Abstract
Pharmaceutical development was at the forefront of efforts to prevent infection with the SARS-CoV-2 virus as well as to treat its often-devastating effects. Drug development, and its multifaceted and multi-disciplined activity toward effective vaccines and drugs, became part of everyday news. I review several key areas of vaccine and drug development that were brought into the public mainstream over the evolution of the pandemic. These include the unprecedented speed of vaccine discovery and development, issues uncovered from early clinical studies, and regulatory concepts that were highlighted throughout the development process. Among these was the importance of pharmacovigilance as each new agent was rapidly deployed to a mostly eager public. Critical challenges around production, packaging, and procurement of product for patient use were often centre stage. Finally, the ever-important need to transition not only from scientific concept to vaccine and drug, but from their authorized and approved use to their implementation in health systems to insure the intended effects both in individuals and populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The COVID-19 global pandemic put drug development, vaccines, and pharmaceutical medicine in the public forefront. |
Issues such as discovery and development, pharmacovigilance, supply chain, and implementation science were mainstream. |
The journey from scientific concept to drug approval was followed by the multifaceted challenges of vaccine implementation into health systems. |
1 Introduction
As the global COVID-19 pandemic unfolded, its rapid spread and often severe manifestations put both the public health profession and general population at an unprecedented state of alert. One can sense a palpable urgency to thwart its devastating medical, social, and economic effects. The frontline fundamental measures of hand washing, physical distancing, personal protection, and crowd avoidance laid the foundation of the battle. These measures were in parallel to efforts focused on development of pharmaceutical products that could prevent infection (i.e., vaccines) as well as therapeutics that could help minimise the manifestations and impact of infection.
The media reported every milestone which, in normal times, would be of limited interest and confined mostly to the pharmaceutical sector. They performed an important service by using their platform to educate on drug development. Conversely, and unfortunately, the spread of false and misleading information by some drowned out credible sources [1]. Table 1 lists some drug development concepts that suddenly appeared in the mainstream.
The primary areas of pharmaceutical development related to development of new therapeutics [2], repurposing existing drugs approved for other indications against COVID-19 infection [3] and development of vaccines [4]. While new therapies and vaccines introduced us to the phased drug approach, repurposing old drugs quickly taught us that efficacy and safety needs to be established in the condition for which it is being used. Given the desperation for interventions, the Emergency Use Authorization (EUA) programme was utilised at a pace never seen previously. Importantly, the complexity of drug development was exemplified as new data led some EUAs to be modified and some even revoked (e.g., hydroxychloroquine).
The global COVID-19 pandemic provided a unique platform for the public and non-research practitioners to learn and appreciate drug development. I will use the discovery, development, and implementation of COVID-19 vaccines over the first 18 months of the global pandemic to highlight those key elements of drug development that became part of our daily lives.
2 Vaccines Science and Development Hits the Mainstream
2.1 Vaccines Seen as the Most Viable Weapon
Vaccines remain an integral part of preventative public health despite controversies in some quarters [5]. Considering the average vaccine development time is about 10 years [6], and the fastest development to date to prevent mumps, took 4 years [7], the public soon had no choice but to accept a hopeful reality for a vaccine while reinforcing the basic public health measures in the interim. The positivity of the journey was nevertheless fostered by many groups, taking many approaches, and regulators willing to fast track any appropriate steps.
The various methods of vaccine development quickly became associated with curiosities about probability of success, effects in various types of patients, and the speed to a realistic availability. With respect to the latter, formulation, packaging, and distribution, which are often behind the scenes, became the leading topic of prime-time news. Discussions ensued on basic factors such as age, gender, race, and pre-existing conditions of the trial cohort that will ultimately determine its value and effectiveness in widespread use. Finally, the ability of the vaccine to prevent infection, reduce or eliminate transmissibility, prevent symptoms, and/or reduce severity of an infection and requirements for hospitalisation and mechanical ventilation are all possible outcomes, each representing a potential question of how the vaccine may work in the real-world setting.
