Abstract
What is patient-centricity? In some contexts, it has been associated with targeting therapies based on biomarkers or enabling healthcare access. There has been a surge in patient-centricity publications, and in many cases for the biopharmaceutical industry, patient engagement is used to endorse pre-held assumptions at a specific moment in time. Rarely is patient engagement used to drive business decisions. Here we describe an innovative partnership between Alexion, AstraZeneca Rare Disease and patients that allowed a deeper understanding of the biopharmaceutical stakeholder ecosystem and an empathic understanding of each patient’s and caregiver’s lived experience. Alexion’s decision to build patient-centricity frameworks resulted in the formation of two unique organisation design platforms: STAR (Solutions To Accelerate Results for patients) and LEAP (Learn, Evolve, Activate and deliver for Patients) Immersive Simulations. These interconnected programmes required cultural, global, and organisational shifts. STAR generates global patient insights that are embedded in drug candidate and product strategies while helping to establish enterprise foundational alignment and external stakeholder engagement plans. LEAP Immersive Simulations produce detailed country-level patient and stakeholder insights that contribute to an empathetic understanding of each patient’s lived experience, support country medicine launches and provide ideas to have a positive impact along the patient journey. Combined, they deliver integrated, cross-functional insights, patient-centric decision making, an aligned patient journey, and 360° stakeholder activation. Throughout these processes, the patient is empowered to dictate their needs and validate the proposed solutions. This is not a patient engagement survey. This is a partnership where the patient co-authors strategies and solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Rather than asking the patient to describe how a treatment improves their quality of life (QoL), the patient is asked what a treatment needs to achieve to improve their overall lived experience. |
For more pharmaceutical companies to embrace patient-centricity, they should move from “we know what you need” to “what do we need to achieve to make you feel better?” |
Strive for drug development becoming driven by patients through an empathic collaboration between the pharmaceutical company and the stakeholder ecosystem. |
1 Introduction
Primary care services are increasingly integrating patient-centred healthcare [1,2,3]. Consequently, patients and their caregivers are seeking transparent healthcare systems and inclusion in the decision-making process [1]. Indeed, our patient authors attest that the patients see themselves as the nucleus of a web, with each stakeholder connected to the patient and each other. These connections can bidirectionally exert influence, but if a strand is broken the whole structure weakens.
Similarly, the pharmaceutical industry has realised that it may not be sufficient to engage patients in surveys or request input for a singular purpose. Research has historically been ‘on’, ‘about’ or ‘for’ patients [4]; creating a disconnect between industry and the end user [5]. Patients are engaged to provide information and insights to help identify opportunities to improve quality of care and outcomes [6,7,8]. Whilst there is no consensus on what patient-centricity means [5, 9, 10], it should begin with deeply and empathetically understanding each patient’s lived disease and healthcare experience [11]. To consistently implement patient-centricity, a coordinated approach must be followed [12] and it is essential that the principles are defined and validated by patients [5].
In the context of rare diseases, lower prevalence rates result in a lack of expertise among healthcare professionals (HCPs). A patient will see an average of seven specialists over an average of 4.8 years before being diagnosed [13]. Further, approved treatments only exist for approximately 5% of rare diseases [14]. Relying on clinical information in the literature may provide an incomplete picture or, at worst, be misleading [11], so the onus is on thoughtful drug development to provide wrap-around treatment, alongside comprehensive education. Because of this, the authors believe that patient engagement is distinct in rare diseases, which may strengthen the engagements where they occur. Therefore, pharmaceutical companies need to evolve from developing drugs to partnering with patients to truly meet their needs [1, 15,16,17].
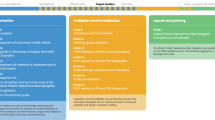
In 2019, Alexion, AstraZeneca Rare Disease (hereafter referred to as Alexion) commenced a journey to becoming a more patient-centric organisation. Alexion identified key patient-centricity principles (Fig. 1) to deeply understand each patient’s lived experience, and demonstrably incorporate their insights into the organisation. This led to the development of STAR (Solutions To Accelerate Results for patients) and LEAP (Learn, Evolve, Activate and deliver for Patients), two complimentary platforms designed to embed patient-centric thinking into all functions and across geographies. Here, we describe these platforms and how they enable patient engagement and end-to-end insight gathering to inform strategies and initiatives. Co-created and endorsed by patients, this work also enhances patient-centricity awareness within the industry to improve the lives of patients living with rare diseases.
