Abstract
Background
Although the efficacy of interval training for improving body composition has been summarized in an increasing number of systematic reviews in recent years, discrepancies in review findings and conclusions have been observed.
Objective
This study aims to synthesize the available evidence on the efficacy of interval training compared with moderate-intensity continuous training (MICT) and nonexercise control (CON) in reducing body adiposity in apparently healthy adults.
Methods
An umbrella review with meta-analysis was performed. A systematic search was conducted in seven databases (MEDLINE, EMBASE, Cochrane Database, CINAHL, Scopus, SPORTDiscus, and Web of Science) up to October 2023. Systematic reviews with meta-analyses of randomized controlled trials (RCTs) comparing interval training and MICT/CON were included. Literature selection, data extraction, and methodological quality assessment (AMSTAR-2) were conducted independently by two reviewers. Meta-analyses were performed using a random-effects model. Subgroup analyses were conducted based on the type of interval training [high-intensity interval training (HIIT) and sprint interval training (SIT)], intervention duration, body mass index, exercise modality, and volume of HIIT protocols.
Results
Sixteen systematic reviews, including 79 RCTs and 2474 unique participants, met the inclusion criteria. Most systematic reviews had a critically low (n = 6) or low (n = 6) AMSTAR-2 score. Interval training demonstrated significantly greater reductions in total body fat percent (BF%) compared with MICT [weighted mean difference (WMD) of − 0.77%; 95% confidence interval (CI) − 1.12 to − 0.32%] and CON (WMD of − 1.50%; 95% CI − 2.40 to − 0.58%). Significant reductions in fat mass, visceral adipose tissue, subcutaneous abdominal fat, and android abdominal fat were also observed following interval training compared to CON. Subgroup analyses indicated that both HIIT and SIT resulted in superior BF% loss than MICT. These benefits appeared to be more prominent in individuals with overweight/obesity and longer duration interventions (≥ 12 weeks), as well as in protocols using cycling as a modality and low-volume HIIT (i.e., < 15 min of high-intensity exercise per session).
Conclusions
This novel umbrella review with large-scale meta-analysis provides an updated synthesis of evidence with implications for physical activity guideline recommendations. The findings support interval training as a viable exercise strategy for reducing adiposity in the general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Interval training demonstrated a small but significantly greater reduction in total body fat percent (BF%) compared with moderate-intensity continuous training (MICT) and significant reductions in fat mass, visceral adipose tissue, subcutaneous abdominal fat, and android abdominal fat compared with nonexercise control. |
Subgroup analyses indicated that both high-intensity interval training (HIIT) and sprint interval training (SIT) resulted in superior BF% loss versus MICT. |
These benefits appeared to be more prominent in individuals with overweight/obesity and longer duration interventions (≥ 12 weeks), as well as in protocols using cycling as a modality and low-volume HIIT (i.e., < 15 min of high-intensity exercise per session). |
1 Introduction
The World Health Organization (WHO) defines excess weight and obesity as abnormal or excess fat accumulation that poses a risk to health. Obesity is an independent risk factor for various noncommunicable diseases, including heart disease, stroke, cancer, chronic respiratory diseases, and type 2 diabetes [1]. Regular physical activity (PA) and exercise play a crucial role in weight management by promoting calorie expenditure, enhancing metabolism, and supporting healthy body composition [1, 2].The current PA guidelines recommend that adults (18–65 years old) should engage in a minimum of 75–150 min of weekly moderate-to-vigorous physical activity (MVPA) to enhance health [2, 3], However, insufficient PA remains a prominent global issue [4]. Therefore, identifying effective, evidence-based, and practical exercise strategies aimed at mitigating the detrimental health consequences of physical inactivity and obesity has important clinical implications.
