Abstract
Background
Clinically validated biomarkers for monitoring of patients with complement-mediated thrombotic microangiopathy (CM-TMA) including atypical hemolytic uremic syndrome (aHUS) are unavailable. Improved characterization of biomarkers in patients with aHUS may inform treatment and monitoring for patients with CM-TMA.
Methods
This analysis used data collected from 55/56 (98.2 %) adult patients with aHUS enrolled in the global Phase III study of ravulizumab (NCT02949128). Baseline (pre-treatment) patient serum, plasma and urine biomarker levels were compared with the maximum observed levels in normal donors and evaluated for associations with pre-treatment plasma exchange/infusion and dialysis status. Biomarkers were also assessed for associations with key clinical measures at baseline and with changes at 26 and 52 weeks from treatment initiation via linear regression analyses.
Results
Complement-specific urine levels (factor Ba and sC5b-9) were elevated in >85 % of patients and are significantly associated with pre-treatment kidney dysfunction. Baseline levels of other evaluated biomarkers were elevated in >70 % of patients with aHUS, except for plasma sC5b-9 and serum sVCAM-1. Lower levels of urine complement markers at baseline are significantly associated with improvements in total urine protein and estimated glomerular filtration rate at 26 and 52 weeks of treatment. Clinical assessment of complement activation by a receiver operating characteristic analysis of Ba and sC5b-9 was more sensitive and specific in urine matrix than plasma.
Conclusion
This analysis identified a set of biomarkers that may show utility in the prognosis of CM-TMA, including their potential for measuring and predicting response to anti-C5 therapy. Further studies are required to enhance patient risk stratification and improve management of these vulnerable patients.
Clinical Trials Registration
NCT02949128, ClinicalTrials.gov.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There is an unmet need for validated biomarkers for determining prognosis and monitoring of patients with CM-TMA. |
Measurements of complement factor Ba and sC5b-9 demonstrate clinical utility in characterizing patients with CM-TMA/aHUS. |
sTNF-RI and thrombomodulin—soluble biomarkers shed from the vascular surface following activation/damage—together with factor Ba, demonstrate potential clinical prognostic utility. |
1 Introduction
Thrombotic microangiopathy (TMA) is a broad clinical term encompassing conditions presenting as the triad of thrombocytopenia, microangiopathic hemolytic anemia, and microvascular thrombosis, typically leading to organ damage/failure [1, 2]. Complement-mediated TMA (CM-TMA) is a subset of TMA disorders driven or exacerbated by complement pathway overactivation with or without presence of complement gene mutations and acquired autoantibodies [2,3,4,5,6]. Atypical hemolytic uremic syndrome (aHUS), often considered the prototypical form of CM-TMA, is caused by dysregulation and overactivation of the alternative pathway of complement, leading to excessive terminal complement activation [4,7, 8]. The complement cascade, including the production of split products, is depicted in Fig. 1.
Despite increasing understanding of the pathophysiology of CM-TMA/aHUS, several clinical challenges exist relating to its differential diagnosis and prognosis. One such challenge is a lack of sensitive, specific, and clinically validated biomarkers that can characterize this rare and heterogenous disease population [2, 7, 8]. While there are some tests and assessments that have been evaluated for clinical utility in this population [9,10,11,12], as described by Fakhouri et al, important methodologic, procedural, scalability and interpretability concerns suggest that further improvement is needed to produce a robust but simple test which does not require specialist laboratories or equipment [13, 14]. Therefore, continued exploration of novel matrices, sample collection protocols, and bioanalytical assays are needed to identify and accurately measure individual and/or combinations of clinically meaningful biomarkers in patients with CM-TMA/aHUS. Identification of a biomarker ‘signature’ in patients with CM-TMA may help inform treatment decisions and therapeutic monitoring of patients with other forms of TMA [13, 15, 16].
The study from Cofiell et al explored the pharmacodynamic effect of complement inhibition – via treatment with the anti-complement C5 monoclonal antibody (mAb) eculizumab – on markers of multiple cellular processes and physiologic pathways seemingly initiated in patients with aHUS, including complement activation, inflammation, vascular/endothelial damage, thrombosis, and renal injury [17,18,19,20,21]. The study found that levels of a subset of these markers were elevated in the majority of patients with aHUS (>70 %) and that, following treatment with eculizumab, the majority returned to normal or near-normal levels when compared to normal donor samples [17].