2 Approach
2.1 The Patient and Public Involvement Statement
Patients were involved early in STAR and at the beginning of LEAP. Facilitated discussion with patients allowed Alexion to gain an in-depth understanding of the Moments that Matter (MTMs), key experiences that significantly impact patients. Immersive Simulations of the stakeholder ecosystem enabled a 360° evaluation of the impact of living with a rare disease; these simulations included all stakeholders and allowed patients, caregivers, and patient advocates an equal voice to all others. Patients and/or patient advocacy group (PAG) leaders who participated in a STAR Therapeutic Patient Forum or LEAP Immersive Experience were invited to provide personal observations and examples of how Alexion shifted its culture from product-focused to patient-centred. Patients were asked to assess the value and limitations of these programmes and feedback was gathered to demonstrate the importance of understanding each patient’s experience. The patient authors may disseminate this manuscript as per their preferred formats and channels (including social media or websites) and, as appropriate, per Alexion compliance guidelines.
2.2 The First Steps in Invoking Fundamental Change
Although plain language communications are increasing, discussions regarding clinical data still largely centre on providing knowledge to HCPs, culminating in a treatment recommendation. Alexion realised that if the goal was to focus on the needs of the patient, drug development needed to be reframed around the question of ‘what does the patient need?’ This shift in viewpoint is aligned with the US Food and Drug Administration non-binding recommendations on ‘Patient-Focused Drug Development: Methods to Identify What Is Important to Patients’ [18].
Alexion conducted a gap analysis to identify the needs within the business and the processes required to be patient-centric during medicine development and commercialisation. The organisational structure was reconsidered, and new functions were created. The driver evolved from ‘what impact will our drug have on symptoms’, to ‘we need to understand what it is like to live with this disease, to engineer meaningful solutions’. This resulted in a cultural shift where the journey starts by actively listening to individual patients.
2.2.1 STAR
STAR is Alexion’s patient value incubator that helps embed patient-centricity into drug development. STAR engages global teams across indications early in the clinical development process and invites patients, caregivers, and stakeholders to partner with Alexion. STAR aims to identify MTMs and prioritise where they can create the greatest value.
STAR Therapeutic Patient Forums utilise four core pillars to deeply understand and empathetically appreciate the MTMs, co-create strategies and solutions, and implement plans (Fig. 2).
Pillar 1: Learn and Align
A comprehensive internal knowledge assessment is conducted, and employees are encouraged to be agnostic of their function to drive an understanding of the disease. This establishes the baseline level of awareness for each patient’s lived experience across disciplines. This assessment is published into a ‘STAR Factbook’, a detailed outline of the experience of living with or managing a rare disease. The STAR Factbook is a key resource for Alexion employees.
Pillar 2: Experience and Prioritise
Learning from external stakeholders about their unique role within the patient journey. Patients, caregivers, and other participants take part in facilitated discussions to capture MTMs, which are characterised as an intense experience, emotion, and/or impact. These insights form the core of the methodology.
Pillar 3: Innovate and Test
The broader, in-depth, understanding developed in the first two pillars informs strategic choices facing the global early medicine strategy and clinical development teams. This creates an opportunity to highlight solutions that could create differentiated stakeholder value.
Pillar 4: Implement and Engage
Finally, the Alexion teams, patients, and stakeholders co-create ideas and plans to positively affect each patient’s lived experience and improve outcomes. STAR connects disparate initiatives and fosters collaboration between teams and patients early in development.
2.2.2 LEAP
Alexion’s LEAP Immersive Simulations enable granular patient insight from late-phase development through to lifecycle management. These experiential interactive simulation sessions are designed to deeply immerse employees in each patient’s lived experience through the MTMs identified in STAR, providing a narrative for an indication as a medicine approaches late phase or regulatory approval.
