Abstract
Introduction
Developing countries can improve their pharmacovigilance systems by analysing their own medication safety data.
Objective
The aims of this study were to characterize Uganda’s reported adverse drug reaction (ADR) onsets in 2012–2015 that were registered on VigiBase® by 31 December 2017, to document delays in international visibility and the influence of covariates on this delay from ADR onsets in 2013 + 2014, to examine data quality, and to illustrate analytical approaches for safety data, particularly for patients receiving antiretroviral therapy (ART).
Methods
International delay was defined as elapsed time from complete ADR onset date to entry date on VigiBase®, with covariates examined using Cox proportional hazards regression. Simple random sampling was used to locate the paper-based ADR forms for data quality assurance. Disproportionality for signal detection focused on serious singleton ADR onsets in patients receiving ART.
Results
Uganda’s VigiBase® had 1018 patient entries with complete ADR onset dates: 260 in 2012, 293 in 2013, 305 in 2014 and 160 in 2015. Only 16% (154/953) of ADR onsets in 2012–2015 were in patients aged < 20 years for whom randomly sampled ADR forms were less fully completed; 87% (889/1018) comprised a singleton sign/symptom; half were serious. Median delay from ADR onset to international visibility was 11 months for ADR onsets in 2013 + 2014, and longest for healthcare professionals other than pharmacists and physicians. Disproportionality for serious ADR onsets in patients receiving ART included anaemia with zidovudine, renal impairment with tenofovir, Stevens–Johnson syndrome with nevirapine and skin rash with efavirenz.
Conclusions
Barely one ADR onset per day was registered on VigiBase® from those submitted to Uganda’s National Pharmacovigilance Centre during 2012–2014; only one in six was from patients aged < 20 years. Paediatric pharmacovigilance requires more emphasis in Uganda. Delays from reported ADR onset to international visibility on VigiBase® need to reduce dramatically. Quality assurance revealed rectifiable data entry deficits. Signal detection performed well for patients receiving ART.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Barely one adverse drug reaction (ADR) onset per day was registered on VigiBase® from those submitted to Uganda’s National Pharmacovigilance Centre during 2012–2014; only one in six were from patients aged < 20 years. |
Median delay from reported ADR onset to international visibility on VigiBase® was 11 months for ADR onsets in 2013 + 2014. |
Quality assurance revealed rectifiable data entry deficits. Nonetheless, our illustrative signal detection analyses performed well for patients receiving antiretroviral therapy. |
1 Introduction
The global visibility of a country’s pharmacovigilance system is evidenced by its involvement in the World Health Organization (WHO) Programme for International Drug Monitoring (PIDM), which had 131 full member countries and 26 associates by 13 March 2018: 35 of 54 African countries were full members and seven were associates [1]. Uganda became a full member of the WHO–PIDM in 2007 and quickly established a regional network by which its National Pharmacovigilance Centre (NPC) disseminates information, conducts training and receives pharmacovigilance reports [1, 2].
Uganda is divided into 127 districts grouped into four administrative regions: central, eastern, northern and western. Uganda’s population was around 40 million in 2014, two-thirds of whom were aged < 20 years [3]. HIV infection, malaria and tuberculosis contribute significantly to morbidity and mortality. Thus, medicines for these three infectious diseases represent a large proportion of reported adverse drug reactions (ADRs)—a pattern that is similar in other sub-Saharan African (SSA) countries [4, 5]. Uganda’s public healthcare sector operates on a referral basis from village health teams through primary care facilities (health centres at levels II, III and IV) to district/general hospitals (49), regional referral hospitals (RRHs) (14) and, at the apex, national referral hospitals (2). Each RRH is a designated regional pharmacovigilance centre responsible for receiving ADR reports from lower-level health facilities and transmitting them to the NPC. Private health facilities have similar structures to public health facilities. There exist 63 private not-for-profit hospitals and 27 private for-profit hospitals.
Given the low rate of spontaneous ADR reporting in Uganda, as in other SSA countries [6, 7], its NPC introduced online ADR reporting in 2015 [8]. The NPC also participated in novel pharmacovigilance initiatives globally, such as the piloting of targeted spontaneous reporting of suspected renal toxicity of tenofovir-based antiretroviral therapy (ART) regimens, which included surveillance of 10,225 patients during 2012–2014 [9]. However, more interventions are required to significantly increase Uganda’s rate of ADR reporting.
Pharmacovigilance in Uganda and other low-resource settings can be strengthened through collaborations that directly promote both the rate and the quality of ADR reporting and, in so doing, augment signal detection. The use of smartphone applications in pharmacovigilance could galvanize ADR reporting in SSA, including from patients and the general public [3, 10]. The 40th Annual Meeting of Representatives of NPCs in the WHO-PIDM was held in Kampala, Uganda, in November 2017, with the theme “Smart safety surveillance where resources are limited” [11]. Besides improving the rate and quality of ADR reporting, SSA countries in the WHO-PIDM also need to demonstrate that they can undertake timely country-specific analyses of extant drug safety data and act on these results to give prompt feedback to ADR reporters, the general public and government/private agencies [12].
We illustrate a series of analytical and pharmacovigilance approaches for Uganda that may be deployed by Africa’s NPCs and other countries with young pharmacovigilance systems as they expand their pharmacovigilance databases and improve feedback to reporters. The aims of this paper are as follows:
-
1.