Using data from patients enrolled in a clinical study of ravulizumab, a long-acting complement C5 inhibitor, the current analysis was designed to evaluate the clinical utility of a subset of these biomarkers. The markers assessed in this study include: soluble vascular cell adhesion molecule 1 (sVCAM-1), released following activation of endothelial cells; soluble thrombomodulin, released following endothelial damage; soluble tumor necrosis factor receptor I (sTNF-RI), a surrogate marker for the pro-inflammatory cytokine tumor necrosis factor alpha (TNFα), released during inflammation; D-dimer, a component of the coagulation cascade released following fibrin clot degradation; complement factor Ba, a cleavage fragment of factor B in the complement cascade and a key indicator of alternative pathway activation; cystatin-C, a soluble marker of renal proximal tubular injury; and soluble C5b-9 (sC5b-9), the soluble form of the terminal effector of complement pathway activation, also known as the membrane attack complex (MAC) [17]. The panel of biomarkers was assessed in a novel combination of blood and/or urine, collected and assayed according to stringent protocols, and the data assessed for clinical correlations to characterize the complement activity signature more accurately in patients with CM-TMA and predict clinical response to C5 blockade.
2 Methods
This was an exploratory analysis based on a prospective biomarker collection and testing strategy designed into the clinical protocol of the Phase III study of ravulizumab in adults with aHUS (NCT02949128). The protocol for this study was approved by the Institutional Review Board or Independent Ethics Committee at each participating center, and the study was conducted in accordance with the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines. The primary and secondary endpoints from the trial have been published previously [22, 23]. Complete TMA response was the primary endpoint, defined as platelet count normalization (≥150 × 109/L), lactate dehydrogenase (LDH) normalization (≤246 U/L), and 25 % or better improvement in serum creatinine (Cr) from baseline. Patients had to meet all TMA criteria concurrently, and each criterion had to be met at two separate assessments obtained at least 28 days apart, and at any measurement in between. In the 26-week initial evaluation period, 30 of 56 (53.6 %) patients achieved complete TMA response. The individual components were achieved by 83.9 %, 76.8 %, and 58.9 % of patients, respectively, and improvement in eGFR by one or more stage was achieved in 68.1 % of patients [22]. Four additional patients achieved complete TMA response in the 52-week extension period (n/N = 34/56; 61 %) [23].
The current exploratory endpoint analysis assessed the potential prognostic utility of blood and urinary biomarkers of complement activation, vascular damage and inflammation, and renal injury in adults with CM-TMA/aHUS. We assessed longitudinal changes in biomarker levels from baseline following ravulizumab treatment for the first 52-week period of the study (initial 26-week evaluation followed by a 26-week extension). All biomarker data were generated in labs adhering to Good Laboratory Practices (GLP) with biomarker assays developed following industry guidelines and validated according to the US Food and Drug Administration (FDA) biomarker guidance [24, 25]. Biomarkers of interest were collected and/or processed to the following matrices for testing: serum for sTNF-RI and sVCAM-1 was collected using a Becton–Dickinson (BD) SST® vacutainer; plasma for Ba, sC5b-9, and thrombomodulin was collected using a BD P100® vacutainer; citrated plasma for D-dimer was collected using a BD vacutainer container 3.2 % sodium citrate solution; and spot urine collections for Ba, sC5b-9, and cystatin-C were collected in a proprietary concentrated cryo-preservative containing protease inhibitor. Urine spot collections were performed under exploratory conditions of random time of day and void volume. To control for these variations in urine collection, urinary biomarkers were normalized against urine creatinine (Cr) concentration. All normal donor serum, plasma, and urine were prospectively collected and stored according to the study protocol. Normal donors were defined as an adult (≥18 years) human, personal information de-identified, and self-reported as healthy per an institutional IRB-approved protocol. All matrices/samples collected were for research use only. All bioanalytical methods were either commercially available or custom solid-phase ligand-binding immunoassays optimized to minimize pre-analytical variability. The long-term storage stability of quality control samples yielded acceptable limits that exceeded the time between collection and testing for all aHUS study samples. A full list of biomarker analysis assays used to test samples collected in this study can be found in the supplementary materials.