During a LEAP Immersive Simulation, participants come to appreciate the diverse perspectives within the stakeholder ecosystem, including patients, carers, physicians, and payers through thoughtfully developed personas. Teams are presented with pivotal scenarios including (but not limited to) symptom onset, diagnosis, and patient access, facilitating real-time reflections and impactful solutions. LEAPs are conducted across countries, in different languages, and Alexion cross-functional country teams are encouraged to ‘take a LEAP’.
Participants engage in role play, focusing on multiple interactions or decision making aligned with real-world choices that a patient, caregiver, or stakeholder need to make. External participants include patients, caregivers, PAGs, and HCPs. Employees are encouraged to put themselves in the shoes of the patient and stakeholders and consider how they would feel in those pivotal moments. As the simulation progresses, participants work together to find solutions to challenges and prioritise strategies for country initiatives. By working collaboratively with all stakeholders at the country level, MTMs can be assessed in the context of the geographical setting and patient demographics, highlighting factors that could go unnoticed at the global level.
2.2.3 Programme Logistics
Patient and other stakeholder involvement in both STAR and LEAP are executed based on appropriate compliance processes. The key compliance considerations that we evaluated included transfer of value, fair market value, honoraria, and necessary policies, processes, and documentation. To ensure both programmes are managed and executed compliantly, Alexion established a patient engagement center of excellence. This team oversees all patient and advocacy activities in partnership with the legal and compliance functions.
Regarding patient engagement, initial outreach is made by Alexion patient advocate professionals who are the formal liaisons to patients, caregivers, and patient advocacy organisations. Once a patient has confirmed interest in participating in one of the programmes, contracting (based on country-specific legal requirements) is completed. Transfer of value such as honoraria is subject to country regulations and reviewed by governance functions. Payment to patients is based on country-specific fair market value guidelines. Engagement in these activities can be a short-term defined period (e.g., 3 months) or a long-term commitment depending on the patient’s preference to be involved in subsequent activities.
The patient’s preference is the key factor in determining whether they participate virtually or in person. In general, most patients engage virtually, via Zoom. Anecdotally, this virtual participation minimises potential burden related to travel and/or disruption of the patient’s healthcare regimen. Virtual participation mitigates the risk of transmitting infections to patients who may be immunocompromised, and also enables patients from different geographies who may speak different languages to participate.
LEAP simulations are developed in English. Country teams translate the simulation into local language prior to conducting their sessions. The output is stored in local language at the country level and shared at the global level in English allowing for an integrated view. For STAR, we utilise the simultaneous language translation function available within the Zoom platform. The meeting materials are translated into the language spoken by participating external stakeholders (i.e., patients, caregivers, physicians, etc.) using a translation software available to AstraZeneca/Alexion employees. The final output is developed and provided in English. The summary reports are developed for and provided for internal review purposes only, therefore are not sent to the external stakeholders.
A visual representation of how STAR and LEAP function, and the interplay between them, can be seen in Fig. 3.
2.3 Feedback Surveys
After every STAR and LEAP event, data are gathered through an anonymous employee survey, which includes quantitative metrics and qualitative feedback via free-text fields.
3 Impact
3.1 STAR
Since launching this initiative in 2019, Alexion has conducted 20 STAR Therapeutic Patient Forums. The identified insights and subsequent strategies and/or solutions have informed a variety of processes, including clinical protocol design, health economics, evidence creation, and pre-commercialisation planning. These function-agnostic forums allow Alexion colleagues to challenge their beliefs and assumptions. Below is an example of the output.
The STAR Patient Forum for generalised myasthenia gravis (gMG)—a rare, chronic autoimmune disease of neuromuscular transmission [19, 20]—identified 24 unique MTMs (Fig. 4). One MTM identified the disconnect between physicians’ versus patients’ perceptions of treatment success. While physicians may perceive gMG to be controlled and the treatment goals attained, patients and their families continue to live with disease- and treatment-related burdens. This highlighted a need to recalibrate treatment goals through the lens of the patient and their family. It also demonstrated that patients with gMG can be extremely resilient, masking impact signs and symptoms and leading to normalisation of the burden. This revealed a need to define well controlled gMG from the patients’ perspective.
This deeper understanding drove an action plan that included a proposed solution to identify ways of facilitating better communications between patients and HCPs. In response, the global Health Economics and Outcomes Research (HEOR) team initiated an innovative study to capture the total disease impact in gMG. It included both direct and indirect measures of disease impact on patients, caregivers, family, healthcare systems, and society, facilitating a 360° evaluation of its total impact.