Describe the characteristics of ADR onsets in 2012–2015 that were internationally visible on VigiBase® by 31 December 2017.
-
2.
Document the international delay distribution of ADR onsets in 2012–2015 and, for ADR onsets in 2013 + 2014, covariate influences on their being internationally visible within 1 year.
-
3.
Illustrate analytical methods for:
-
3.1
appraising how peer-reviewed published ADR reports on pharmacovigilance are represented in Uganda’s VigiBase®,
-
3.2
quality control of the initial-stage assessment of ADR onsets reported to the NPC and
-
3.3
signal detection by profiling three specific ART medicines with known associated ADR onsets, and for patients whose ART medication excluded the trio of drugs, and by highlighting almost surely iatrogenic ADR onsets.
-
3.1
2 Methods
2.1 Study Approach
The research was conducted over 10 days in February 2018. The pharmacist/statistician team (RK/SMB) obtained approval from the NPC team (VN/HBN) to gain access to Uganda’s anonymised ADR reports that had been registered on the Uppsala Monitoring Centre (UMC) de-duplicated international database, VigiBase®, by 31 December 2017. This review focused on ADR onsets with known start dates in 2012–2015. For simplicity and to minimize loss of generality, we analysed only ADR onsets whose VigiBase® serial number began UG-NDA, and we did not analyse dosages [13]. See the Electronic Supplementary Material (ESM) for details of how pharmacovigilance reports are processed in Uganda’s VigiBase®. An initial data-orientation exercise provided insight into how to structure the subsequent formal data analyses. Key areas for formal analyses included (1) characteristics of the ADR onsets and their seasonal reporting patterns, (2) elapsed time from ADR onset to international registration on VigiBase® and the covariate influences on delayed international visibility, (3) data quality, (4) VigiBase® registration of exceptional ADRs from Uganda that were already published in peer-reviewed journals, (5) illustration of signal detection analyses for serious singleton ADR onsets stratified by ART status and (6) pharmacovigilance of antituberculosis medications.
2.2 Delayed International Registration of Adverse Drug Reaction (ADR) Onsets and Covariate Influences
We defined international delay as the time between ADR onset and the date of entry on VigiBase®, which marked its international visibility. We documented the distribution of international delays for ADRs in four separate calendar years: 2012, 2013, 2014 and 2015. Separation was necessary because the delay distribution for ADR onsets in 2015 was incompletely observed as the maximum observable delay was only 2 years for ADR onsets as late as 31 December 2015.
In any registry, the sequence of event dates is a key issue. In deriving delay distributions, simplifications were made with minimal loss of generality. First, only complete ADR onset dates (year-month-day) were considered. Second, if the ADR onset comprised more than one sign/symptom, each with its own complete onset date, then the latest complete onset date (within same year as the earliest) was recorded as the ADR onset date.
Only for 2013 + 2014 ADR onsets did we examine how their delay distribution was influenced by five covariates: patient’s sex, patient’s age group, qualification of reporter, seriousness of suspected ADR and whether the patient encountered a multiplicity of completely dated signs/symptoms. We used proportional hazards regression to analyse the joint influence of this set of covariates on the achievement of international delays of less than 1 year.
2.3 Quality Assurance of 45 Randomly Sampled ADR Forms
For quality assurance, RK/SMB also reviewed three sets of ADR forms to explore the influence of reporter’s qualification, tendency for only a single sign/symptom to be described and relative infrequency of ADR reports for individuals aged < 20 years. We illustrate the value of simple random sampling for quality control via three random samples, each of 15 ADR forms, for ADR onsets in 2013 + 2014 by (1) 61 pharmacist reporters, (2) 62 physician reporters and (3) 92 individuals aged < 20 years. The quality of ADR form completion was subjectively scored from 1 (low) to 5 (high) by RK and compared with VigiBase®. Besides the quality score, we abstracted data on whether the patient had received ART, whether the reporter described a multiplicity of reactions, whether dose details were provided and whether other medications were used besides the “suspected” drug. We also recorded the date of ADR onset, the date the ADR form was received by the NPC and the presence/absence of signatures from first and second ADR causality assessors.
2.4 Appraisal of Uganda’s VigiBase® for Exceptional Peer-Reviewed Published ADRs
We also investigated exceptional ADRs in the SSA literature, namely, (1) ADR onsets mentioning paralysis (to look for cases linked to intramuscular injection of quinine into the gluteal muscle, as first reported by Ugandan surgeons [14]) and (2) ADR onsets mentioning diclofenac (as per the report by Kiguba et al. [12] detailing a healthcare professional’s [HCP] suspicion of diclofenac-related haemoptysis in Uganda when only two such case reports had been published internationally).
2.5 Illustrative Signal Detection Approaches
2.5.1 Iatrogenic ADR Onsets
We inspected exceptional Medical Dictionary for Regulatory Activities (MedDRA) terms associated with serious singleton ADRs for which the ADR was almost surely iatrogenic. We focused on instances where only a single drug was reported since even a single serious ADR onset may be sufficient to occasion a pharmacovigilance alert. Where several drugs were used, we identified the co-prescribed suspect and concomitant drugs. Exceptional MedDRA terms that almost surely indicate an iatrogenic ADR include gynaecomastia in an otherwise healthy male patient, acute tardive dyskinesias or erythema multiforme [15].