2.2 Vaccine Platforms and Their Development
While the pre-clinical proof of concept studies is different with drugs and vaccines, the choosing of a platform for the vaccine was a critical first step with many scientific teams in industry and academia taking different approaches. Interestingly, the two main approaches of a viral vector or a messenger RNA platform were in some regard repurposing of the platform for a COVID-19 vaccine. For example, the Pfizer/BioNtech mRNA platform capitalised on years of research on its potential utility where mRNA would instruct cells to produce proteins. This was being considered for a potential HIV vaccine but also for other conditions associated with diabetes. When the sequence of the SARS-CoV-2 virus was reported in January of 2020, teams of scientists went to work and the spike protein on the surface of the virus was the target. The second mRNA vaccine that was approved from Moderna also utilizes this platform after years of study for utility in other diseases.
The viral vector platform had been used with other vaccines in the past and was the basis for the third vaccine approved. The Johnson and Johnson vaccine was the first approved in the USA with a viral platform and utilised adenovirus 26 (Ad26). In the recent past, Johnson & Johnson used Ad26 to develop vaccines for Ebola and other diseases. Thus, they had experience in engineering AD26 and could initiate a programme rapidly.
The AstraZeneca vaccine, approved in several countries outside the USA, also uses a viral platform; specifically, one consisting of a replication-deficient chimpanzee adenoviral vector ChAdOx1, containing the SARS-CoV-2 structural surface glycoprotein antigen (spike protein; nCoV-19) gene.
3 Initial Clinical Studies Fuel Optimism and Faith in Science
3.1 Vaccine Concepts Rapidly Proved in Man
The development timeline of the first approved vaccine illustrates the remarkable achievements conveyed to an eager public and scientific community. Early studies, that were conducted combining Phase 1 and Phase 2 objectives of initial safety and proof of concept, demonstrated that an mRNA approach to immunity was possible as evidenced by development of antibodies to the viral spike protein as well as an immune T-cell response [8, 9]. There were no side effects that precluded advancing development rapidly.
Rapid development proceeded with the launched a Phase 2/3 trial with 30,000 volunteers, which was further expanded to 44,000 participants. This expansion reinforced both safety observations but also potential efficacy in a wider age group as well as those with pre-existing condition.
In addition to the large Pfizer/BioNTech trial, several other sponsors similarly proceeded to Phase 3 in rapid succession [10], giving further hope to thwarting the pandemic.
3.2 Phase 3 Milestones Achieved
In mid-November of 2020, nearly 1 year since the pandemic virus was first identified, preliminary reports of the Pfizer/BioNTech Phase 3 study showed a 95% effect rates with no obvious safety issues [11, 12]. This news came at a time when infection rates in most jurisdictions were at an all-time high with growing concern of overwhelming surge capacity of acute and critical care [13]. With the possibility of a harvestable pharmaceutical in sight, the Phase 3 results of the Moderna vaccine [14] were reported soon after with similar efficacy results.
Positive Phase 3 data from the Johnson and Johnson [15] vaccine gave further hope as did the viral vector approach of AstraZeneca/ Oxford University [16, 17], although these later data were subjected to a call for further evaluations [18, 19].
3.3 All Hurdles in the Limelight
Potential safety signals in both viral vector programmes led to trial pauses while they were evaluated. A Phase 3 trial initiated in August was halted in September 2020 to evaluate a case of symptoms of traverse myelitis [20]. Another Phase 3 trial in 60,000 volunteers started in September 2020 was paused in October to evaluate a patient incident before resuming shortly thereafter [21]. These events highlighted the fact that clinical trials are to establish both safety and efficacy and that halting trials, allowing an independent Data Safety Monitoring Board (DSMB) to review adverse event cases, is a careful and established procedure [22, 23].
Atypical regulatory principles were also illustrated by the announcement that a Russian-developed vaccine would be available for administration prior to the ultimate publication of the Phase 2 trial [24] and absence of Phase 3 data [25, 26]. This approval was relabelled a “conditional registration certificate,” with the Phase 3 report ultimately showing an efficacy rate of 92% [27].
As all of these Phase 3 trials were approaching completion, the media were constantly balancing promises from politicians and realities from the developers. In order to reinforce faith in proper drug development, several chief executives of the research-based pharmaceutical industry issued a pledge not to submit their respective firm’s vaccine for licensing until safety and efficacy is established in Phase 3 trials [28]. While this did not preclude justifiable fast tracking of regulatory procedures, it did reinforce that the established drug development process is rooted in improving and protecting public health. This was then followed by a self-defence statement by Food and Drug Administration (FDA) regulators promising to uphold the scientific integrity and independence of their work [29].