Empowering patients has become core to the Alexion gMG strategic disease awareness plan, resulting in many patient-centred actions (Fig. 5), along with tangible outcomes including the development of the gMG STAR Factbook, which serves as an educational primer to Alexion colleagues as they prepare to participate in the STAR Therapeutic Patient Forums. A key realisation is that it is insufficient to request patient feedback once a drug is developed, as this misses vital opportunities to include the patient voice in the development programme.
Feedback from Therapeutic Patient Forums demonstrate that participants consider them to be engaging, productive, and meaningful. When asked whether they agreed that participating in STAR was a valuable use of their time, 96% of participants agreed or strongly agreed; the remainder somewhat agreed. Free-text feedback was also encouraged; an example was “I am proud Alexion continues to find creative ways for us to better understand patient needs!”.
3.2 LEAP
Since launching in 2019, LEAP has expanded to include experiential learning sessions for indications across haematology, nephrology, neurology, and metabolic rare diseases. The LEAP Immersive Simulation fosters a patient-centric mindset and ensures that patient and stakeholder perspectives and insights in a country underpin indication commercialisation and lifecycle management (Fig. 6). LEAP focuses on the nuances created by geographical variation, generating a deepened ecosystem understanding, leading to solutions as demonstrated below.
A LEAP Immersive Simulation was conducted in a country that has a multi-payer healthcare system. The simulation focused on paroxysmal nocturnal haemoglobinuria (PNH), a rare, chronic, and devastating intravascular haematological disorder that is potentially life-threatening [21, 22]. This identified an opportunity to re-examine patient reimbursement criteria, which was largely based on data generated to support regulatory approval. With an HCP and patient in attendance, the team validated the concept of developing payer-oriented materials to enable physicians to advocate for patient access based on recent real-world evidence. A second initiative focussed on expediating specialist referrals and time-to-diagnosis through digital public disease awareness materials on differential diagnostic testing.
Tangible outcomes were also evident from the neuromyelitis optica spectrum disorder (NMOSD) LEAP. NMOSD is a rare, severely disabling, complement-mediated autoimmune neuroinflammatory disease of the central nervous system characterised by devastating and unpredictable attacks/relapses that cause immediate and irreversible damage [23]. Putting themselves in the shoes of an HCP, the team gained improved understanding on the requirement for vaccination prior to C5 inhibitor therapy initiation. As a result, focused materials and resources were developed to enable experienced treating physicians to deliver peer-to-peer education related to vaccination requirements, ensuring that patients are adequately vaccinated against Neisseria meningitidis at 2 weeks prior to receiving eculizumab or ravulizumab unless the risk of delaying therapy outweighs the risk of developing a meningococcal infection. Another team incorporated the PAG perspective in the development of patient materials for NMOSD to raise awareness among the patient and caregiver community, resulting in the development of patient/caregiver toolkits in collaboration with the PAG who attended the LEAP session. These toolkits included educational cards which patients and caregivers could present to emergency room clinicians to help quickly inform care, while minimising the need to personally inform and educate all emergency room staff on their condition.
To date, over 3500 employees from 45 countries and 75 external stakeholders have participated in LEAP. By actively listening and learning, participants were able to empathetically walk in the patient’s shoes, validated by feedback such as “Patient health is not the only thing that is affected when they're diagnosed with a rare disease; it also changes a lot of other aspects of their lives”.
Internal validation of one LEAP demonstrated value to 93% of colleagues and provided new insight for 89% of colleagues. However, the ultimate judge must be the patient. During their participation in LEAP, our patient authors realised that those working to serve the rare disease community often considered it to be a calling, not just a job. By sharing their experiences, they provided insights to help Alexion employees perform their jobs more effectively and with greater meaning, humanising their work. Different functions came together to listen and learn about the patient experience directly, challenging their assumptions. LEAP facilitated patient empowerment, with one author stating, “the inclusion made me feel like my voice and journey mattered and provided me with the opportunity of being involved with something bigger than myself. It proved that the landscape of patient insights was evolving for the better and that our advocacy work was becoming increasingly important”.
