 2022;249:123375. https://doi.org/10.1016/j.talanta.2022.123375.
Adam MH, Mohamoud JH, Mohamood AS, Mohamed AA, Garba B, Dirie NI. Seroprevalence of anti-SARS-CoV-2 antibodies in Benadir Region, Somalia. Vaccines (Basel). 2022. https://doi.org/10.3390/vaccines10020220.
Osaigbovo II, Igbarumah IO, Muoebonam EB, Obaseki DE. Setting up a molecular diagnostic laboratory for SARS-CoV-2 testing: Experience of a single centre in a resource-constrained setting. Afr J Lab Med. 2021;10(1):1326. https://doi.org/10.4102/ajlm.v10i1.1326.
Gartner K, Meleke H, Kamdolozi M, Chaima D, Samikwa L, Paynter M, et al. A fast extraction-free isothermal LAMP assay for detection of SARS-CoV-2 with potential use in resource-limited settings. Virol J. 2022;19(1):77. https://doi.org/10.1186/s12985-022-01800-7.
Xing W, Wang J, Zhao C, Wang H, Bai L, Pan L, et al. A highly automated mobile laboratory for on-site molecular diagnostics in the COVID-19 pandemic. Clin Chem. 2021;67(4):672–83. https://doi.org/10.1093/clinchem/hvab027.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Conflicts of Interest
Yuh Ping Chong, Kay Weng Choy, Christian Doerig, and Chiao Xin Lim report no conflicts of interest relevant to this manuscript.
Ethics approval
Not applicable.
Approval for participation & publication
Not applicable.
Data availability
Data availability is not applicable to this article as no new experimental results were created in this study.
Code availability
Not applicable.
Author Contributions
Conception and design: YPC, KWC, CXL. Administrative support: None. Provision of study materials or patients: None. Collection and assembly of data: None. Data analysis and interpretation: YPC, CXL. Manuscript writing: All authors. Final approval of the manuscript: All authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chong, Y.P., Choy, K.W., Doerig, C. et al. SARS-CoV-2 Testing Strategies in the Diagnosis and Management of COVID-19 Patients in Low-Income Countries: A Scoping Review. Mol Diagn Ther 27, 303–320 (2023). https://doi.org/10.1007/s40291-022-00637-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-022-00637-8