2.1 Statistical Analysis
Baseline serum, plasma, and urine biomarker levels in patients with aHUS – assessed prior to treatment initiation – were compared with biomarker levels obtained from normal donor samples; normal serum and plasma samples were obtained from Alexion in-house donors, BioIVT (Westbury, NY, USA) and Sanguine Biosciences (Waltham, MA, USA); and normal urine samples were obtained from Alexion in-house donors. Statistical analysis was performed using SAS V9.4 (SAS Institute Inc., Cary, NC, USA).
Differences between baseline biomarker levels in adults with aHUS and normal donors were assessed via use of the Wilcoxon rank sum test for differences between groups. The distribution of each biomarker was explored and a log2 transformation was used to achieve normality prior to inclusion in statistical models. Samples with biomarker levels below the limit of assay quantitation were assigned a value equal to one-half times the lower limit of quantitation for that assay. For urine biomarkers normalized to Cr, at least one quantifiable result was required for the normalized result to be included in the analysis. Longitudinal biomarker levels and changes in levels from baseline following treatment with ravulizumab, were evaluated using a mixed model for repeated measures (MMRM) analysis, with log-transformed biomarker level as the dependent variable, and fixed categorical effect of visit and fixed continuous effect of log-transformed baseline level as a covariate.
Baseline biomarkers were assessed in the context of plasma exchange (PE)/plasma infusion (PI) and dialysis status at baseline. Baseline biomarkers were also evaluated for associations with key clinical measures of TMA—platelet count, LDH concentration, estimated glomerular filtration rate (eGFR), and urine protein/creatinine ratio (UPCR)—via Spearman correlation coefficients between baseline biomarker levels and baseline clinical measures, and between baseline biomarker levels and change in clinical measures at 26 and 52 weeks from treatment initiation. Simple linear regression analyses were also performed, with both the log-transformed baseline clinical measure as the dependent variable and the log-transformed baseline biomarker level as the independent variable, as well as with the 26- and 52-week changes from baseline in clinical variable as the dependent variable and the log-transformed baseline biomarker level as the independent variable. For associations with baseline clinical measures, every 2-fold increase in baseline biomarker results in an increase (or decrease) in the lab value by a factor of two raised to the regression coefficient. When assessing associations with the change in clinical measures at 26 and 52 weeks, every 2-fold increase in baseline biomarker results in the change in lab value increasing (or decreasing) by the regression coefficient. A two-sided t test was used to test for significance of the regression coefficient.
Results were stratified by complete TMA response status (defined as: normalization of platelet count, normalization of LDH, and ≥25 % improvement in serum Cr from baseline, met concurrently at two separate assessments, at least 4 weeks apart) at 26 and 52 weeks.
Logistic regression analysis was performed to assess any relationship between identified biomarkers at baseline and complete TMA response. Patients were included in this analysis set if the endpoint of complete TMA response was confirmed by assessment at 26 and 52 weeks. For the logistic regression analysis, clinical response was set as the dependent variable with the log of the baseline biomarker level as the independent variable. Odds ratios (ORs) and 95 % confidence intervals (CI) were reported, with ORs representing the increased (or decreased) odds of achieving the efficacy response for every 2-fold increase in baseline biomarker; statistical significance of the predictor was assessed via Wald testing.
Baseline levels of Ba and sC5b-9, in urine and plasma, from aHUS patients and normal donors, were input into CombiROC (an online tool to help researchers identify the optimal combination of markers and threshold setting through a simple analytical method) to generate receiver operating characteristic (ROC) curves for analysis of sensitivity and specificity [26, 27].
3 Results
3.1 Baseline Demographics
A total of 56 adults with aHUS were enrolled in this clinical trial, 55 of whom were included in the current biomarker analyses; patient demographics and disease characteristics are outlined in Table 1. The median age at the time of first infusion was 40.1 years, and the majority were female (66 %) and White (52 %).