Recent bibliometric evidence has highlighted interval training as an emerging exercise strategy for improving health-related fitness in the general population compared with traditional training methods [5]. Interval training has attracted widespread attention among health and fitness professionals over the past decade and has been ranked among the top trends in the American College of Sports Medicine (ACSM) Worldwide Survey of Fitness Trends since 2013 [6]. Interval training typically involves repeated bouts of high-intensity exercise, interspersed with active or inactive periods of rest or recovery [7, 8]. Interval training is commonly classified as high-intensity interval training (HIIT) or sprint interval training (SIT) [2, 9], although various iterations appear in the literature. In a health context, HIIT can be characterized as intermittent bouts of exercise performed above moderate intensity (typically up to 4 min), primarily falling within the classification of vigorous intensity exercise (e.g., ~ 80–95% maximal heart rate) [8]. On the other hand, SIT represents a particularly intense variant of interval training that can be distinguished as repeated sprints at supramaximal intensities, typically performed with “all-out” efforts lasting ≤ 30 s [9].
Until recently, interval training has been recognized as an alternative option to traditional exercise approaches like moderate-intensity continuous training (MICT) in various authoritative PA guidelines worldwide, including those by the ACSM [2], the USA [10] and the United Kingdom [11]. However, it is important to note that these guidelines often lack a clear distinction between HIIT and SIT. For instance, the US guidelines mention the absence of universally accepted durations for the “maximal-effort” period, recovery period, ratio of the two, number of cycles per session, overall session duration, and the specific relative intensity at which the maximal-effort component should be performed during interval training [10]. Similarly, the UK guidelines state that data on HIIT are still emerging, and that further investigation is necessary to determine the optimal amount and form of HIIT to recommend [11]. These acknowledged limitations in authoritative guidelines underscore the need for additional research to comprehensively analyze the effectiveness of interval training, encompassing both HIIT and SIT, on body composition outcomes. Furthermore, while HIIT has received more attention in the literature and may be deemed more suitable for wider populations [7], SIT may still be considered a feasible option for relatively active and healthy individuals if appropriately designed [12, 13]. Therefore, including SIT in evaluating the overall efficacy of interval training allows for a broader range of interventions that are relevant and applicable to different populations.
While original studies exploring the efficacy of interval training in improving body adiposity in both general or populations with overweight/obesity have been conducted and summarized in an increasing number of systematic reviews in recent years, discrepancies in review findings and conclusions have been observed. For instance, some systematic reviews revealed the benefits of interval training in improving body composition, such as reducing whole-body fat and visceral fat when compared with MICT [14,15,16], whereas contrasting findings from other reviews have indicated a lack of significant differences [17, 18]. These systematic reviews, often focused on specific population subgroups (e.g., average healthy or individuals with overweight/obesity), interval training regimens (e.g., HIIT/SIT), intervention duration (e.g., short-term/long-term), comparator groups (e.g., MICT/ nonexercising control), or on specific anthropometric outcomes, pose challenges for healthcare professionals and researchers to understand the total body of evidence for interval training in the management of body fat reduction.
In this regard, umbrella reviews (also termed overviews of systematic reviews or meta-reviews) have been proposed as an effective approach to present a comprehensive overview of evidence synthesis on a given topic. Umbrella reviews summarize existing evidence from systematic reviews, making them a comprehensive means to inform guidelines. To the best of our knowledge, only one umbrella review has previously been conducted regarding the efficacy of interval training across the general population [19]. While that review suggested that interval training, in the form of HIIT, is effective and safe for improving cardiometabolic health and anthropometric measures, the results were described narratively without additional statistical analysis (i.e., quantitative meta-analysis). In addition, the article included systematic reviews of both randomized controlled trials (RCTs) and nonrandomized trials that mostly compared HIIT with an active control, but it did not report whether and how this form of interval training was superior to a nonactive control. High quality RCTs encompassing various forms of interval training and including both active and nonactive control groups would be required to provide further insights on the full range of benefits of interval training. Considering the substantial increase in evidence published from past systematic reviews and meta-analyses in recent years [20], an umbrella review that can address the aforementioned research gaps to further establish the benefits, compliance, and applications of interval training interventions among the general population appears timely. Therefore, we set out to undertake the most comprehensive synthesis of evidence to date regarding the effect of interval training on body composition and adiposity in adults.