2.5.2 Disproportionality
For serious singleton ADRs reported to Uganda’s NPC, the majority had received ART so that signal detection for serious ADRs was necessarily stratified by the presence/absence of ART. We illustrate signal detection using the disproportionality method, introduced by Finney [16,17,18,19]. We concentrate primarily on the stratum of serious singleton ADR onsets in 2012–2014 experienced by Ugandan patients who had received ART.
Initially, we illustrate the power of disproportionality for three ART medicines licensed well before 2012 and with known serious ADRs as follows: anaemia (zidovudine), renal impairment/increased blood creatinine (tenofovir) and Stevens–Johnson syndrome (SJS) (nevirapine). We profile each of these trio drugs (zidovudine, tenofovir, nevirapine) using selected MedDRA terms including anaemia, renal impairment/increased blood creatinine and SJS. However, we also investigated hepatocellular damage and the MedDRA terms ‘rash’, ‘rash: specified’ and ‘urticaria’. Profiles are also shown for patients receiving ART who received none of the trio of zidovudine, tenofovir, nevirapine and for patients receiving ART who received/did not receive co-trimoxazole (subsequently referred to as sulfamethoxazole; trimethoprim), an antibacterial.
We coded every patient with a serious singleton ADR onset according to whether the patient had received (1 = yes) or not (0 = no) ART, zidovudine, tenofovir, nevirapine or any of the trio drugs (zidovudine, tenofovir, nevirapine).
We compare the major profiles of MedDRA terms for serious singleton ADR onsets in 2012–2014 in patients receiving ART who received at least one of the above trio versus those who received only non-trio ART. We describe the simple exploratory methods by which we tracked down the source of the detected signal (using Fisher’s exact test).
2.6 Pharmacovigilance of Antituberculosis Medications
The NPC team took close interest in the pharmacovigilance of antituberculosis medications, including bedaquiline, a new molecule recently introduced globally [20], and kanamycin for multidrug-resistant tuberculosis (MDR-TB) [21,22,23]. Thus, these drugs were factored into our illustrative analyses.
3 Results
3.1 Characteristics of ADR Onsets
After excluding 12 patient entries coded as UG-UNEPI and 150 with incomplete onset dates (39 in 2012, 27 in 2013, 49 in 2014, 35 in 2015), 1018 patient entries were registered on Uganda’s VigiBase® for 1 January 2012 to 31 December 2017 with a complete latest onset date in 2012–2015. Of these patient entries, 260 ADR onset dates were in 2012, 293 were in 2013, 305 were in 2014 and only 160 were in 2015 (Table 1). The 1018 ADR onsets included 18 fatalities and a foetal death (whose mother had received efavirenz).
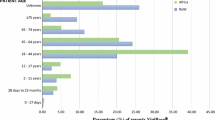
Table 1 also summarizes, for each ADR onset year, the sex and age group of patients whose ADR onset was reported, the reporter’s qualification, whether the registered ADR onset was serious and whether a single or multiple signs/symptoms were registered. Females accounted for 72% of ADR onsets in 2012 (183/253; 95% confidence interval [CI] 67–78), a higher percentage than in 2013 + 2014 (65%, 383/586; 95% CI for difference 0–14) and 2015 (53%, 83/157; 95% CI for difference 10–29). Only 16% (43/273; 95% CI 12–21) of Uganda’s internationally registered ADR onsets in 2012–2015 related to patients aged < 20 years.
Qualification of the reporter, when known, was registered most often as ‘other HCP’ (74%). The annual contribution of pharmacists varied from 12 to 43 ADR onsets and that by physicians ranged from 11 to 58. For ADR onsets in 2013 + 2014, pharmacists and physicians contributed about equally (62 and 61 ADR onsets, respectively).
Around half of all reported ADR onsets were serious; the only temporal change was in 2015 when the proportion of serious ADR onsets was higher (71%, 114/160). A remarkably high proportion of registered ADR onsets in 2012–2015 (87%, 889/1018) comprised a singleton sign/symptom (Table 1), which differed by qualification of reporter, with 70% (65/93) of ADR onsets being singleton if reported by pharmacists versus 95% (124/130) by physicians and 89% (574/644) by other HCPs; and 83% (126/151) if the reporter’s qualification was unknown.
We observed seasonality in the reporting of ADR onsets. Table S1 in the ESM shows significant month-to-month heterogeneity in reported ADR onsets for 2012–2014, with high counts in the rainy months (October, November, April, May)—averaging 0.93 per day (Poisson 95% CI 0.83–1.03)—and low counts in December and January, coincident with vacation—averaging 0.53 per day (Poisson 95% CI 0.42–0.63).
3.2 Delayed International Registration of ADR Onsets and Covariate Influences
Considerable delays could occur between ADR onset date and the report’s entry date on VigiBase®. Delays of more than 1 year were not infrequent, with some longer than 3 years (Table 1).
