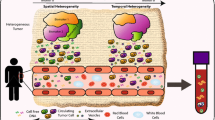
Abstract
The global liquid biopsy industry is expected to exceed $US5 billion by 2023. One application of liquid biopsy technology is the diagnosis of disease using biomarkers found in blood, urine, stool, saliva, and other biological samples from patients. These biomarkers could be DNA, RNA, protein, or even a cell. More recently, the use of cell-free DNA from plasma is emerging as an important minimally invasive tool for clinical diagnosis. The development of technology has increased the diversity of its application. Here, we discuss how liquid biopsies have been used in the clinic, and how personalized medicine are likely to use liquid biopsies in the near future.
Similar content being viewed by others
Change history
17 February 2020
In the original publication of the article, the name of the fourth author, which previously read "Howard Chan”, should read as "Howard Chang". The original article has been updated.
References
Reportlinker. New York. https://www.prnewswire.com/news-releases/global-liquid-biopsy-market-outlook-to-2020-300220260.html (2016).
Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531.
Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146.
Krebs MG, Hou JM, Ward TH, Blackhall FH, Dive C. Circulating tumour cells: their utility in cancer management and predicting outcomes. Ther Adv Med Oncol. 2010;2:351–65. https://doi.org/10.1177/1758834010378414.
Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4:650–61.
Neumann MHD, Bender S, Krahn T, Schlange T. ctDNA and CTCs in liquid biopsy—current status and where we need to progress. Comput Struct Biotechnol J. 2018;16:190–5.
Bidard F-C, Michiels S, Riethdorf S, Mueller V, Esserman LJ, Lucci A, Naume B, Horiguchi J, Gisbert-Criado R, Sleijfer S, et al. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. J. Natl. Cancer Inst. 2018;110:560–7.
Potdar PD, Sen K. Profiling of circulating tumor cells in liquid biopsies from metastatic cancer patients. J Cancer Metastasis Treat. 2017;3:6–15.
Pan B-T, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78.
Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and α-granules. Blood. 1999;94:3791–9.
Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–20.
Stevens GL, Scheer WD, Levine EA. Detection of tyrosinase mRNA from the blood of melanoma patients. Cancer Epidemiol Biomark Prev. 1996;5:293–6.
Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. https://doi.org/10.1016/j.ygyno.2008.04.033.
Otandault A, Anker P, Al Amir Dache Z, Guillaumon V, Meddeb R, Pastor B, Pisareva E, Sanchez C, Tanos R, Tousch G, et al. Recent advances in circulating nucleic acids in oncology. Ann. Oncol. 2019;30:374–84.
Zhang X, Hong R, Chen W, Xu M, Wang L. The role of long noncoding RNA in major human disease. Bioorg Chem. 2019;92:103214.
Bach DH, Lee SK, Sood AK. Circular RNAs in cancer. Mol Ther Nucleic Acids. 2019;16:118–29.
Pastorino U, Boeri M, Sestini S, Sabia F, Silva M, Suatoni P, Verri C, Cantarutti A, Sverzellati N, Corrao G, Marchianò A, Sozzi G. Blood microRNA and LDCT reduce unnecessary LDCT repeats in lung cancer screening: results of prospective BioMILD trial. International Association for the Study of Lung Cancer, World Conference on Lung Cancer (2019).
Mandel P, Metais P. Title in other language. C R Seances Soc Biol Fil. 1948;142:241–3.
Wan JC, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223.
Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer. 2002;2:210.
Underhill HR, et al. Fragment length of circulating tumor DNA. PLoS Genet. 2016;12:e1006162.
Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579.
Köhn L, Johansson M, Grankvist K, Nilsson J. Liquid biopsies in lung cancer—time to implement research technologies in routine care? Ann Transl Med. 2017;5:278.
Lo YM, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–7. https://doi.org/10.1016/s0140-6736(97)02174-0.
Canick JA, Palomaki GE, Kloza EM, Lambert-Messerlian GM, Haddow JE. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat Diagn. 2013;33:667–74. https://doi.org/10.1002/pd.4126.
Hui L, Bianchi DW. Noninvasive prenatal DNA testing: the vanguard of genomic medicine. Annu Rev Med. 2017;68:459–72. https://doi.org/10.1146/annurev-med-072115-033220.
Suciu ID, Toader OD, Galeva S, Pop L. Non-invasive prenatal testing beyond trisomies. J Med Life. 2019;12:221–4.
Taylor-Phillips S, et al. Accuracy of non-invasive prenatal testing using cell-free DNA for detection of Down, Edwards and Patau syndromes: a systematic review and meta-analysis. BMJ Open. 2016;6(1):e010002.
FDA. List of cleared or approved companion diagnostic devices (in vitro and imaging tools) (2018).
Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O’Connell A, Messineo MM, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698–705.
Denis JA, Guillerm E, Coulet F, Larsen AK, Lacorte JM. The role of BEAMing and digital PCR for multiplexed analysis in molecular oncology in the era of next-generation sequencing. Mol Diagn Ther. 2017;21(6):587–600.
Olmedillas-López S, García-Arranz M, García-Olmo D. Current and emerging applications of droplet digital PCR in oncology. Mol Diagn Ther. 2017;21(5):493–510.