4 Regulatory Use Authorisations: Concepts of Safety and Efficacy
The decision-making process for authorisation, even during the pandemic, followed a thorough data review by FDA scientists after unblinding, analysis and reporting of studies by the sponsoring firm. The FDA also utilises an independent advisory committee who also review the data carefully, discuss it openly in a public forum, and vote on questions posed to them by the FDA.
4.1 Emergency Use Authorisations with High Efficacy Vaccines
By the 1-year anniversary of the global pandemic declaration, there were three vaccines authorised for emergency use by FDA with others, including the AstraZeneca vaccine, also available for use in other jurisdictions. The importance of multiple product authorisations giving implementation flexibility proved extraordinarily valuable given the need for rapid global immunisation as well as subsequent hurdles driven by careful pharmacovigilance [30]. This science brought hope to the public as well as the medical profession. For industry scientists, it confirmed their vocational commitment that the answer to the pandemic was in science.
Given that the FDA published guidelines that required at least 50% efficacy for vaccine approval [31], the initial reports of Phase 3 data demonstrating 90–95% effectiveness were remarkable (Table 2). The efficacy rates of the mRNA vaccines against infection somewhat dampened the efficacy reports of 66% with the first approved viral vector agent; however, these data instructed the public on the important aspects of trial comparisons, including the concept of endpoints and period of conduct. Specifically, Johnson and Johnson trial highlighted the importance of endpoints beyond infectivity, choosing to focus on severity of illness, hospitalisation, mortality and death. These were of particular importance given the ever-stressed acute-care hospitals and their 'head-to-head’ studies. In the Johnson & Johnson pivotal study, for example, the trial was conducted at later dates when infectivity was peaking (September–December 2020) and in USA, Latin America, and South Africa. FDA analysis [32] showed an overall efficacy rate of 72% in the USA and 64% in South Africa, where a concerning variant was emerging. Furthermore, 86% efficacy was observed against severe forms of COVID-19 in the USA, and 82% against severe disease in South Africa. This lower risk of vaccinated persons in contracting severe disease or dying was particularly welcome in those jurisdictions where ‘flattening the curve’ of serious infections would reduce burden on the healthcare system (e.g., intensive care unit [ICU] beds).
4.2 Efficacy and Effectiveness
As vaccine trials were being reported, a great deal of attention was given to the concepts of efficacy and effectiveness. The vaccine efficacy rate is calculated from observations in a clinical trial by subtracting the placebo infection risk by the intervention infection risk, and subsequently dividing the difference by the placebo infection risk. Multiplying this number by 100 will provide us with the efficacy rate in percentage form. In other words, the percentage change of infection risk between the placebo group and intervention group is calculated. After a vaccine is authorised for emergency use or ultimately has an approved New Drug Application (NDA), clinical epidemiologists continue to assess vaccine effectiveness—i.e., how a vaccine works in real-world conditions. The goal is to understand how a vaccine protects people outside of strict clinical trial settings with a variety of study designs employed [33].
5 The Vaccine to Vaccination Implementation is Complex
5.1 Translational Science Illustrated
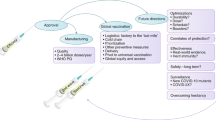
The landmark of established vaccine efficacy in the COVID-19 pandemic confirmed the unique skills of the pharmaceutical industry in translational science, i.e., bridging scientific knowledge to a tangible therapy, device or diagnostic (Fig. 1). Often referred to as “the valley of death” based on the difficulty of such translational goals, the pharmaceutical industry not only triumphed, but did so in record time with the team of clinical investigators and clinical trial volunteers.