2 Methods
2.1 Search Strategy
This overview of systematic reviews was performed in accordance with the Preferred Reporting Items for Overviews of Reviews (PRIOR) statement [21] and registered in the PROSPERO database (CRD42023490819). Seven databases were searched (MEDLINE, EMBASE, Cochrane Database, CINAHL, Scopus, SPORTDiscus, and Web of Science) using subject heading, keyword, and medical subject headings (MeSH) term searches for “systematic review,” “meta-analysis,” “HIIT,” and “body adiposity.” Database searches were limited to peer-reviewed systematic review articles published in English language from inception to 1 October 2023. The reference lists of the selected review articles were also examined for other potentially eligible papers. The detailed search strategy is presented in Supplementary Table 1.
2.2 Selection Procedure and Eligibility Criteria
The population, interventions, comparators, outcomes, and study type (PICOS) framework was used to develop the inclusion criteria.
2.2.1 Types of Population
The population of interest was men and women aged 18 years or above, who were not suffering from any kind of acute or chronic disease, except for obesity. No exclusion criteria were applied to participants’ baseline fitness. Individuals who simultaneously have obesity and related comorbidities (e.g., cardiovascular diseases and type 2 diabetes) were excluded.
2.2.2 Types of Interventions
The term “interval training” has been used extensively in the literature to describe a variety of different high-intensity protocols that vary in the number and intensity of intervals, the time and nature (active or passive) of recovery periods, and total volume [8]; therefore, the definitions used in the present review are based on a general classification scheme for interval training put forward by Weston et al. [9]. The two most common protocols, HIIT and SIT, were differentiated based on the exercise intensity and unique characteristics observed in previous interval training studies. HIIT is generally defined as “near maximal” efforts performed at an intensity that elicits ≥ 80% maximal heart rate (HRmax) or peak oxygen uptake, whereas SIT is characterized by repeated “all-out” sprints at supramaximal intensities (i.e., > 100% peak oxygen uptake) interspersed with recovery periods. Studies were eligible irrespective of interval training modality (e.g., treadmill running, cycling, or body-weight exercises), settings (e.g., clinical, laboratory, or community facility) or dose (frequency and duration).
2.2.3 Type of Comparators
In this overview of reviews, studies with no comparison groups were excluded. RCTs that involved MICT and/or nonexercise control (CON) comparison groups were included. MICT describes “traditional” exercise protocols performed continuously at a steady state for a set duration (usually 20–60 min) [9]. Moderate intensity is defined as intensity that induces a heart rate response of 60–79% HRmax or that elevates the rate of oxygen consumption to 40–59% of peak oxygen uptake [9].
2.2.4 Types of Outcomes
The results quantitatively reported from each embedded RCT included at least one of the following outcomes: total body fat percentage (BF%), body mass (BM), fat mass (FM), body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR), lean mass (LM), fat-free mass (FFM), visceral adipose tissue (VAT), and abdominal fat (AF).
2.2.5 Types of Studies
Systematic reviews (with or without meta-analyses) of RCTs were selected.
2.3 Selection of Literature and Data Extraction
Search results were imported into EndNote X10 (Clarivate, Philadelphia). Two reviewers (EP and JHL) independently screened the titles and abstracts of the retrieved citations from the seven electronic databases, removed duplicates, and determined eligible systematic reviews based on our inclusion criteria. For each eligible citation from our previous step, full texts of the embedded citations were obtained. Inter-reviewer disagreements were resolved by consensus or arbitration by a third reviewer (R.H.). Data from included systematic reviews were extracted in duplicate by two independent reviewers (E.P. and J.H.L.) using a standardized extraction form. The extracted data included the lead author, year of publication, design of original studies, population characteristics (age and sex), number of original studies, and participants included, description of interval training interventions (protocols, frequency, and duration), comparison groups, and outcomes.
