Abstract
Background
There are several pharmacogenetic algorithms to determine the warfarin doses required in patients treated for thromboembolism, but they only explain 60 % of dose variation, suggesting that other genes may influence the dose required.
Objectives
This study aimed to evaluate the impact of clinical factors and CYP2C9*2, CYP2C9*3, VKORC1-1639G>A, MDR1 3435C>T, APOE* ε4, and UGT1A1(TA)n polymorphisms on the warfarin dose required, especially in those individuals requiring a high warfarin dose.
Methods
We studied 116 Brazilian patients who received warfarin therapy for thromboembolism. Associations between dose variability and age, body mass index (BMI), gender, use of warfarin antagonists, and genetic polymorphisms were examined.
Results
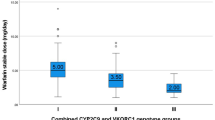
CYP2C9*2, CYP2C9*3, VKORC1-1639G>A, and APOE *ε4 were associated with lower warfarin doses. Of these subjects, 21 % required a warfarin dose higher than 70 mg/week, which was associated with a BMI greater than 25 kg/m2, use of warfarin antagonists, and the presence of the MDR1 3435T allele and UGT1A1(TA) 7 polymorphism. These individuals were considered to exhibit warfarin resistance. The individuals with the MDR1 3435TT genotype required a dose 21 % higher than that required by 3435CT and 3435CC individuals. The UGT1A1(TA) 7 allele was positively correlated with the warfarin dose.
Conclusion
CYP2C9*2, CYP2C9*3, VKORC1-1639G>A, and APOE *ε4 were associated with lower warfarin doses, while MDR1 3435C>T and UGT1A1(TA) n polymorphisms were associated with a requirement for higher doses. This is the first study to evaluate warfarin resistance, APOE *ε4 and UGT1A1(TA) n genotypes in the Brazilian population, and the association of these two genotypes with warfarin dose required.
Similar content being viewed by others
References
Jorgensen AL, FitzGerald RJ, Oyee J, Pirmohamed M, Williamson PR. Influence of CYP2C9 and VKORC1 on patient response to warfarin: a systemic review and meta-analysis. PLoS One. 2012;7:e44064. doi:10.1371/journal.pone.0044064.
The International Warfarin Pharmacogenetics Consortium. Estimation of the warfarin dose with clinical and pharmacogenetic Data. N Engl J Med. 2009;360:753–64.
Johnson JA, Gong L, Whirl-Carrillo M, Gage BF, Scott SA, et al. Clinical Pharmacogenetics implementation consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90:625–9.
Kangelaris KN, Stephen B, Nussbaum RL, Garcia DA, Tice JA. Genetic testing before anticoagulation? A systematic review of pharmacogenetic dosing of warfarin. J Gen Intern Med. 2009;24:1171.
Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121:23–34.
Wadelius M, Chen LY, Lindh JC, Eriksson N, Ghori MJ, et al. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009;113:784–92.
D’Andrea G, D’Ambrosio RL, Perna PD, Chetta M, Santacroce R, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–9.
Botton MR, Bandinelli E, Rohde LE, Amon LC, Hutz MH. Influence of genetic, biological and pharmacological factors on warfarin dose in a Southern Brazilian population of European ancestry. Br J Clin Pharmacol. 2011;72:442–50.
Kohnke H, Scordo MG, Pengo V, Padrini R, Wadelius M. Apolipoprotein E (APOE) and warfarin dosing in an Italian population. Eur J Clin Pharmacol. 2005;61:781–3.
Cavallari LH, Butler C, Langaee TY, Wardak N, Patel SR, et al. Association of apolipoprotein e genotype with duration of time to achieve a stable warfarin dose in african-american patients. Pharmacotherapy. 2011;31:785–92.
Kimmel SE, Christie J, Kealey C, Chen Z, Price M, et al. Apolipoprotein E genotype and warfarin dosing among Caucasians and African Americans. Pharmacogenomics J. 2008;8:53–60.
Almeida VCO, Ferreira ACS, Ribeiro DD, Gomes KBB, Fernandes APSM, et al. Association of the C3435T polymorphism of the MDR1 gene and therapeutic doses of warfarin in thrombophilic patients. J Thromb Haemost. 2011;9:2120–2.
Bratton SM, Mosher CM, Khallouki F, Finel M, Court MH, et al. Analysis of R- and S-hydroxywarfarin glucuronidation catalyzed by human liver microsomes and recombinant UDP-glucuronosyltransferases. J Pharmacol Exp Ther. 2012;340:46–55.
Sinxadi M, Blockman M. Warfarin resistance. Cardiovasc J Afr. 2008;19:215–7.
Loebstein R, Dvoskin I, Halkin H, Vecsler M, Lubetsky A, et al. A coding VKORC1 Asp36Tyr polymorphism predisposes to warfarin resistance. Blood. 2007;109:2477–80.