3.2 Baseline Biomarker Level Comparison Between Patients with aHUS and Normal Donors
A comparison of baseline biomarker levels in adult patients with aHUS and normal donors is presented in Table 2. In urine, creatinine-normalized levels of the proximal tubular injury marker cystatin-C, complement alternative pathway-specific marker factor Ba, and terminal pathway-specific marker sC5b-9 were elevated in >85 % of patients compared with levels in normal donors. In plasma, endothelial cell damage marker thrombomodulin, coagulation marker D-dimer and factor Ba levels were elevated in >70 % of patients but sC5b-9 levels were elevated in only 21 % of patients compared with levels in normal donors. In serum, inflammation marker sTNF-RI levels were elevated in 96 % of patients but endothelial cell activation marker sVCAM-1 levels were elevated in only 9 % of patients compared with normal donors. In summary, most biomarkers were elevated in most patients with aHUS, with the exception of plasma sC5b-9 and serum sVCAM-1.
3.3 Longitudinal Changes in Biomarkers from Baseline Following Treatment with Ravulizumab
In ravulizumab-treated adults with aHUS, a pharmacodynamic effect was observed for both blood and urine biomarkers (Fig. 2), including significant reductions in normalized urine sC5b-9/Cr, Ba/Cr and cystatin-C/Cr levels compared with baseline at all collection timepoints (Fig. 2B). Serum sTNF-RI and plasma D-dimer levels also decreased significantly from baseline levels until day 127 (Fig. 2A).
Violin plots of biomarker observed levels over time to 52 weeks. A Biomarkers in blood. B Biomarkers in urine. Ravulizumab dose was determined by bodyweight and given at a loading dose at baseline, dose 2 at Day 15, maintenance dose at Day 71 and once every 8 weeks thereafter. Horizontal lines represent the 25 %, median and 75 % quartiles. P-values are calculated from a mixed model for repeated measures analysis with biomarker as dependent variable, and fixed categorical effect of visit and fixed continuous effect of baseline value as covariate. The null hypothesis that the mean change from baseline equals zero was tested against the alternative hypothesis that the mean change does not equal zero
3.4 Baseline Biomarker Associations with Pre-Treatment Plasma Exchange/Plasma Infusion (PE/PI) and Dialysis
When baseline biomarkers were assessed for any associations with pre-treatment PE/PI status, patients who received PE/PI within 7 days of the first infusion of ravulizumab had significantly lower plasma sC5b-9 levels compared with patients who did not (Supplementary Table 1). Patients who received dialysis within 5 days of treatment initiation had significantly higher plasma Ba, serum sTNF-RI, and urine Ba/Cr than patients who did not (Supplementary Table 2). No other baseline biomarker levels were associated with PE/PI status (Supplementary Tables 1 and 2).
3.5 Baseline Biomarker Associations with Baseline Clinical Measures
Increased plasma Ba, plasma thrombomodulin, and serum sTNF-RI were all significantly associated with lower baseline eGFR and higher baseline UPCR values (Table 3). Urine cystatin-C/Cr, sC5b-9/Cr, and Ba/Cr were also associated with lower baseline eGFR and higher UPCR values. No significant associations between any biomarker levels at baseline and baseline platelet count were identified.
3.6 Baseline Biomarker Associations with Changes in Clinical Measures
Analysis of the change in clinical measures at 26 and 52 weeks using simple regression against baseline biomarker levels found no significant associations between baseline biomarker levels and change in platelet count at either timepoint (Supplementary Table 3). Baseline urine sC5b-9/Cr levels were significantly associated with change in LDH at 26 weeks, but not at 52 weeks (Supplementary Table 4). Increased plasma Ba, thrombomodulin and sC5b-9, serum sTNF-RI and sVCAM-1, and urine cystatin-C/Cr and Ba/Cr were significantly inversely associated with the change from baseline in eGFR at both 26 and 52 weeks (Table 4 and Fig. 3). Baseline urine sC5b-9/Cr was significantly associated with change in UPCR at 52 weeks (n = 32, regression coefficient estimate 14.63, p = 0.0324, correlation coefficient 0.0528; data not shown).
Line plot of eGFR and biomarker change from baseline over time by complete TMA response status at 52 weeks. A Biomarkers in blood. B Biomarkers in urine. Change from baseline for blood and urine biomarkers were compared to the clinical measure of eGFR out to 52 weeks. The p values are presented in figure for each of the biomarkers. P-value from a linear regression analysis with the 52-week change from baseline in eGFR as the dependent variable and log(2) of the baseline biomarker level and log(2) of the 52-week biomarker level as the independent variables. eGFR estimated glomerular filtration rate, TMA thrombotic microangiopathy
Furthermore, the time and magnitude of the change for each biomarker in comparison to the change in clinical measure varied between complete responders and non-responders. For example, in complete responders, urine sC5b-9/Cr levels decreased dramatically relative to baseline by Day 71 and overall remained low despite the sharp elevated peak at Day 127 (due to a single sample, as observed in Fig. 3B); whereas in non-responders, urine sC5b-9/Cr remained unchanged through the course of treatment (Fig. 3B).