Drug development for COVID-19 infection involves bridging the “First Valley of Death” from scientific concept (Viral Identification) to a tangible drug or vaccine. This was achieved with Emergency Use Approval of an effective vaccine in record time. Bridging the “Second Valley of Death” with a COVID-19 vaccine was complicated by supply chain logistics and vaccine access hurdles, including hesitancy. Repurposing an approved drug for COVID-19 infection was partially successful, but seriously tarnished by lack of leadership and misinformation. Illustrations in this figure were created with Flaticon.com
The public often heard ‘it is vaccination, not vaccines which are needed to thwart the pandemic’. This journey from approved drug to patient health and happiness has also been termed “the second valley of death” as sometimes useful therapies are not integrated into health systems to have their intended effects. In the case of COVID-19 vaccines, this second translation involves packaging, distribution, acceptance and optimal delivery of care, e.g., decisions on vaccination sequence to various cohorts. This is a crucial aspect of the public health objective of herd immunity, which occurs when most of the population is immune to the viral infection, thus providing protection to those who are not immune. Herd immunity could be achieved if, for example, 8 out of 10 individuals developed immunity. In this case, 4 out of 5 people (80%) who contact someone with infection will not get ill nor will they spread the disease to others. Thus, spread is controlled.
5.2 Production, Packaging, and Procurement
As approvals of vaccine were imminent, modelling experts noted that their trial efficacy rates were important, but perhaps not as important as factors related to implementation effectiveness and background epidemic severity [34]. Implementation issues related to manufacturing and deployment delays, vaccine hesitancy or greater epidemic severity. Thus, the general public was exposed to other complexities of the process that are both unnoticed, and frankly, taken for granted.
Formulation of drugs, along with their packaging and preservation along the distribution pathway is complex and can present enormous challenges. First, vaccines being produced with new gene-based mRNA technology requires the solution to be stored at sub-zero temperatures including deep freezing during transport. This presented unique challenges considering millions of glass vials needed to be produced for transportation thousands of miles between logistic hubs – all while being kept at up to −80 degrees Celsius. Many logistics companies were preparing for such challenges in parallel with Phase 3 trials with assembly of “freezer farms” to assist with an unprecedented global scale logistics challenge [35, 36].
While freezers were one challenge, a global shortage of dry ice due to lower production of ethanol (its by-product carbon dioxide) during the pandemic presented another. Additionally, conventional glass commonly contains boron, which can lead to content contamination. Therefore, the need for pharmaceutical grade glass to withstand cracking in frozen conditions and maintain purity must be secured in parallel with clinical development to avoid delays in distribution once approved for distribution.
The cold-chain challenges were being addressed in parallel by the industry in cooperation with established logistics specialists in the public and private sectors as well as with major airlines. In parallel came the development of global positioning system (GPS) tracked temperature-controlled containers to allow for real-time monitoring [37].
Toward the end of this value chain comes ultimate administration protocols, most often complicated by the need for a second dose. While this passing of the baton to government authorities represents a clear milestone, the industry continued to scale-up procedures for continuity of supply and conduct life-cycle management (e.g., development of a powder formulation to simplify transport and storage) [38]. The reality of supply hurdles was illustrated by the quality control issues at one manufacturing facility [39] and seriousness of proper logistic planning was illustrated by alterations in EUA-approved dosing regimens [40] that were admittedly driven by procurement problems causing debatable public health trade-offs [41]. These dosing adjustments away from the authorised label uncouple real-world observations from clinical trial learnings.
5.3 Pharmacovigilance
Perhaps the most important aspect following emergency use is pharmacovigilance. Despite trial observations in tens of thousands of volunteers in clinical trials, real-world dosing in millions of individuals will uncover more rare events. This was illustrated with the rare, but potentially serious, observations of a vaccine-related variant of heparin-induced immune thrombotic thrombocytopenia [42, 43] (termed VITT), which resulted in a temporary pause in authorisation. This vigilance led to a temporary pause of the EUA while the FDA and Centers for Disease Control and Prevention (CDC) evaluated 13 cases among over 8 million doses of vaccine administered to that point. Following this in-depth review, administration was resumed [44].
6 Conclusions
The drug-development process is complex. The development of therapies to prevent and/or treat COVID-19 infection became a vital need during our global pandemic. While we relied on frontline measures of hand washing, facemasks, physical distancing, and crowd avoidance, the daily media was focused on drugs and vaccines with late-breaking news highlighting areas of drug development not commonplace in prime-time broadcasts. We have reviewed the steps along this journey, reinforcing the value of the research-based pharmaceutical industry and associated regulatory bodies. The months ahead will undoubtedly witness important new information from pharmacovigilance and real-world effectiveness studies along with new vaccines and therapeutics as our knowledge of the SARS-CoV-2 virus and its variants evolves.