Table 2 explores the possible influence of five covariates on the typical delay from ADR onset in 2013 + 2014 to its international visibility on Uganda’s VigiBase®. Neither patient sex nor ADR seriousness appeared to influence the median delay. However, the delay distribution for serious ADR onsets was longer tailed. Pharmacist reporters achieved the shortest median delay (186 days) versus that for physicians (315 days); and other HCPs had the longest median delay (415 days). There is some suggestion that median delay to international visibility increased with the patient’s age group, being lowest for patients aged < 20 years (262 days) and highest for patients aged ≥ 50 years (414 days). Delay until international visibility was considerably shorter if the registered ADR onset had a multiplicity of signs/symptoms (see also Fig. 1).
Table 3 shows that qualification of reporter, multiplicity of signs/symptoms of suspected ADR onset and age group were the most influential covariates in determining whether a reported ADR onset in 2013 + 2014 was internationally visible within 1 year of the patient’s ADR experience. Only qualification of reporter was significant at the 1% level and showed that the shortest delays were achieved when the reporter was either a pharmacist or the qualification was unknown. By far the longest delays occurred when the reporter was ‘other HCP’.
3.3 Quality Assurance of 45 Randomly Sampled ADR Forms
Only 29 of the 45 randomly selected ADR forms were located (64%, 95% CI 50–78). The running mean for the percentage of located ADR forms that cited multiple reactions was (6 + 4 + 5)/(11 + 10 + 8), or 52% (15/29, 95% CI 33–70) (see Table S2 in the ESM), which is significantly higher than recorded in Uganda’s VigiBase®. Only seven of the 15 ADR forms with multiple reactions were correctly registered as a multiplicity on VigiBase®. The six multiple reactions reported by pharmacists were all registered on VigiBase® as multiple reactions. By contrast, only one of the nine multiple reactions recorded by physicians or for individuals aged < 20 years was registered on VigiBase® as a multiplicity (p = 0.0014, Fisher’s exact test).
Mean ± standard deviation (SD) RK subjective score (out of 5) for quality of reporting was 4.3 ± 1.01 for pharmacists, 4.2 ± 0.63 for physicians but only 3.0 ± 0.76 for the eight patients aged < 20 years. The pooled SD was 0.83. Thus, the standard error for comparison of scores for the youngest patients versus for ADR reports completed by physicians or pharmacists was 0.34. The quality of completion of ADR reports for children and young adults appeared significantly lower than that of reports from physicians and pharmacists (t test on 26 df − 3.6; p = 0.002).
3.4 Appraisal of Uganda’s VigiBase® for exceptional Peer-Reviewed Published ADRs
Diclofenac-linked haemoptysis: Ten ADR reports in Uganda’s international database to 31 December 2017 mentioned diclofenac as potentially involved in an ADR. Eight of the ten ADR forms were located; none included a description that related, even remotely, to haemoptysis [12]. Of the eight forms, five had an ADR causality assessor sheet; four of the five assessor sheets were signed by the first assessor, but none was counter signed.
Quinine-linked limb paralysis: Of six listed cases, two had UG-UNEPI 2009 codes (one from Kamuli District in eastern Uganda) and one an UG-UNEPI 2011 code (region not specified). None of these three ADR forms was located. The other three cases had UG-NDA codes, two for 2011 (both from Kamuli District, and both of which were located) but the third, provided electronically, was for 2013 (from Kayunga District in eastern Uganda).
The two located ADR forms from Kamuli District related to intramuscular injection of quinine for the treatment of malaria. Neither specified the injection site (buttock or thigh). The reporter, the first assessor and the date (11 March 2011) was the same for both reports; neither report was co-signed.
ADR onset date for the first case (female, aged 32 years, treated during 10–12 November 2010) was not specified on the form but was entered on VigiBase® as the period of quinine treatment (10–12 November 2010). The reporter specified the route as intramuscular, but the route was entered incorrectly on VigiBase® as ‘intrameningeal’. Anaphylaxis and paralysis of right lower limb were reported on the ADR form, but only paralysis was registered on VigiBase®. This first report, dated 17 December 2010, was received at the NPC on 2 March 2011 and first assessed on 11 March 2011. Other drugs used were paracetamol, metronidazole, oral rehydration solution and ciprofloxacin, three of which were entered on VigiBase®; oral rehydration solution was not entered.
The second case (male, aged 2 years) received intramuscular quinine during 16–18 December 2010. Severe local reaction and paralysis of the limb were reported on the ADR form, but only limb paralysis was registered on VigiBase®. Limb paralysis began on 19 December 2010. Other drugs administered were diclofenac for inflammation and paracetamol for pyrexia, but neither was entered on VigiBase®. This second report, dated 3 January 2011, was received at the NPC on 2 March 2011 and was also first assessed on 11 March 2011 by the same assessor who dealt with the first report, on the same day. The route was entered correctly as intramuscular on VigiBase®.
The 2013 electronic ADR report related to three ART medications (tenofovir, lamivudine, nevirapine) received for over a year by a 47-year-old female patient. She experienced paraesthesia of both lower limbs for 5 months (onset dated May 2012) and renal impairment. All three were suspected drugs. Unlike the ADR form, VigiBase® listed paraesthesia as the only ADR.
3.5 Illustrative Signal Detection Approaches
3.5.1 MedDRA Terms Linked to Three or More Singleton ADR Onsets
Table 4 lists the MedDRA terms linked to three or more fully dated singleton ADR onsets in 2012–2014. The listed MedDRA terms account for 267 (77%) of the 346 serious singleton ADR onsets and for 271 (69%) of the 395 not labelled as serious ADR onsets.