Considering that some of the systematic reviews included might have contained certain component RCTs that did not meet our inclusion criteria (e.g., “contamination” of RCTs with ineligible participants, interventions, or outcomes), every component RCT from the included reviews was further screened by two reviewers (E.P. and J.H.L.) independently to ensure relevance. The inclusion criteria for the RCTs in the umbrella review remained consistent with the aforementioned criteria. Inter-reviewer disagreements were resolved by consensus or arbitration by a third reviewer (R.H.). Subsequently, data from eligible RCTs were extracted, including the first author, year of publication, characteristics of participants, and sample size. The intervention features were also extracted to assist the reviewers in subcategorizing the interval training.
2.4 Critical Appraisal of Systematic Reviews and Randomized Controlled Trials
Critical appraisals of both systematic reviews and RCTs were independently performed by the two reviewers (E.P. and J.H.L.), and discrepancies were resolved through discussions. Discrepancies were resolved by consensus or arbitration by a third reviewer (R.H.).
2.4.1 Methodological Quality Assessment of Included Systematic Reviews
The methodological quality of the included reviews was assessed by two independent reviewers (E.P. and J.H.L.) in duplicate using the A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) tool [22]. The AMSTAR-2 tool involves 16 items, with each item scored as yes, partial yes or no. Seven items are considered “critical” and nine “noncritical” [22]. The critical domains are protocol registration, adequacy of search strategy, justification for excluding individual studies, risk of bias assessment, appropriateness of meta-analysis methods, use of risk of bias during interpretation, and assessment of publication bias. Reviews were rated as “high confidence” (zero critical weakness and less than three noncritical weaknesses), “moderate” (one critical weakness and less than three noncritical weaknesses), “low” (greater than one critical weakness and less than three noncritical weaknesses), or “critically low” (greater than one critical weakness and greater than or equal to three noncritical weaknesses) [22].
2.4.2 Methodological Quality Assessment of Included Randomized Controlled Trials
The methodological quality of the included RCTs was also independently assessed by two reviewers (E.P. and J.H.L.) using the modified physiotherapy Evidence Database (PEDro) scale. The original PEDro scale used an 11-point scale, but due to the impracticality of blinding participants and investigators in supervised exercise interventions, we opted to exclude assessment items related to blinding (numbers 5, 6, and 7 in the scale) as in previous exercise-related systematic reviews. Consequently, the modified 8-point PEDro scale has a maximum value of 7 (excluding the first item from the total score). The qualitative methodological ratings were adjusted as follows: “excellent” (6–7 points), “good” (5 points), “moderate” (4 points), and “poor” (0–3 points).
2.5 Umbrella Review Synthesis Methods
The overlap in component RCTs that were included across all eligible reviews was assessed using the corrected covered area (CCA) method [23]. A CCA of 100% indicates that every review included in our umbrella review comprised the same component RCTs, while a CCA of 0% indicates that every review in our umbrella review included entirely unique RCTs. The following cutoffs were used to quantify the CCA: 0–5%, “slight overlap;” 6–10%, “moderate;” 11–15%, “high;” and > 15%, “very high” overlap [23].
Meta-analyses from eligible component RCTs were conducted using Review Manager software (RevMan 5.4; Cochrane Collaboration, Oxford, United Kingdom). The absolute change in mean difference and standard deviation of the outcome value from postintervention between groups (interval training versus MICT/CON) in each study was calculated and pooled using the random-effects model. For studies that compared multiple intervention groups with a single comparison group (or vice versa), the sample size of the shared comparison group was split to avoid double counting [24]. Weighted mean differences (WMDs) with 95% confidence intervals (CIs) were used to synthesize continuous outcomes and create forest plots, except for VAT and AF outcomes, where standardized mean differences (SMDs) were used. Subgroup analyses were conducted based on the type of interval training (HIIT or SIT), intervention duration (< 12 weeks or ≥ 12 weeks), body mass index (BMI 18.5–24.9 or ≥ 25 kg/m2), exercise mode (cycling or running/walking/jogging), and HIIT volume (< 15 min and ≥ 15 min of high-intensity exercise per session). The heterogeneity of included RCTs was assessed using the I2 statistic, in which values of < 25%, 50%, and 75% were considered indicative of low, moderate, and high heterogeneity, respectively. Inverse variance weighting was used to compensate for the heterogeneity of sample sizes between studies. Publication bias was assessed by creating a funnel plot and observing the presence of asymmetries or missing sections.