Taken together, baseline biomarker levels and changes in clinical measures showed few associations, except those associated with change in eGFR, and urine sC5b-9/Cr at 26 weeks with LDH and 52 weeks with UPCR. The extent of these changes varied between complete responders and non-responders.
3.7 Baseline Biomarker Associations with Complete TMA Response
Barbour et al 2021 evaluated the long-term efficacy and safety of ravulizumab during the extension period of the currently assessed ravulizumab study (median follow-up duration 76.7 [0.6–118.3] weeks) and observed a complete TMA response in >60 % of patients [23].
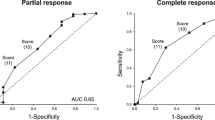
When assessed by logistic regression analysis, increased plasma thrombomodulin, serum sVCAM-1, serum sTNF-RI, and plasma Ba levels prior to treatment were found to be significantly associated with a lower likelihood of achieving complete TMA response at 52 weeks (Fig. 4).
Logistic regression analysis of complete TMA response at 52 weeks of treatment based on baseline biomarker levels. Odds ratios are derived from a logistic regression analysis with the response variable as the dependent variable and the log of the baseline biomarker level as the independent variable and represent the increased (or decreased) odds of achieving the efficacy response for every 2-fold increase in baseline biomarker. CI confidence interval, Cr creatinine, eGFR estimated glomerular filtration rate, LDH lactate dehydrogenase, TMA thrombotic microangiopathy
3.8 Clinical Sensitivity and Specificity of Complement Baseline Biomarker Levels
Individual analysis of plasma Ba, plasma sC5b-9, urine Ba/Cr and urine sC5b-9/Cr showed some overlap between normal control range and baseline patient values (Fig. 5A). Use of the combined CombiROC analysis for both Ba plus sC5b-9 values resulted in the highest clinical specificity and sensitivity in both urine and plasma matrices (Fig. 5B, C and D).
Receiver operating characteristic curves of Ba and sC5b-9 levels in patients versus NDs. A Dot plots for individual sC5b-9 and Ba levels in urine (ng/mg Cr) and plasma. B CombiROC curves for combined biomarkers in urine and plasma. C Violin plots of prediction probabilities for combined biomarkers in urine and plasma. D Summary table for combined biomarkers. Cr creatinine, FN false negative, FP false positive, ND normal donor, ROC receiver operating characteristic, TN true negative, TP true positive
By standard, individual ROC analysis, the area under the curve was considerably larger in urine than in plasma for sC5b-9 (0.72 vs 0.52) whereas for Ba it was larger in plasma (0.67 vs 0.80) (Fig. 5 and S1A). More false-positive (FP) and false-negative (FN) results occurred in plasma sC5b-9 than urine sC5b-9/Cr (32 FP, 19 FN vs 0 FP, 5 FN); however, the distributions for Ba levels in urine and plasma were very similar, with few false results (Fig. S1B).
4 Discussion
Urine Ba and sC5b-9 show potential for use as biomarkers of CM-TMA. Their nearly ubiquitous elevation in adults with aHUS compared with the maximum observed levels in normal donors means they have high specificity for CM-TMA disorders. Notably, urine sC5b-9 was completely undetectable in normal samples. Their prognostic reliability in urine, particularly in combination, was further evidenced by the CombiROC curves shown in Figure 5. However, these two biomarkers alone may not be sufficient; other clinical or biomarker measurements may be needed to develop a full understanding of individual patient profiles across all CM-TMA subsets.
Matrix was also relevant to other sC5b-9 associations. Baseline levels of urine sC5b-9 were found to be elevated over normal donor levels to a much greater extent than plasma sC5b-9 levels. Additionally, baseline urine but not plasma sC5b-9 was significantly associated with change in UPCR at 52 weeks, and urine but not plasma sC5b-9 levels decreased significantly over the entire 52 weeks of treatment. These observations, along with individual marker ROC analyses (Fig. S1) of sC5b-9 in urine versus plasma, point to urine being the more clinically meaningful matrix. Understanding the differential utility of biomarkers in different matrices is particularly important, as identification of optimized, non-invasive assessments of complement activity, which do not rely on complex, ex vivo assays, and analyses remains a key goal in this patient population.