3.5.2 Iatrogenic ADR Onsets
Table 5 lists exceptional MedDRA terms, including those indicted as iatrogenic [15], that account for a further 27 singleton ADR onsets in the serious and ‘not labelled as serious’ categories. In Tables 4 and 5, the following iatrogenic ADR onsets are notable: anaphylactic shock with benzylpenicillin; two reports of erythema multiforme in patients who received sulfamethoxazole; trimethoprim (one of whom also received nevirapine); six reports of gynaecomastia in males, all of whom received efavirenz; and tardive dyskinesia in three patients who received metoclopramide (one of whom also received ciprofloxacin and metronidazole).
Table 5 also highlights two reports of Fanconi syndrome in which tenofovir was the suspected drug, although one patient also received two other ART medicines together with sulfamethoxazole; trimethoprim; one report of foetal death (mother had received efavirenz); and four reports of visual impairment/blurred vision/reduced visual acuity in relation to the antituberculosis medications ethambutol (three cases) or ethionamide (one case).
3.5.3 Disproportionality
In Table 4, the MedDRA terms occurring disproportionately in association with serious ADR onsets included anaemia, renal impairment and increased blood creatinine, and SJS. They are precisely the serious ADR onsets that occur disproportionately among patients with serious singleton ADR onsets who were receiving ART.
Table 6 shows clear signals of the following associations in Uganda’s 252 serious singleton ADR onsets in 2012–2014 for patients receiving ART: zidovudine with anaemia (zidovudine 44% [42/96] vs. no zidovudine 0% [0/156]), tenofovir with renal impairment (tenofovir 32% [24/75] vs. no tenofovir 1% [2/177]) and nevirapine with SJS (nevirapine: 25% [31/123] vs. no nevirapine: 4% [5/129]). Notice also ‘rash’, which is otherwise unspecified.
We now focus on the columns of Table 6 headed ‘Any of the trio’. Intriguingly, there is a signal that the 29 patients receiving ART who did not receive zidovudine, tenofovir or nevirapine had a significantly increased risk (38% [11/29]) of ‘rash: specified’ or ‘urticaria’ versus trio patients (11% [25/223]) who reported having a serious singleton ADR (Chi squared on 1 degree of freedom 14.96, p < 0.001).
Table 7 lists the medicines received and the serious ADR onsets experienced by non-trio cases. Efavirenz was prescribed for 23/29 non-trio patients, including all 11 whose serious singleton ADRs were ‘rash: specified’ (6) or ‘urticaria’ (5). Gynaecomastia in a 60-year-old male and foetal death were also listed against efavirenz.
3.6 Pharmacovigilance of Antituberculosis Medications
Tables S3 and S4 in the ESM show how we investigated drug–drug interactions between antituberculosis medications and each of zidovudine, sulfamethoxazole; trimethoprim and the trio of zidovudine; tenofovir; nevirapine; see also ototoxicity in patients who received MDR-TB treatment.
4 Discussion
This paper coincides with the tenth anniversary of the NPC and focuses on over 1000 reports of ADR onsets that occurred during 2012–2015 for which ADR onset dates were complete and the corresponding individual case safety reports (ICSRs) were internationally reported by 31 December 2017 on VigiBase®, the global ICSR database managed by the UMC [24].
Despite a population of 40 million and highly prevalent HIV, malaria and tuberculosis, completely dated ADR onsets reported to Uganda’s NPC were fewer than one per day in 2012–2014 and fewer than eight per million of population annually, despite Uganda’s membership since 2007 of the WHO-PIDM [1, 25]. Moreover, to stimulate reporting, NPC has undertaken targeted reporting of ADRs for patients receiving ART [9] and follow-up of an annual, national cohort of patients who receive treatment for MDR-TB. The NPC’s excellent work on targeted surveillance for ADR onsets in over 10,000 patients who received tenofovir allowed estimation of both the incidence of renal impairment and the under-ascertainment rate by spontaneous reporting [9].
In addition to the low rate of ADR notifications to the NPC, median delay from ADR onset to its international visibility on VigiBase® was 11 months for ADR onsets in 2013 + 2014. Registration delays arise for a variety of reasons, including to improve data quality and ensure that the NPC’s internationally reported suspected ADR onsets meet the required entry criteria set by the UMC. Delays matter primarily because they slow the detection of safety signals and consequent recommendations on harm-reduction measures. The contribution of the NPC’s regional centres, mostly busy RRHs, to the overall delay from ADR onset to international visibility should be assessed. Historically, we have evidenced a delay of 2 months before the NPC received two within-region reports of paralysis following intramuscular quinine injection.