References
Bauchner H, Malani PN, Sharfstein J. Reassuring the public and clinical community about the scientific review and approval of a COVID-19 vaccine. JAMA. 2020;324(13):1296.
United States Food and Drug Administration. Coronavirus Treatment Acceleration Program (CTAP). Silver Spring (MD): U.S Food and Drug Administration. 2021. https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap. Accessed 24 Jun 2021.
Farne H, Kumar K, Ritchie AI, Finney LJ, Johnston SL, Singanayagam A. Repurposing Existing drugs for the treatment of COVID-19. Ann Am Thorac Soc. 2020;17(10):1186–94.
Heaton PM. The Covid-19 vaccine-development multiverse. N Engl J Med. 2020;383(20):1986–8. https://doi.org/10.1056/NEJMe2025111.
Larson HJ. Stuck. How vaccine rumors start—and why they don’t go away. New York: Oxford University Press; 2020.
International Federation of Pharmaceutical Manufacturers & Associations. Complex Journey of as vaccine. The steps behind developing a new vaccine. IFPMA. 2019. https://www.ifpma.org/wp-content/uploads/2019/07/IFPMA-ComplexJourney-2019_FINAL.pdf.
Roos D. How a new vaccine was developed in record time in the 1960s. History.com. 2020. https://www.history.com/news/mumps-vaccine-world-war-ii. Accessed 24 Jun 2021.
Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Baily R, Swanson KA, Li P, Kouty K, Kalina W, Cooper D, Fontes-Garfias C, Shi P-Y, Türeci Ö, Tompkins KR, Walsh EE, Frenck R, Falsey AR, Dormizer PR, Gruber WC, Sahin U, Jansen KU. Phase I/II study of COVID-19RNA vaccine BNT162b1 in adults. Nature. 2020;590(7844):E26–E26. https://doi.org/10.1038/s41586-020-2639-4.
Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci O, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U, Gruber WC. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2021;383(25):2439–50.
Zimmer C, Corum J, Wee S-L. Coronavirus Vaccine Tracker. New York, NY: The New York Times. 2021. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html. Accessed 24 Jun 2021.
Pfizer Corporation. Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 vaccine candidate, meeting all primary efficacy endpoints. Pfizer News. 2020. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine. Accessed 24 Jun 2021.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Marc GP, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15.
US Centers for Disease Control and Prevention. Novel Coronavirus Reports. Atlanta, GA: MMWR. 2020. https://www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html. Accessed 24 Jun 2021.
Moderna, Inc. Moderna Completes Enrollment of Phase 3 COVE Study of mRNA Vaccine Against COVID-19 (mRNA-1273). Cambridge, MT: Moderna Inc. Press Release. 2020. https://investors.modernatx.com/news-releases/news-release-details/moderna-completes-enrollment-phase-3-cove-study-mrna-vaccine. Accessed 24 Jun 2021.
Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Damme WV, Leroux-Roels I, Berghmans PJ, Kimmel M, Damme PV, Hoon JD, Smith W, Stephenson KE, De Rosa SC, Cohen KW, McElrath MJ, Cormier E, Scheper G, Barouch DH, Hendriks J, Struyf F, Douoguih M, Hoof JV, Schuitemaker H. Interim Results of a phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med. 2021;NEJMoa2034201.
AstraZeneca. AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19 [Internet]. AstraZeneca Press Release. 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html. Accessed 24 Jun 2021.
Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, McGregor A, Morrison H, Mujadidi YF, Nana A, O’Reilly PJ, Padayachee SD, Pitella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Torok ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ, Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020;397(10269):99–111.
Robbins R, Meuller B. After admitting mistake, AstraZeneca Faces Difficult Questions About Its Vaccine. New York, NY: New York Times. 2020. https://www.nytimes.com/2020/11/25/business/coronavirus-vaccine-astrazeneca-oxford.html. Accessed 24 Jun 2021.
Mancini DM, Ahuja A, Cookson C. Doubts raised over AstraZeneca-Oxford vaccine data [Internet]. London, England: Financial Times. 2020. https://www.ft.com/content/4583fbf8-b47c-4e78-8253-22efcfa4903a. Accessed 24 Jun 2021.
