Abstract
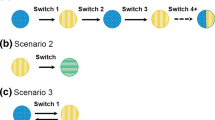
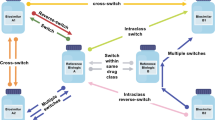
Under the US Biologics Price Competition and Innovation Act of 2009 (BPCI), the development of biosimilar (test) products provides affordable alternatives to innovative biological (reference) products for the general patient population. However, in practice, as the number of biosimilar products available on the market increases, whether these biosimilars can be used interchangeably is a concern. Thus, using switching studies to evaluate the risk of reduced efficacy and increased safety concerns with and without switch(es) in the development of biosimilar products is of interest. For this purpose, the US FDA, in its recent draft guidance on interchangeability, suggested using a 2 × 2 crossover design (RT, RR) to evaluate a single switch and (RTR, RRR) and (RTRT, RRRR) to evaluate multiple switches. In this article, we examine the statistical properties, analyses, and sample size requirements of these switching study designs. We also investigate the relative efficiencies of these switching designs compared with the complete n-of-1 trial design.
Similar content being viewed by others
References
Endrenyi L, Declerck P, Chow SC. Biosimilar Product Development. London: Taylor & Francis; 2017.
BPCI. Biologic price, competition, and innovation act. Washington DC: United States Congress; 2009.
FDA. Guidance on Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. Silver Spring: The United States Food and Drug Administration; 2015.
FDA (2017, 2019). Guidance for industry—considerations in demonstrating interchangeability with a reference product. The United States Food and Drug Administration, Silver Spring.
Chow S, Song F, Cui C. On hybrid parallel–crossover designs for assessing drug interchangeability of biosimilar products. J Biopharm Stat. 2017;27:265–71.
PHS (1944). Public Health Services Act. Passed by United States Congress and enacted July, 1944. Washington DC.
FDA. Guidance on statistical approaches for evaluation of bioequivalence. Rockville: Center for Drug Evaluation and Research, U.S. Food and Drug Administration; 2001.
FDA. Guidance on bioavailability and bioequivalence studies for orally administrated drug products—general considerations. Rockville: Center for Drug Evaluation and Research, U.S. Food and Drug Administration; 2003.
Chow SC, Liu JP (2008) Design and analysis of bioavailability and bioequivalence studies. 3rd ed, vol 25. New York: Taylor & Francis, pp. 237–241.
Chow SC. Biosimilars: design and analysis of follow-on biologics. 1st ed. Boca Raton: Chapman and Hall/CRC; 2013.
Haidar SH, Davit B, Chen ML, Conner D, Lee L, Li QH, Lionberger R, Makhlouf F, Patel D, Schuirmann DJ, Yu LX. Bioequivalence approaches for highly variable drugs and drug products. Pharm Res. 2008;25(1):237–41.
Chow SC, Xu H, Endrenyi L, Song FY. A new scaled criterion for drug interchangeability. Chin J Pharm Anal. 2015;35(5):844–8.
Lillie EO, Patay B, Diamant J, Issell B, Topol E, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Personal Med. 2011;8(2):161–73. https://doi.org/10.2217/pme.11.7.
Davidson KW, Cheung YK, McGinn T, Wang YC (2018) Expanding the role of n-of-1 trials in the precision medicine era: action priorities and practical consideration. Commentary. National Academy of Medicine, Washington DC. http://doi.org/10.31478/201812d.
Chen KW, Chow SC, Li G. A note on sample size determination for bioequivalence studies with higher-order crossover designs. J Pharmacokinet Biopharm. 1997;25:753–65. https://doi.org/10.1023/a:1025738019069.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this research article.
Conflict of interest
Dr. Shein-Chung Chow and Dr. Sang Joon Lee have no conflicts of interest that are directly relevant to the content of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chow, SC., Lee, S.J. Design and Analysis of Biosimilar Switching Studies. Pharm Med 33, 379–388 (2019). https://doi.org/10.1007/s40290-019-00296-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-019-00296-x