Registration on VigiBase® within 1 year of ADR onset was less likely for reports submitted by HCPs other than pharmacists and physicians but more likely if the VigiBase®-registered ICSR comprised multiple clinical signs/symptoms. Other HCPs may have to negotiate institutional reporting hierarchies before the NPC is informed; alternatively, reports by other HCPs may be subject to more time-consuming cross-checks to pass quality assurance at the NPC. In total, 90% of registered ADR onsets reported by physicians or other HCPs, compared with 70% of those by pharmacists, described a single sign/symptom. As the first stage of assessment at the NPC is performed by intern pharmacists, it is interesting that our random sampling of ADR forms revealed that pharmacists’ descriptions of multiple signs/symptoms was more faithfully represented on VigiBase® than were those described by physicians or pertaining to patients aged < 20 years. The NPC team confirmed a tendency for NPC’s assessors to summarize the reporter’s account of signs/symptoms, yet the text and commentary conveyed in actual ADR reports could be additionally insightful. The NPC’s current training on the use of MedDRA classifications emphasises that each sign/symptom should be coded as detailed by the reporter. Random sampling can be used to check that the NPC’s training has indeed changed practice. The proportion of ADR onsets recorded as serious was highest in 2015: with only half the reports registered, the NPC may have prioritized registering serious ADR onsets.
Reporting suspected ADR onsets at the extremes of age is particularly important [18]. Older patients with comorbidities may have been excluded from licensing trials. Medicines initially licensed for use in adults may have undergone less detailed study in children before being approved for paediatric use, or their use could be off-label. Only one in six reported ADR onsets in 2012–2015 and registered on Uganda’s VigiBase® by 31 December 2017 pertained to patients aged < 20 years, and their randomly sampled ADR forms were less well completed. Only two ADR onsets per million of population aged < 20 years (46/23.6) were reported annually. Paediatric pharmacovigilance requires more emphasis in Uganda where, in 2014, 59% of the population was aged < 20 years [3].
We showed significant seasonality in reported ADR onsets, with a substantially higher rate during the rainy months of October, November, April and May and a low reported ADR onset rate in December and January. The former is likely to be disease related, whereas the December and January low may be a reporting deficit during the Christmas and New Year vacation. Seasonal variation in the spontaneous reporting of suspected ADRs is well-documented in the developed world and should be heeded to mitigate false-positive safety signals for new ADRs when comparison periods are short [26].
For patients receiving ART, we illustrated signal detection using disproportionality for three selected drugs (zidovudine, tenofovir, nevirapine) with different known serious ADRs (anaemia, renal impairment, SJS). We presented a MedDRA terms profile for each selected drug and for patients who received none of these three. Our illustration of signal detection worked very well; perhaps too well. Each selected drug had been licensed for more than a decade by 2014, so reporters were probably already familiar with each drug’s most serious ADRs. Thus, the reporting of ADR onsets may have more been evidence of the reporters’ knowledge than illustrative of the undoubted potential of Ugandan HCPs to recognise novel serious ADRs [12].
The serious ADR onsets reported to the NPC for the 29 non-trio patients were sufficient for signal detection related to ‘rash: specified’ and ‘urticaria’. Even this demonstration may have been aided by some spillover from training that reporters could have received in association with NPC’s piloting in 2013 + 2014 of stimulated reporting of ADR onsets for patients who received tenofovir [9]. Moreover, pharmacist RK (but not statistician SMB) already knew that patients receiving efavirenz are at risk of maculopapular rash and urticaria.
Signals will be amplified if reports from stimulated ADR recognition in specifically designed cohorts are amalgamated with spontaneous reports. Ideally, stimulated ADR reports from cohort studies should be specifically identifiable in VigiBase®, but they are not.
Besides deploying disproportionality separately for patients who received ART, we illustrated other approaches to pharmacovigilance, notably, searching—within Uganda’s VigiBase®—for safety reports on published pharmacovigilance signals from SSA [12]; review of ADR fatalities (including foetal death); and inspection of rare serious ADRs, which are almost always iatrogenic [15] and, thus, indict the suspected drug (if it is truly the only medication that the patient received). However, WHO-PIDM countries with few ADR reports can also use global evidence from the UMC’s web-based VigiLyze to support analysis of their national databases [27].
Our purposive, randomly sampled look-backs to actual ADR forms for quality assurance revealed deficits, including that other drugs a patient was receiving did not feature on the VigiBase® entry. Hence, before a rare, serious iatrogenic ADR can be taken as indictment of the suspected drug, it is necessary to check information on the reporter’s ADR form to ensure that other drugs were not documented by the reporter.
Look-back was challenging at the NPC, as a third of randomly sampled ADR forms could not be located. Electronic submission may alleviate the misfiling of paper forms but must allow sufficient space for comment, otherwise context and detail previously written down by reporters may be lost. For the pair of quinine reports from Kamuli District, 2 months passed before they were received at the NPC, whereupon intramuscular was entered differently, the correct entry being made on the basis of the form’s comment section (where route was identified as intramuscular) despite ‘route’ being coded by the reporter as ‘not applicable’. Both reports cited a singleton reaction (paralysis) on VigiBase®, even though both forms mentioned two reactions. Nonetheless, this pair of ADR reports illustrated prompt recognition of serious, specific and exceptional ADR onsets and their timely reporting.
ADR reports were sufficient for a causal link to be declared for efavirenz and gynaecomastia, tenofovir and Fanconi syndrome, sulfamethoxazole; trimethoprim and erythema multiforme, and metoclopramide and tardive dyskinesia. The US FDA issued a warning about the latter in August 2011 [28]. NPCs should maintain a national list of ‘almost surely iatrogenic’ MedDRA terms, against which each newly reported ADR onset can be checked. If necessary, the associated medication can then be added to the current watchlist of drugs on which the NPC is conducting enhanced surveillance.