3.3 Patient Insight Forums
Another tangible impact of STAR and LEAP was the need to facilitate ongoing conversations and feedback between Alexion and the patient community, resulting in the development of Patient Insight Forums. With these forums, Alexion continues to meaningfully engage with patients and caregivers through a feedback loop of insights and information—collated via direct, two-way dialogue—to deliver on our patient-centricity ambitions. These forums provide a unique way for us to engage patients, and for patients to engage with each other, versus other insight-gathering initiatives such as market research.
In an international forum on PNH, patients, caregivers, and PAGs engaged via a secure, social media-style platform to discuss critical topics with Alexion, including clinical protocols and patient support programmes. The virtual format allowed patients to participate any time over a 2-week period. Employees served as moderators, directly interacting with 15 patients; 1052 comments were logged over 10 days in response to 58 questions.
The event also provided a space for patients to meet each other, and bond over shared experiences. The Patient Insights Forum is globally available for all diseases and includes ad hoc country involvement.
4 Analysis
Alexion, in partnership with patients, has developed a novel and systematic approach to ensure that a patient-centric culture is fully embedded within its operating model, as well as its drug development process. Alexion’s patient-centric focus is a cross-functional, continuous process that evolves with the changing landscape (Fig. 3). By exploring with patients and caregivers how, if, and when their needs are being met, Alexion has ensured that its focus and aspiration are on helping to enable patients to live the life that they desire.
In keeping with this ethos, this manuscript has been co-created and co-authored by patients, PAG leads who participated in the STAR and/or LEAP experiences, and Alexion. This represents a shift from viewing a disease as a biochemical pathway to be altered, to understanding that any intervention is flawed if it is not centred on addressing patient’s needs and empowering them to confirm when these needs are met.
Patient-centricity is not a novel concept. As early as 2009 there were calls to provide an integrated healthcare system to address patients’ comfort and needs [24], closely followed by the medical community urging an empathic understanding of healthcare costs, patient concerns, and patient emotions to improve QoL [25, 26]. Yet at the same time, care for patients was being defined by symptoms and disease markers deemed important by the HCP, rather than allowing patients to dictate what was important to them [27, 28].
Unlike other disease areas, rare diseases represent smaller patient populations. Coupled with low disease awareness amongst the medical community, the patient experience of getting a diagnosis, accessing treatment, and education may be exacerbated. These aspects of rare disease are drivers for our patient-centricity focus and perhaps what makes our STAR and LEAP programmes unique and invaluable. The design of these key frameworks represents strategic and innovative partnerships where patient insights are at the forefront of decision making and business strategies, because the needs of these patients may not be understood as readily as in other diseases.
The notion that HCPs and regulators are the only experts engaged by the healthcare industry raised concerns from our patient authors. Patient engagement should also be viewed as ‘expert’ engagement and subjected to the same regulations as HCP engagement, including transparently declaring patient input, crediting the source, and appropriately compensating patients.
When considering patient engagement, factors such as internet access or individual digital savviness need to be considered, otherwise patient-centricity can only be claimed for a subset of patients. Likewise, enrolment and retention in clinical studies should consider the patient perspective on trial design, access to study sites, and demographic diversity. Our patient authors expressed concern that patients are effectively silenced by regulations, technology, and circumstance, and that “those in a position of power have a responsibility to cast the net wide in order to serve the needs of all patients”.
There remains a need for comprehensive and thoughtful patient engagement to understand the experience of living with a rare disease for people in communities of colour; factors precluding engagement include mistrust of the healthcare system, health literacy, and cultural or religious differences [29, 30]. However, this understanding remains essential to ensure richer insights that represent a broader patient population, which has been recognised in the recent FDA draft guidance [31].
Our partnership is cognisant that some of these limitations still apply to both STAR and LEAP. Navigating these processes may be amplified for patients for whom English is not their first language, people with disabilities, or patients with family and/or work commitments. Consequently, there needs to be a continuous assessment of patient engagement approaches to address these barriers, while also considering the appropriate governance for patient-related activities.