Further differences, including matrix-dependent differences, were also observed between markers. For example, factor Ba and sC5b-9 in plasma were found to be significantly associated with PE/PI or dialysis status prior to treatment with ravulizumab, while baseline plasma but not urine sC5b-9 was associated with patients on PE/PI within 7 days of treatment initiation but not with dialysis treatment. In contrast, baseline plasma and urine Ba were significantly associated with dialysis status within the 5 days prior to treatment initiation but not with the use of PE/PI (Supplementary Table 1). Although plasma sC5b-9 has previously been proposed as a potential biomarker of interest for CM-TMA, and indeed appears to show substantial utility in conditions such as hematopoietic stem cell transplant-associated TMA, this analysis found it less informative [28]. Other studies have also reported conflicting results around its utility. For example, Noris et al have previously reported that plasma sC5b-9 is not suitable as a biomarker of aHUS based on an analysis of their cohort of patients; while both Volokhina et al and Cataland et al found that plasma sC5b-9 was elevated in all patients with acute aHUS and advocated for its use [12, 29,30,31]. A 2015 study by Bu et al, suggested that plasma sC5b-9 had a very high positive predictive rate (89 %) but a comparatively low negative predictive rate (46 %) in their cohort of patients with aHUS, concluding that plasma sC5b-9 levels are indicative of active disease, but should not be used in isolation [29]. Bu et al also noted the large inconsistencies in plasma sC5b-9 assay results across the studies of Noris, Volkohina and Cataland; and highlighted potential discrepancies in aHUS diagnostic criteria, specimen collection and storage protocols, and different detection kits and methodologies as possible reasons for the substantially different results and conclusions recorded by these groups [29]. Given the discordant results and interpretations from the literature, combined with our results discussed above, plasma sC5b-9 levels are generally less significantly associated with clinical measures and outcomes of CM-TMA/aHUS than urinary sC5b-9 [12, 29,30,31].
The results of these analyses support the use of urinary sC5b-9 as a potential biomarker of CM-TMA/aHUS. The results are aligned with the overall conclusions of Bu et al, suggesting that the utility of sC5b-9 assessments may be heightened when combined with other markers of TMA disease or complement dysfunction. Prime candidates from this study include Ba, sTNF-RI, sVCAM-1, and thrombomodulin as identified in the logistic regression response plot (Fig. 4) for predicting complete CM-TMA response [29]. Further analysis may be warranted to determine the pharmacodynamic effect, if any, of complement C5 inhibitor treatment on sC5b-9 levels and/or half-life measurements in both plasma and urine samples.
As discussed, this study was designed to build on the work conducted by Cofiell et al, who evaluated a panel of biomarkers and identified a subset which were elevated at baseline in the majority of patients with aHUS who were subsequently treated with eculizumab [17]. While the patients in this study all received ravulizumab, the assessed biomarkers showed similar, although not identical, patterns of baseline elevation and response to treatment across both studies [17]. This is not unexpected, as eculizumab and ravulizumab have both been shown to be potent terminal complement inhibitors [32]. However, there are significant methodologic differences between the ravulizumab and eculizumab clinical study designs, such as differences in dosing frequencies and the use of weight-based dosing only in the ravulizumab trial. These differences, coupled with the inherently heterogenous presentation and progression of aHUS in these two patient populations, complicate any formal statistical comparisons of biomarker levels at baseline or during treatment.
Limitations to this study include the relatively small cohorts of both normal donors and patients with CM-TMA/aHUS and that not all patients consented to optional genetic testing, which further limited the power and feasibility of assessing any possible associations between pathogenic genetic variants and the evaluated biomarkers. Another challenge is the labile nature of the complement cascade and potential for ex vivo autoactivation. However, this is one of the first studies to prospectively design the assessment of a specific panel of blood and urine biomarkers in patients with CM-TMA/aHUS, with a particular focus on their potential clinical utility as prognostic markers and predictors of treatment response.