4.1 Limitations
We analysed Uganda’s de-duplicated VigiBase® and did not review the NPC’s methodology (exact or probabilistic; regional or national) for recognising duplicate reports. Likewise, we did not review whether the ADR onset, as registered on VigiBase®, was a superset or an intersection of the information conveyed by its several reporters. For surety, we also focused on completely dated ADR onsets. Further investigation of the excluded 15% of ADR onsets could be warranted.
Second, because ADR onsets mostly registered only a single sign/symptom, our analysis concentrated on serious single ADR onsets in 2012–2014. See Table S5 in the ESM for multiple signs/symptoms.
Third, as regional pharmacovigilance centres are not registered on VigiBase®, we could not analyse regional variations, either in ADR reporting rate per million of regional population or in regional contribution to the delay from ADR onset to international visibility on Uganda’s VigiBase®.
Fourth, disproportionality for signal detection within the stratum of patients receiving ART may have performed well, despite the limited number of reported ADR onsets (fewer than 1000), because the NPC had undertaken various initiatives to target (or stimulate) the reporting of serious ADR onsets in patients receiving ART, notably tenofovir.
Fifth, until Uganda’s VigiBase® differentiates between ADR onsets from spontaneous versus targeted reporting, the performance of disproportionality for signal detection within the subset of spontaneously reported serious ADR onsets cannot be formally assessed and may be less incisive than appears in this paper.
Sixth, our three random samples, each of 15 ADR onsets, delivered answers that were less precise than we had intended them to be because one-third (16/45) of the sampled ADR forms could not be located. Future random sampling for quality assurance by Uganda’s NPC and others should take into account that a non-negligible proportion of ADR forms may be difficult to locate, for example, if incorrectly filed.
Finally, with only 10 days for data orientation, quality assurance, formal analyses and discussion of recommendations, the analyses presented are illustrative rather than comprehensive. In particular, we performed no dose-related analyses [13].
4.2 Recommendations
Uganda’s academic pharmacists, physicians and surgeons need to demonstrate strong professional commitment to, and leadership in, pharmacovigilance by ensuring that they themselves promptly report to Uganda’s NPC all serious ADR onsets, particularly in young patients. Support from Uganda’s academic professional cadres could increase the rate at which ADR onsets are reported to the NPC by a factor of ten: from < 8 to > 80 per million of population; and from under one report per day to at least ten per day.
In addition, the NPC needs to dramatically reduce the delay from reported ADR onset to international visibility on Uganda’s VigiBase®. Further investigation into how the median delay of 11 months arises (institutional hierarchies, regional centre delays, quality assurance at NPC) should identify and resolve key contributions to the overall delay. Delay in international visibility hinders signal detection and interventions to reduce harm to patients. Moreover, delay in processing ADR reports also delays feedback to reporters, who should feel valued by the NPC for their efforts in drug safety [12].
We embarked upon these analyses partly from a keenness to assess the potential for Uganda’s ADR report form, which is already available for online completion [8], to be used in a convenient app that might enhance ADR reporting to the NPC by HCPs, just as the UK’s Yellow Card App has done [3]. Currently, Uganda’s ADR report form is used both for spontaneous reporting of ADRs in the general population of patients and for stimulated reporting in well-defined cohorts of patients being treated for disease X, receiving medication Y, or defined by covariate Z (e.g. age group, region, or healthcare facility). In principle, the same set of spontaneous versus stimulated reporting options applies when introducing an ADR-reporting app for Uganda. From a methodological standpoint, randomized introductions of an ADR-reporting app for Uganda could allow its performance to be quantified in the chosen leadership settings.
5 Conclusions
Barely one ADR onset per day was registered on VigiBase® from those submitted to Uganda’s National Pharmacovigilance Centre during 2012–2014; with only one in six from patients aged < 20 years. Paediatric pharmacovigilance requires more emphasis in Uganda, where three-fifths of the population is aged < 20 years. Delay from reported ADR onset to international visibility on VigiBase® needs to reduce dramatically. Quality assurance revealed rectifiable data entry deficits. Signal detection performed well for patients receiving ART.
References
WHO Collaborating Centre for International Drug Monitoring. Members of the WHO Programme for International Drug Monitoring. 2018. https://www.who-umc.org/global-pharmacovigilance/members/who-programme-members/. Accessed 22 Mar 2018.
National Pharmacovigilance Centre. What is current practice of Pharmacovigilance in Uganda? 2012. https://africapv2012.files.wordpress.com/2012/04/day-2_5_country-presentations-nras_h-ngadije-uganda-compatibility-mode.pdf. Accessed 22 Mar 2018.
Uganda Bureau of Statistics. National Population and Housing Census 2014—Main Report. 2016. http://www.ubos.org/2016/03/24/census-2014-final-results/. Accessed 23 Mar 2018.
Dheda M. Perspectives on the emergence of pharmacovigilance in Public Health Programmes in South Africa. Pharm Med. 2016;30(4):213–9.
Olowofela A, Fourrier-Réglat A, Isah AO. Pharmacovigilance in Nigeria: an overview. Pharm Med. 2016;30(2):87–94.
