Abstract
Objectives
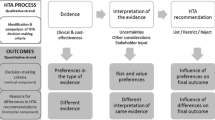
Health Technology Assessment (HTA) agencies produce recommendations that guide public funding of pharmaceuticals, based on various criteria. We explored factors that may contribute to explaining differences in coverage decisions by the National Institute for Health and Care Excellence (NICE) in England and Wales, the Scottish Medicines Consortium (SMC), the Dutch College voor Zorgverzekeringen (CVZ), and the French Haute Autorité de Santé (HAS).
Methods
A dataset of 977 HTA decisions made in 2004–2009 was created. A three-category outcome variable was used (decision to ‘recommend’, ‘restrict’ or ‘not recommend’ a technology). Multivariate analyses explored impacts of clinical, economic, process and socio-economic variables in their decision making.
Results
Relative to the CVZ and adjusting for a range of confounders, technologies were more likely to be recommended by NICE and HAS, and restricted or not-recommended by the SMC. Recommendation was significantly associated (p ≤ 0.10) with several variables: strength of clinical evidence (number of trials, use of active comparator-arm, demonstration of clinical superiority) orphan status and indication for cancer. Simultaneous assessment of multiple rather than single pharmaceuticals was associated with increased probability of restriction.
Conclusions
In this European multi-HTA study, appraisal outcomes differed significantly across HTA bodies. A range of evidence and non-evidence factors were associated with HTA decisions, confirming the value of comprehensive, multivariate analyses. Nevertheless, a large proportion of variance in HTA decisions remained unexplained, suggesting that greater transparency of decision making is needed, along with associated further research.



Similar content being viewed by others
Notes
While prices of 1A listed technologies are pre-defined based on already existing reference technologies, it is the manufacturer that submits a proposal with a price in the anticipated range according to the therapeutic differentiation of the product, and it is not the CVZ who asks for a price reduction to meet the reference price rule as a result of the appraisal. In addition, both 1A and 1B result in similar coverage from the patient perspective. This is why we decided to consider both 1A and 1B listings recommendations by the CVZ.
It should be noted that since 2005, CVZ has increasingly used cost-effectiveness evidence in its decision making, in particularly for technologies evaluated for 1B listing and specific instances. However, because of the fact that in the sample of CVZ decisions between 2004 and 2009, only 11 % of technologies (<30) were supported by reported ICER data, it was not included in base-case model 2.
References
European Commission. Amended proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on the transparency of measures regulating the prices of medicinal products for human use and their inclusion in the scope of public health insurance systems. COM(2013) 168 final/2. 20-3-2013.
Sorenson C, Drummond M, Kanavos P. Ensuring value for Money in Health Care: the role of HTA in the European Union. 2008. Cornwall, UK, World Health Organization 2008, on behalf of the European Observatory on Health Systems and Policies.
Bellanger MM, Cherilova V, Paris V. The “Health Benefit Basket” in France. Eur J Health Econ. 2005;Suppl:24–9.
Sandier S, Paris V, Polton D. Health Care Systems in Transition—France 2004. 2004. Copenhagen, Denmark, WHO Regional Office for Europe on behalf of the European Observatory on Health Systems and Policies.
Clement FM, Harris A, Li JJ, Yong K, Lee KM, Manns BJ. Using effectiveness and cost-effectiveness to make drug coverage decisions: a comparison of Britain, Australia, and Canada. JAMA. 2009;302(13):1437–43.
Dakin HA, Devlin NJ, Odeyemi IA. “Yes”, “No” or “Yes, but”? Multinomial modelling of NICE decision-making. Health Policy. 2006;77(3):352–67.
Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13(5):437–52.
Mason AR, Drummond MF. Public funding of new cancer drugs: is NICE getting nastier? Eur J Cancer. 2009;45(7):1188–92.
Al MJ, Feenstra T, Brouwer WB. Decision makers’ views on health care objectives and budget constraints: results from a pilot study. Health Policy. 2004;70(1):33–48.
Menon D, Stafinski T, Stuart G. Access to drugs for cancer: Does where you live matter? Can J Public Health. 2005;96(6):454–8.
OECD. Health technologies and decision making. Paris: OECD; 2005.
Vuorenkoski L, Toiviainen H, Hemminki E. Decision-making in priority setting for medicines—a review of empirical studies. Health Policy. 2008;86(1):1–9.
Bryan S, Williams I, McIver S. Seeing the NICE side of cost-effectiveness analysis: a qualitative investigation of the use of CEA in NICE technology appraisals. Health Econ. 2007;16(2):179–93.
Packer C, Simpson S, Stevens A. International diffusion of new health technologies: a ten-country analysis of six health technologies. Int J Technol Assess Health Care. 2006;22(4):419–28.
Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med. 1998;13(10):716–7.
Buxton MJ. How much are health-care systems prepared to pay to produce a QALY? Eur J Health Econ. 2005;6(4):285–7.
Ross J. The use of economic evaluation in health care: Australian decision makers’ perceptions. Health Policy. 1995;31(2):103–10.
Kanavos P, Nicod E, van den Aardweg S, Pomedli S. The impact of health technology assessments: an international comparison. Euro Observer. 2010;12(4):1–7.
Barbieri M, Hawkins N, Sculpher M. Who does the numbers? The role of third-party technology assessment to inform health systems’ decision-making about the funding of health technologies. Value Health. 2009;12(2):193–201.
Lexchin J, Mintzes B. Medicine reimbursement recommendations in Canada, Australia, and Scotland. Am J Manag Care. 2008;14(9):581–8.
Fischer KE. A systematic review of coverage decision-making on health technologies—evidence from the real world. Health Policy. 2012;107:218–30.
Cerri KH, Knapp M, Fernandez JL. Decision making by NICE: examining the influences of evidence, process and context. Health Econ Policy Law. 2013;21:1–23.
Cerri KH, Knapp M, Fernandez JL. Public funding of pharmaceuticals in the Netherlands: investigating the effect of evidence, process and context on CVZ decision-making. Eur J Health Econ. 2013;15(7):681–95.
Acknowledgments
The authors would like to thank Michael Drummond and Alistair McGuire for their valuable comments on the methods used and interpretation of analyses that were performed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the conduct or publication of this study.
Conflicts of interest
KC was an employee of Bristol-Myers Squibb during the time this research was conducted. MK has received funding from NICE for PSSRU’s part in the NICE Collaborating Centre for Social Care. JLF has no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cerri, K.H., Knapp, M. & Fernandez, JL. Untangling the Complexity of Funding Recommendations: A Comparative Analysis of Health Technology Assessment Outcomes in Four European Countries. Pharm Med 29, 341–359 (2015). https://doi.org/10.1007/s40290-015-0112-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-015-0112-8