5 Conclusion
Fundamentally, patient-centricity is the ability to listen and learn, then drive through to co-creating a new future. It is a multi-directional, continual communication process between industry, patients, and stakeholders that elicits change at every level of the organisation. Finally, patient-centricity is of little value unless it is genuinely endorsed. Therefore, the patient authors will have the final words: “You are not a patient-centric organisation until the patients tell you that you are!”
References
WHO. Patient Engagement: Technical Series on Safer Primary Care. 2016 [cited 2022 10 May]; Available from: https://apps.who.int/iris/bitstream/handle/10665/252269/9789241511629-eng.pdf;sequence=1?msclkid=aca9d8afd03a11ec9e5780c6fbb59d2c
Davis RE, Jacklin R, Sevdalis N, Vincent CA. Patient involvement in patient safety: what factors influence patient participation and engagement? Health Expect. 2007;10(3):259–67.
Chalasani M, Vaidya P, Mullin T. Enhancing the incorporation of the patient’s voice in drug development and evaluation. Res Involv Engagem. 2018;4:1–10.
Hamakawa N, Kogetsu A, Isono M, Yamasaki C, Manabe S, Takeda T, et al. The practice of active patient involvement in rare disease research using ICT: experiences and lessons from the RUDY JAPAN project. Res Involv Engagem. 2021;7(1):1–15.
Yeoman G, Furlong P, Seres M, Binder H, Chung H, Garzya V, et al. Defining patient centricity with patients for patients and caregivers: a collaborative endeavour. BMJ Innov. 2017;3(2):76–83.
Benbow JH, Rivera DR, Lund JL, Feldman JE, Kim ES. Increasing Inclusiveness of patient-centric clinical evidence generation in oncology: real-world data and clinical trials. Am Soc Clin Oncol Educ Book. 2022;42:1–11.
Roberts JC, Recht M, Gonzales SE, Stanley J, Denne M, Caicedo J, et al. Incorporating the patient voice and patient engagement in GOAL-Hēm: advancing patient-centric hemophilia care. Res Pract Thromb Haemost. 2022;6(1): e12655.
Hoos A, Anderson J, Boutin M, Dewulf L, Geissler J, Johnston G, et al. Partnering with patients in the development and lifecycle of medicines: a call for action. Ther Innov Regul Sci. 2015;49(6):929–39.
Trier H, Valderas JM, Wensing M, Martin HM, Egebart J. Involving patients in patient safety programmes: a scoping review and consensus procedure by the LINNEAUS collaboration on patient safety in primary care. Eur J Gen Pract. 2015;21(sup1):56–61.
Francisco R, Brasil S, Pascoal C, Jaeken J, Liddle M, Videira PA, et al. The road to successful people-centric research in rare diseases: the web-based case study of the Immunology and Congenital Disorders of Glycosylation questionnaire (ImmunoCDGQ). Orphanet J Rare Dis. 2022;17(1):1–17.
Morel T, Cano SJ. Measuring what matters to rare disease patients—reflections on the work by the IRDiRC taskforce on patient-centered outcome measures. Orphanet J Rare Dis. 2017;12(1):171.
EUMA. European Union Medicines Agency Network Strategy to 2020. Working together to improve health. . 2015 [cited 2022 10 May]; Available from: https://www.ema.europa.eu/en/documents/other/eu-medicines-agencies-network-strategy-2020-working-together-improve-health_en.pdf
Shire. Rare disease impact report: insights from patients and the medical community. 2013; Available from: https://globalgenes.org/wp-content/uploads/2013/04/ShireReport-1.pdf
Dunoyer M. Rare disease inequities: Opportunities for change. Fortune.
Kaufmann P, Pariser AR, Austin C. From scientific discovery to treatments for rare diseases—the view from the national center for advancing translational sciences—office of rare diseases research. Orphanet J Rare Dis. 2018;13(1):196.
Wilson P, Mathie E, Keenan J, McNeilly E, Goodman C, Howe A, et al. Health Services and Delivery Research. ReseArch with Patient and Public invOlvement: a RealisT evaluation—the RAPPORT study. Southampton (UK): NIHR Journals Library; 2015.
Flatau T, Greenfield J, Dickie B, Rayner O, Matthews H, Wise J. Medical research charities and biopharmaceutical companies as partners in patient-centred R&D. Pharmaceut Med. 2022;36(5):279–86.