Aagaard L, Strandell J, Melskens L, Petersen PS, Holme Hansen E. Global patterns of adverse drug reactions over a decade: analyses of spontaneous reports to VigiBase. Drug Saf. 2012;35(12):1171–82.
Ndomondo-Sigonda M, Miot J, Naidoo S, Dodoo A, Kaale E. Medicines regulation in Africa: current state and opportunities. Pharm Med. 2017;31(6):383–97.
National Pharmacovigilance Centre. Annual Pharmacovigilance Report July 2015–June 2016. 2016. http://health.go.ug/content/annual-pharmacovigilance-report-national-drug-authority. Accessed 10 May 2018.
Ndagije H, Nambasa V, Namagala E, Nassali H, Kajungu D, Sematiko G, et al. Targeted spontaneous reporting of suspected renal toxicity in patients undergoing highly active anti-retroviral therapy in two public health facilities in Uganda. Drug Saf. 2015;38(4):395–408.
Pharmacovigilance Programme of India. Important events of the Pharmacovigilance Programme of India. Mobile App for ADRs reporting—an effective tool 2015. http://ipc.nic.in/writereaddata/mainlinkFile/File453.pdf. Accessed 22 Mar 2018.
Organization WH. 40th Annual Meeting of Representatives of National Pharmacovigilance Centres participating in the WHO Programme for International Drug Monitoring. 2017. http://www.who.int/medicines/regulation/medicines-safety/npvc-meeting/en/. Accessed 22 Mar 2018.
Kiguba R, Karamagi C, Waako P, Ndagije HB, Bird SM. Rare, serious, and comprehensively described suspected adverse drug reactions reported by surveyed healthcare professionals in Uganda. PLoS One. 2015;10(4):e0123974.
Ndagije HB, Nambasa V, Manirakiza L, Kusemererwa D, Kajungu D, Olsson S, et al. The burden of adverse drug reactions due to artemisinin-based antimalarial treatment in selected Ugandan health facilities: an active follow-up study. Drug Saf. 2018;41(8):753–65.
Ekure J. Gluteal fibrosis. A report of 28 cases from Kumi Hospital, Uganda. East Cent Afr J Surg. 2006;12(1):144–7.
Rawlins MD. Spontaneous reporting of adverse drug reactions. II: uses. Br J Clin Pharmacol. 1988;26(1):7–11.
Finney DJ. Statisical logic in the monitoring of reactions to therapeutic drugs. Methods Inf Med. 1971;10(4):237–45.
Finney DJ. Problems, data, and inference. J R Stat Soc Ser A (General). 1974;137(1):1–23.
Simpson JM, Bateman DN, Rawlins MD. Using the adverse reactions register to study the effects of age and sex on adverse drug reactions. Stat Med. 1987;6(7):863–7.
Wilson AM, Thabane L, Holbrook A. Application of data mining techniques in pharmacovigilance. Br J Clin Pharmacol. 2004;57(2):127–34.
United States Food and Drug Administration. Accelerated Approval—SIRTURO (Bedaquiline) 100 mg Tablets. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/204384Orig1s000ltr.pdf. Accessed 10 May 2018.
Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JC, Anderson LF, Baghaei P, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821–34.
Farmer PE. Better and safer treatment for multidrug-resistant tuberculosis. Lancet. 2018;392(10150):798–800.
Reuter A, Furin J. Reducing harm in the treatment of multidrug-resistant tuberculosis. Lancet. 2018;392(10150):797–8.
WHO Collaborating Centre for International Drug Monitoring. What is VigiBase? 2018. https://www.who-umc.org/vigibase/vigibase/. Accessed 22 Mar 2018.
Ampadu HH, Hoekman J, de Bruin ML, Pal SN, Olsson S, Sartori D, et al. Adverse drug reaction reporting in Africa and a comparison of individual case safety report characteristics between Africa and the rest of the world: analyses of spontaneous reports in VigiBase(R). Drug Saf. 2016;39(4):335–45.
Marrero O, Hung EY, Hauben M. Seasonal and geographic variation in adverse event reporting. Drugs Real World Outcomes. 2016;3(3):297–306.
Uppsala Monitoring Center. Getting the answers you need. 2018. https://www.who-umc.org/vigibase/vigilyze/. Accessed 15 May 2018.
United States Food and Drug Administration. Warning: Tardive Dyskinesia. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/017854s058lbl.pdf. Accessed 22 Mar 2018.
Author information
Authors and Affiliations
Contributions
RK and SMB conceived of the study and participated in its design, implementation and statistical analysis and the drafting of the manuscript. VN and HBN provided access to the data and participated in the drawing of inferences. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Funding
Open access was funded by the National Drug Authority in Uganda.The authors gratefully acknowledge Grant support from the Cambridge-Africa ALBORADA Research Fund.
Conflict of interest
SMB holds GSK shares. RK, VN, HBN and SMB otherwise have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kiguba, R., Ndagije, H.B., Nambasa, V. et al. Adverse Drug Reaction Onsets in Uganda’s VigiBase®: Delayed International Visibility, Data Quality and Illustrative Signal Detection Analyses. Pharm Med 32, 413–427 (2018). https://doi.org/10.1007/s40290-018-0253-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-018-0253-7