TaqMan™ Liquid Biopsy dPCR Assay. https://www.thermofisher.com/order/catalog/product/A44177?SID=srch-hj-A44177#/A44177?SID=srch-hj-A44177. Accessed 30 Dec 2019.
Baer C, Kern W, Koch S, Nadarajah N, Schindela S, Meggendorfer M, Haferlach C, Haferlach T. Ultra-deep sequencing leads to earlier and more sensitive detection of the tyrosine kinase inhibitor resistance mutation t315i in chronic myeloid leukemia. Haematologica. 2016;101(7):830–8.
Chen M, Zhao H. Next-generation sequencing in liquid biopsy: cancer screening and early detection. Hum Genomics. 2019;13(1):34.
Phallen J, Sausen M, Adleff V, Leal A, Hruban C, White J, Anagnostou V, Fiksel J, Cristiano S, Papp E, et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med. 2017;9(403):eaan2415.
Razavi P, et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat Med. 2019;25(12):1928–37.
Oncomine™ Pan-Cancer Cell-Free Assay. https://www.thermofisher.com/order/catalog/product/A37664?SID=srch-srp-A37664#/A37664?SID=srch-srp-A37664. Accessed 30 Dec 2019.
AVENIO ctDNA Analysis Kits. https://sequencing.roche.com/en-us/products-solutions/by-category/assays/ctdna-analysis-kits.html. Accessed 30 Dec 2019.
TruSight Oncology 500 ctDNA. https://www.illumina.com/products/by-type/clinical-research-products/trusight-oncology-500-ctdna.html. Accessed 30 Dec 2019.
Cohen JD et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science eaar3247 (2018).
Chan KA, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513–22.
Tie J, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8:346ra392-346ra392.
Garcia-Murillas I, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Science translational medicine. 2015;7:302ra133-302ra133.
Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci. 2003;100:776–81.
Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Chalmers ZR, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34.
Gandara DR, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24:1441.
Liu XS, Mardis ER. Applications of immunogenomics to cancer. Cell. 2017;168:600–12.
Garcia-Garijo A, Fajardo CA, Gros A. Determinants for neoantigen identification. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.01392.
Nielsen M, et al. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci. 2003;12:1007–17.
Lundegaard C, et al. NetMHC-3.0: accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8–11. Nucleic Acids Res. 2008;36:W509–12.
Lundegaard C, Lund O, Nielsen M. Accurate approximation method for prediction of class I MHC affinities for peptides of length 8, 10 and 11 using prediction tools trained on 9mers. Bioinformatics. 2008;24:1397–8.
Merker JD, Oxnard GR, Compton C, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. Arch Pathol Lab Med. 2018;142:1242–53.
Benincasa G, Mansueto G, Napoli C. Fluid-based assays and precision medicine of cardiovascular diseases: the ‘hope’ for Pandora’s box? J Clin Pathol. 2019;72(12):785–99.
Zhang X, Karunathilaka N, Senanayake S, Subramaniam VN, Chan W, Kostner K, Fraser J, Atherton JJ, Punyadeera C. The potential prognostic utility of salivary galectin-3 concentrations in heart failure. Clin Res Cardiol. (2019). https://doi.org/10.1007/s00392-019-01557-0(epub ahead of print).
Lehmann-Werman R, Neiman D, Zemmour H, et al. Identification of tissue-specific cell death using methylation patterns of circulation DNA. Proc Natl Acad Sci USA. 2016;113(13):E1826–34.
Roels S, Costa OR, Tersey SA, Stangé G, De Smet D, Balti EV, Gillard P, Keymeulen B, Ling Z, Pipeleers DG, Gorus FK, Mirmira RG, Martens GA. Combined analysis of GAD65, miR-375, and unmethylated insulin DNA following islet transplantation in patients with T1D. J Clin Endocrinol Metab. 2019;104(2):451–60.
Lim B, Tsolaki M, Soosaipillai A, Brown M, Zilakaki M, Tagaraki F, Fotiou D, Koutsouraki E, Grosi E, Prassas I, Diamandis EP. Liquid biopsy of cerebrospinal fluid identifies neuronal pentraxin receptor (NPTXR) as a biomarker of progression of Alzheimer’s disease. Clin Chem Lab Med. 2019;57(12):1875–81.
Frost & Sullivan, Growth opportunities in the global liquid biopsy market, (2018).
Funding
Paper is supported by the Shenzhen Science and Technology programs (Grant no. JCYJ20150529151839281), the Guangdong Natural Science Foundation (Grant no. 2016A030313381), and the Shenzhen Health and Family Planning Commission (Grant no. SZSM201612041) to S.W. and Y.Z.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Dake Chen, Tao Xu, Shubin Wang, Howard Chang, Tao Yu, Yu Zhu, and Jian Chen have no conflicts of interest that are directly relevant to the content of this review/study.
Additional information
The original version of this article was revised as the name of the fourth author, which previously read “Howard Chan”, should read as “Howard Chang”.
Rights and permissions
About this article
Cite this article
Chen, D., Xu, T., Wang, S. et al. Liquid Biopsy Applications in the Clinic. Mol Diagn Ther 24, 125–132 (2020). https://doi.org/10.1007/s40291-019-00444-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-019-00444-8