Harrington DJ, Gorska R, Wheeler R, Davidson S, Murden S, et al. Pharmacodynamic resistance to warfarin is associated with nucleotide substitutions in VKORC1. J Thromb Haemost. 2008;6:1663–70.
Micromedex. Drug-Reax System (2010) Internet database. Available at: www.micromedexsolutions-com.ez27.periodicos.capes.gov.br/micromedex2/librarian/PFDefaultActionId/evidencexpert.ShowDrugInteractionsResults. Accessed 17 June 2013.
Sakaeda T, Nakamura T, Horinouchi M, Kakumoto M, Ohmoto N, et al. MDR1 genotype-related pharmacokinetics of digoxin after single oral administration in healthy Japanese subjects. Pharm Res. 2001;18:1400–4.
Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosultransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism. Proc Natl Acad Sci. 1998;95:8170–4.
White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:1–8.
Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44:62–9.
Jensen BP, Chin PK, Roberts RL, Begg EJ. Influence of adult age on the total and free clearance and protein binding of (R)- and (S)-warfarin. Br J Clin Pharmacol. 2012;74:797–805.
Perini JA, Struchiner CJ, Silva-Assunção E, Santana IS, Rangel F, et al. Pharmacogenetics of warfarin: development of a dosing algorithm for brazilian patients. Clin Pharmacol Ther. 2008;84:722–8.
Watzka M, Geisen C, Bevans CG, Sittinger K, Spohn G, et al. Thirteen novel VKORC1 mutations associated with oral anticoagulant resistance: insights into improved patient diagnosis and treatment. J Thromb Haemost. 2011;9:109–18.
Vianna JR, Perini JA, Rondinelli E, Suarez-Kurtz G. CYP2C9 genotypes and the pharmacokinetics of tenoxicam in Brazilians. Clin Pharmacol Ther. 2004;76:18–26.
Limdi NA, Wadelius M, Cavallari L, Eriksson N, Crawford DC, International Warfarin Pharmacogenetics Consortium, et al. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood. 2010;115:3827–34.
Daly AK. Pharmacogenomics of anticoagulants: steps toward personal dosage. Genome Med. 2009;1:10.
Sconce EA, Daly AK, Khan TI, Wynne HA, Kamali F. APOE genotype makes a small contribution to warfarin dose requirements. Pharmacogenet Genomics. 2006;16:609–11.
Kohnke H, Sorlin K, Granath G, Wadelius M. Warfarin dose related to apolipoprotein E (APOE) genotype. Eur J Clin Pharmacol. 2005;61:381–8.
The Pharmacogenomics Knowledgebase - PharmGKB (homepage na Internet). Stanford: Stanford University; (atualizada em 23 de maio de 2013; acesso em 20 de junho de 2013). Clinical PGx. Disponível em http://www.pharmgkb.org/gene/PA126.
Kim Y, Smith A, Wu AH. C3435T polymorphism of MDR1 gene with warfarin resistance. Clin Chim Acta. 2013;425:34–6.
Issac MS, El-Nahid MS, Wissa MY. Is there a role for MDR1, EPHX1 and protein Z gene variants in modulation of warfarin dosage? A study on a cohort of the Egyptian population. Mol Diagn Ther. 2014;18(1):73–83.
Marques SC, Ikediobi ON. The clinical application of UGT1A1 pharmacogenetic testing: Gene–environment interactions. Hum Genomics. 2010;4:238–49.
Fertrin KY, Gonçalves MS, Saad ST, Costa FF. Frequencies of UDP- glucuronosyltransferase 1 (UGT1A1) gene promoter polymorphisms among distinct ethnic groups from Brazil. Am J Med Genet. 2002;108:117–9.
Stojiljkovic M, Patrinos GP, Pavlovic S. Clinical applicability of sequence variations in genes related to drug metabolism. Curr Drug Metab. 2011;12(5):445–54.
Santos PCJL, Soares RAG, Santos DBG, Nascimento RM, Coelho GL. CYP2C19 and ABCB1 gene polymorphisms are differently distributed according to ethnicity in the Brazilian general population. BMC Med Genet. 2011;12:13.
Suarez-Kurtz G. Population diversity and the performance of warfarin dosing algorithms. Br J Clin Pharmacol. 2011;72:451–3.
Acknowledgments
The current study was supported by the Universidade Federal de Minas Gerais and Instituto Hermes Pardini.
Disclosure of Conflicts of interest
V.C. Oliveira Almeida, D.D. Ribeiro, K.B. Gomes, and A.L. Brunialti Godard have no conflicts of interest that are directly related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira Almeida, V.C., Ribeiro, D.D., Gomes, K.B. et al. Polymorphisms of CYP2C9, VKORC1, MDR1, APOE and UGT1A1 Genes and the Therapeutic Warfarin Dose in Brazilian Patients with Thrombosis: A Prospective Cohort Study. Mol Diagn Ther 18, 675–683 (2014). https://doi.org/10.1007/s40291-014-0121-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-014-0121-4