5 Conclusion
The results of this analysis highlight a key set of biomarkers with potential prognostic utility in the management of CM-TMA/aHUS, particularly when measured in urine. These data also demonstrate changes in biomarker levels in response to anti-complement C5 therapy and their potential utility for predicting treatment response. While it would be of significant interest to compare the assessed biomarkers with the various clinical manifestations of CM-TMA in other disease settings, this was not within the scope of the current study. Further assessment and validation of these biomarkers in larger patient populations and in patients with other forms of TMA are needed to guide patient risk stratification and clinical management decisions; prospective acquisition and assessment of samples from patients with other forms of CM-TMA is currently ongoing. This analysis also highlights the need for stringent sample collection, storage, and optimized methodology to reduce pre-analytical variability, allowing for more accurate measurement of biomarker levels to identify patients with complement activity above the range of normal donor levels.
Change history
04 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40291-023-00643-4
References
Arnold DM, Patriquin CJ, Nazy I. Thrombotic microangiopathies: a general approach to diagnosis and management. CMAJ. 2017;189(4):E153–9.
Gavriilaki E, Anagnostopoulos A, Mastellos DC. Complement in thrombotic microangiopathies: unraveling Ariadne’s thread into the labyrinth of complement therapeutics. Front Immunol. 2019;10:337.
Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361(17):1676–87.
Raina R, Krishnappa V, Blaha T, Kann T, Hein W, Burke L, et al. Atypical hemolytic-uremic syndrome: an update on pathophysiology, diagnosis, and treatment. Ther Apher Dial. 2019;23(1):4–21.
Asif A, Nayer A, Haas CS. Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment with eculizumab. J Nephrol. 2017;30(3):347–62.
Lemaire M, Noone D, Lapeyraque A-L, Licht C, Frémeaux-Bacchi V. Inherited kidney complement diseases. Clin J Am Soc Nephrol. 2021;16(6):942.
Thurman JM, Nester CM. All things complement. Clin J Am Soc Nephrol CJASN. 2016;11(10):1856–66.
Frazer-Abel A, Kirschfink M, Prohászka Z. Expanding horizons in complement analysis and quality control. Front Immunol. 2021;12:697313.
Galbusera M, Noris M, Gastoldi S, Bresin E, Mele C, Breno M, et al. An ex vivo test of complement activation on endothelium for individualized eculizumab therapy in hemolytic uremic syndrome. Am J Kidney Dis. 2019;74(1):56–72.
Palomo M, Blasco M, Molina P, Lozano M, Praga M, Torramade-Moix S, et al. Complement activation and thrombotic microangiopathies. Clin J Am Soc Nephrol CJASN. 2019;14(12):1719–32.
Gavriilaki E, Yuan X, Ye Z, Ambinder AJ, Shanbhag SP, Streiff MB, et al. Modified Ham test for atypical hemolytic uremic syndrome. Blood. 2015;125(23):3637–46.
Noris M, Galbusera M, Gastoldi S, Macor P, Banterla F, Bresin E, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124(11):1715–26.
Fakhouri F, Frémeaux-Bacchi V. Monitoring complement activation: the new conundrum in thrombotic microangiopathies. Clin J Am Soc Nephrol CJASN. 2019;14(12):1682–3.
Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Fremeaux-Bacchi V, Kavanagh D, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91(3):539–51.
Raina R, Sethi SK, Dragon-Durey MA, Khooblall A, Sharma D, Khandelwal P, et al. Systematic review of atypical hemolytic uremic syndrome biomarkers. Pediatr Nephrol. 2022;37(7):1479–93.
Blasco M, Guillén E, Quintana LF, Garcia-Herrera A, Piñeiro G, Poch E, et al. Thrombotic microangiopathies assessment: mind the complement. Clin Kidney J. 2020;14(4):1055–66.
Cofiell R, Kukreja A, Bedard K, Yan Y, Mickle AP, Ogawa M, et al. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood. 2015;125(21):3253–62.
Waters AM, Licht C. aHUS caused by complement dysregulation: new therapies on the horizon. Pediatr Nephrol. 2011;26(1):41–57.
Cataland SR, Wu HM. How I treat: the clinical differentiation and initial treatment of adult patients with atypical hemolytic uremic syndrome. Blood. 2014;123(16):2478–84.
Weitz IC. Complement the hemostatic system: an intimate relationship. Thromb Res. 2014;133(Suppl 2):S117–21.
Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177(7):4794–802.
Rondeau E, Scully M, Ariceta G, Barbour T, Cataland S, Heyne N, et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2020;97(6):1287–96.
Barbour T, Scully M, Ariceta G, Cataland S, Garlo K, Heyne N, et al. Long-term efficacy and safety of the long-acting complement C5 inhibitor ravulizumab for the treatment of atypical hemolytic uremic syndrome in adults. Kidney Int Rep. 2021;6(6):1603–13.
Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res. 2006;23(2):312–28.
US Food and Drug Administration. Biomarker Qualification: Evidentiary Framework Guidance for Industry and FDA Staff. Draft Guidance. 2018; https://www.fda.gov/media/122319/download. Accessed 15 Feb 2022.
Mazzara S, Rossi RL, Grifantini R, Donizetti S, Abrignani S, Bombaci M. CombiROC: an interactive web tool for selecting accurate marker combinations of omics data. Sci Rep. 2017;7(1):45477.
Protein Microarray and Bioinformatics Facilities INGMReEII, Milan, Italy. CombiROC. 2017; V1.2; last updated 10/02/2017]; V1.2: http://combiroc.eu/. Accessed 29 Mar 2022.
Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM, et al. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29(3):191–204.
Bu F, Meyer NC, Zhang Y, Borsa NG, Thomas C, Nester C, et al. Soluble c5b–9 as a biomarker for complement activation in atypical hemolytic uremic syndrome. Am J Kidney Dis. 2015;65(6):968–9.
Volokhina EB, Westra D, van der Velden TJAM, van de Kar NCAJ, Mollnes TE, van den Heuvel LP. Complement activation patterns in atypical haemolytic uraemic syndrome during acute phase and in remission. Clin Exp Immunol. 2015;181(2):306–13.
Cataland SR, Holers VM, Geyer S, Yang S, Wu HM. Biomarkers of terminal complement activation confirm the diagnosis of aHUS and differentiate aHUS from TTP. Blood. 2014;123(24):3733–8.
Tomazos I, Hatswell AJ, Cataland S, Chen P, Freemantle N, Lommele Å, et al. Comparative efficacy of ravulizumab and eculizumab in the treatment of atypical hemolytic uremic syndrome: An indirect comparison using clinical trial data. Clin Nephrol. 2022;97(5):261–72.
Acknowledgments
This analysis was funded by Alexion, AstraZeneca Rare Disease, Boston, MA. Alexion, AstraZeneca Rare Disease, Boston, MA was responsible for the collection, management, and analysis of information contained in the study. Alexion, AstraZeneca Rare Disease contributed to data interpretation, preparation, review, and approval of the manuscript for submission. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication. The sponsor and investigators thank the patients and their families for their participation in, and support for, this clinical study. The authors would like to acknowledge Alexander T. Hardy, PhD, Helen Bristow, BSc, and Kate Booth, PGCE, of Bioscript, Macclesfield, UK for providing medical writing support with funding from Alexion, AstraZeneca Rare Disease; Radha Narayan, PhD, of Alexion, AstraZeneca Rare Disease, for critical review of the manuscript, and Jimmy Wang and John Kaye, of Alexion, AstraZeneca Rare Disease, for their contributions to the statistical analyses of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was wholly funded by Alexion, AstraZeneca Rare Disease, Boston, MA, USA, and did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Consent to participate
As described in the original study publication.
Consent to publish
As described in the original study publication.
Ethics approval
As described in the original study publication.
Conflicts of interest
All authors are employees and stockholders of Alexion, AstraZeneca Rare Disease, Boston, MA, USA.
Author contributions
All authors met ICMJE authorship criteria, contributed to the study conception and design, material preparation, data collection, critical review of the draft manuscript and approval of the final manuscript.
Data availability
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level, deidentified clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability, and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cammett, T.J., Garlo, K., Millman, E.E. et al. Exploratory Prognostic Biomarkers of Complement-Mediated Thrombotic Microangiopathy (CM-TMA) in Adults with Atypical Hemolytic Uremic Syndrome (aHUS): Analysis of a Phase III Study of Ravulizumab. Mol Diagn Ther 27, 61–74 (2023). https://doi.org/10.1007/s40291-022-00620-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-022-00620-3