Wu KJ, Thomas K. AstraZeneca Pauses Vaccine Trial for Safety Review. New York, NY: New York Times. 8 Sep 2020. https://www.nytimes.com/2020/09/08/health/coronavirus-astrazeneca-vaccine-safety.html. Accessed 24 June 2021.
Hughes V, Thomas K, Zimmer C, Wu KJ. Johnson & Johnson halts coronavirus vaccine trial because of sick volunteer. New York, NY: New York Times. 2020. https://www.bizjournals.com/sacramento/news/2020/10/13/johnson-johnson-halts-vaccine-trial-due-to-sick.html. Accessed 24 Jun 2021.
US Food and Drug Administration. Guidance for Clinical Trial Sponsors. Establishment and Operation of Clinical Trial Data Monitoring Committees. 2006. https://www.fda.gov/media/75398/download. Accessed 24 Jun 2021.
Australian Government, National Health and Medical Research Council. Data Safety Monitoring Board (DSMBs). Canberra, Australian Capital Territory (ACT): National Health and Medical Research Council. 2018. https://www.australianclinicaltrials.gov.au/sites/default/files/content/For%20researchers/Data%20Safety%20Monitoring%20Boards_1.pdf. Accessed 11 Aug 2021.
Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, Grousova DM, Erokhova AS, Kovyrshina AV, Botikov AG, Izhaeva FM, Popova O, Ozharovskaya TA, Esmagambetov IB, Favorskaya IA, Zrelkin DI, Voronina DV, Lubenets NL, Egorova DA, Shmarov MM, Nikitenko NA, Morozova LF, Smolyarchuk EA, Kryukov EV, Babira VF, Borisevich SV, Naroditsky BS, Gintsburg AL. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–97.
The National Research Center for Epidemiology and Microbiology. The first interim data analysis of the Sputnik V vaccine against COVID-19 phase III clinical trials in the Russian Federation demonstrated 92% efficacy. SpuntnikV. 2020. https://sputnikvaccine.com/newsroom/pressreleases/the-first-interim-data-analysis-of-the-sputnik-v-vaccine-against-covid-19-phase-iii-clinical-trials-/. Accessed 24 Jun 2021.
Callaway E. Russia announces positive COVID-vaccine results from controversial trial. Nature News. 2020. https://doi.org/10.1038/d41586-020-03209-0.
Corum J, Zimmer C. How gamaleya’s vaccine works. New York, NY: The New York Times. 2021. https://www.nytimes.com/interactive/2021/health/gamaleya-covid-19-vaccine.html. Accessed 11 Aug 2021.
Rowland R. Vaccine CEOs issue safety pledge amid Trump’s quest for pre-election approval. Washington, DC: The Washington Post. 2020. https://www.washingtonpost.com/business/2020/09/08/vaccine-safety-pledge-ceos/. Accessed 24 Jun 2021.
Cavazzoni P, Marks P, Mayne S, McMeekin J, Shuren J, Solomon S, Woodcock J, Zeller M. We are committed to making decisions guided by the best evidence. Our approach has been and must remain the gold standard that all can rely upon. USA Today. 2020. https://www.usatoday.com/story/opinion/2020/09/10/sound-science-to-meet-covid-challenges-fda-career-officials-column/5756948002/. Accessed 24 Jun 2021.
Chandler RE. Optimizing safety surveillance for COVID-19 vaccines. Nat Rev Immunol. 2020;20(8):451–2. https://doi.org/10.1038/s41577-020-0372-8.
United States Food and Drug Administration. Development and Licensure of Vaccines to Prevent COVID-19 Guidance for Industry. Silver Spring (MD): U.S Food and Drug Administration. 2020. https://www.fda.gov/media/139638/download. Accessed 24 Jun 2021.
US Food and Drug Administration. Advisory Committee Meeting. Vaccines and related biological products advisory committee February 26, 2021, meeting announcement. Silver Spring (MD): U.S Food and Drug Administration. 2021. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-february-26-2021-meeting-announcement#event-materials. Accessed 24 Jun 2021.