FDA. Patient-Focused Drug Development: Methods to Identify What Is Important to Patients. 2022 [cited 2022 30 July]; Available from: https://www.fda.gov/media/131230/download
Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116(11):2843–54.
Nesargikar PN, Spiller B, Chavez R. The complement system: history, pathways, cascade and inhibitors. Eur J Microbiol Immunol (Bp). 2012;2(2):103–11.
Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–11.
Jang JH, Kim JS, Yoon SS, Lee JH, Kim YK, Jo DY, et al. Predictive factors of mortality in population of patients with paroxysmal nocturnal hemoglobinuria (PNH): results from a Korean PNH Registry. J Korean Med Sci. 2016;31(2):214–21.
Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflam. 2012;19(9):14.
van der Vinne E. The ultimate goal of disease management: improved quality of life by patient centric care. Int J Integr Care. 2009;10(9): e89.
Bhutani J, Bhutani S, Kumar J. Patient-centric care: managing celiac disease. Indian J Endocrinol Metab. 2013;17(1):177.
Pai M, Yadav P, Anupindi R. Tuberculosis control needs a complete and patient-centric solution. Lancet Glob Health. 2014;2(4):e189–90.
Brand CS. Management of retinal vascular diseases: a patient-centric approach. Eye (Lond). 2012;26(Suppl 2):S1-16.
Waldman SA, Terzic A. Patient-centric clinical pharmacology advances the path to personalized medicine. Biomark Med. 2011;5(6):697–700.
Goler Blount L. Historical discrimination, malpractice and lingering mistrust block the path to significant diversity in rare disease research. Diversity Elusive in Rare Disease Research [Briefings for Journalists] 2021 20/12/2021 [cited 2022 3 August]; Available from: https://nationalpress.org/topic/diversity-elusive-in-rare-disease-research/
Abdel-Rahman SM, Wimes MP, Curran T. A call to action: issuing a diversity and inclusion challenge to research organizations. Clin Transl Sci. 2021;14(6):2095–8.
FDA. Diversity Plans to Improve Enrollment of Participants From Underrepresented Racial and Ethnic Populations in Clinical Trials” Guidance for Industry Draft Guidance. 2022 14/04/2022 [cited 2022 4 August]; Available from: https://www.regulations.gov/document/FDA-2021-D-0789-0002
Acknowledgements
We would like to thank Yiyi Xia (Director, STAR Ideation & Strategy at Alexion, AstraZeneca Rare Disease) for her contribution to the initial stages of the article and providing key information. We also thank Joel Hastings (Director, Patient Advocacy—US at Alexion, AstraZeneca Rare Disease) for his contribution in establishing Alexion’s Patient Insight Forum expertise. Finally, we thank and recognise the global Patient Experience Team for being expert models of patient-centricity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
All activities and analysis were funded by Alexion, AstraZeneca Rare Disease. Medical writing and editorial support, funded by Alexion, AstraZeneca Rare Disease, was provided by Catherine Hoare, PhD, Bioscript Medical, Macclesfield, UK. Alexion, AstraZeneca Rare Disease reviewed and provided feedback on the manuscript.
Conflicts of interest
Rohita Sharma and Judy Campagnari are employees of, and hold stocks in, Alexion, AstraZeneca Rare Disease. Judy Campagnari also has patents pending. Lelainia Lloyd has been the recipient of grants, honoraria, support for attending meetings and equipment, materials, drugs, medical writing, gifts or other services from Alexion, AstraZeneca Rare Disease; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from the MS Society of Canada; and is on the editorial board for the Journal of Medical Imaging and Radiation Sciences. Sumaira Ahmed and Wendi Huff have no competing interests to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed to drafting and revising the article, provided their final approval of all content and agree to be accountable for all aspects of the work. Yiyi Xia (Director, STAR Ideation & Strategy at Alexion, AstraZeneca Rare Disease) contributed to the initial stages of the article and provided key information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sharma, R., Ahmed, S., Campagnari, J. et al. Embedding Patient-Centricity by Collaborating with Patients to Transform the Rare Disease Ecosystem. Pharm Med 37, 265–273 (2023). https://doi.org/10.1007/s40290-023-00474-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-023-00474-y