The United States Centers for Disease Control. Ensuring COVID-19 vaccines work. Atlanta, GA: Centers for Disease Control and Prevention. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness.html. Accessed 24 Jun 2021.
Paltiel AD, Schwartz JL, Zheng A, Walensky RP. Clinical outcomes of a COVID-19 vaccine: implementation over efficacy. Health Aff. 2021;40(1):42–52.
Gelles D. How to ship a vaccine at –80°C, and other obstacles in the Covid fight [Internet]. New York, NY: New York Times. 2020. https://www.nytimes.com/2020/09/18/business/coronavirus-covid-vaccine-cold-frozen-logistics.html. Accessed 24 Jun 2021.
Hopkins JS. Covid-19 vaccine race turns deep freezers into a hot commodity. New York, NY: The Wall Street Journal. 2020. https://www.wsj.com/articles/covid-19-vaccine-race-turns-deep-freezers-into-a-hot-commodity-11599217201. Accessed 24 Jun 2021.
Robbins R, Gelles D. How Pfizer plans to distribute its vaccine (It’s Complicated). New York, NY: The New York Times. 2020. https://www.nytimes.com/2020/11/12/business/pfizer-covid-vaccine-coronavirus.html?referringSource=articleShare. Accessed 24 Jun 2021.
Kansteiner F. Amid cold chain blues, Pfizer looks to powder vaccine formula in 2021: report. Fierce Pharma. 2020. https://www.fiercepharma.com/vaccines/amid-cold-chain-blues-pfizer-looks-to-powder-vaccine-formula-2021-report. Accessed 24 Jun 2021.
United States Food and Drug Administration. FDA continues important steps to ensure quality, safety and effectiveness of authorized COVID-19 vaccines. Silver Spring (MD): United States Food and Drug Administration. 2021. https://www.fda.gov/news-events/press-announcements/fda-continues-important-steps-ensure-quality-safety-and-effectiveness-authorized-covid-19-vaccines. Accessed 24 Jun 2021.
Aiello R. Vaccine manufacturers concerned about provinces delaying second doses: Anand [Internet]. CTV News. 2021. https://www.ctvnews.ca/politics/vaccine-manufacturers-concerned-about-provinces-delaying-second-doses-anand-1.5269040. Accessed 24 Jun 2021.
Kadire SR, Wachter RM, Lurie N. Delayed second dose versus standard regimen for Covid-19 vaccination. N Engl J Med. 2021;384(9):e28(1)-e28(3). https://doi.org/10.1056/NEJMclde2101987.
Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;2021:NEJMoa2104840.
Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;2021:NEJMoa2104882.
United States Food and Drug Administration. FDA and CDC lift recommended pause on Johnson & Johnson (Janssen) COVID-19 vaccine use following thorough safety review. Silver Spring (MD): U.S Food and Drug Administration. 2021. https://www.fda.gov/news-events/press-announcements/fda-and-cdc-lift-recommended-pause-johnson-johnson-janssen-covid-19-vaccine-use-following-thorough. Accessed 24 Jun 2021.
Pfizer Inc. A Phase 1/2/3, Placebo-controlled, randomized, observer-blind, dose-finding study to evaluate the safety, tolerability, immunogenicity, and efficacy of SARS-CoV-2 RNA vaccine candidates against COVID-19 in healthy individuals. New York, NY: Pfizer. 2020. https://cdn.pfizer.com/pfizercom/2020-11/C4591001_Clinical_Protocol_Nov2020.pdf.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Eng K Med. 2020;383:2603–15. https://doi.org/10.1056/NEJMoa2034577.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;383:403–16. https://doi.org/10.1056/NEJMoa2035389.
Sadoff J, Gray G, Vandebosch A, Cardenas V, Shukavrev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;383:2187–201. https://doi.org/10.1056/NEJMoa2101544.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received.
Conflict of interest
No conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
T.W–Conception of work, Manuscript Writing, Responsible Author. T.W has read and approved the final paper and agrees to be accountable for the work.
Rights and permissions
About this article
Cite this article
Witek, T.J. How the Global COVID-19 Pandemic Brought Drug and Vaccine Development into the Public Mainstream. Pharm Med 35, 287–295 (2021). https://doi.org/10.1007/s40290-021-00402-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-021-00402-y