3 Results
3.1 Overview of Search Results
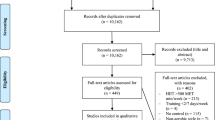
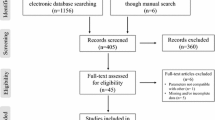
Out of the 542 records identified, 16 systematic reviews were included in this overview for the subsequent literature screening for eligible RCTs (see Fig. 1 for PRISMA flowchart). Table 1 provides an overview of the characteristics of all the reviews. A total of 432 original studies were listed in the included systematic reviews, with a CCA of 2.9%, indicating slight overlap.
Of the 432 embedded RCTs, we excluded 139 duplicates and 216 studies (see Fig. 2 for flowchart and reasons for exclusions in Supplementary Table 4). A total of 77 eligible RCTs met our inclusion criteria from the included systematic reviews. Two additional RCTs were identified by checking the reference lists, resulting in 79 RCTs included in our overview for data extraction. Supplementary Table 2 summarizes the characteristics of all included studies [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103]. Evaluation of funnel plots showed no evidence of publication bias. The majority of the original studies specifically involved individuals with overweight/obesity based on a BMI ≥ 25 kg/m2 (N = 57), while others involved participants with a BMI of 18–24.9 kg/m2 (N = 11), and a small number did not report (N = 6) or classify (N = 5) BMI.
3.2 Participant Characteristics
The meta-analysis included a total of 2474 unique participants. They were assigned to the following groups: HIIT (n = 717), SIT (n = 485), MICT (n = 755), and CON (n = 517). Among the original studies that reported participants’ sex, the male (n = 1179) and female (n = 1225) ratio was similar. Among the RCTs that reported drop-out, a total of 376 participants (~ 15%) who initially enrolled in the studies were not included in the data analysis. In accordance with the inclusion criteria, participants were apparently healthy adults without acute or chronic diseases, with a mean age range of 18 to 73.5 years.
3.3 Intervention Characteristics
The intervention characteristics of eligible RCTs are summarized in Supplementary Table 2. The duration of interventions ranged from 13 days to 16 weeks, with 29 studies lasting ≥ 12 weeks and 50 studies lasting < 12 weeks. The interventions generally had a frequency of 2–7 days per week, with each session lasting 8–70 min. Various exercise modalities were used in interval training, including cycling, running, aquatic treadmill running, all-extremity air-baked ergometer, circuit strength training, TRX, boxing drills (heavy bag, focus mitts, circular body weight footwork drills, skipping), circuit-based dynamic body-weight exercise, and walking/ jogging. The total set/bouts in the interval training protocols ranged from 2 to 80 times. Both passive and active recovery protocols were employed, with work-to-rest ratio ranging from 2:1 to 1:9.
3.4 Methodological Quality of Included Reviews
Table 2 provides a summary of the AMSTAR-2 scores. The majority of the reviews had a critically low (n = 6) or low (n = 6) score, while two reviews had a moderate score. Specifically, only 37.5% of reviews referred to a predefined methodology (item 2). None of the studies provided a list of excluded studies with reasons for exclusions (item 7) or reported on the sources of funding for the included studies (item 10). All studies accounted for risk of bias (RoB) when interpreting the results (item 9) and 69% discussed heterogeneity (item 14). Among the reviews that conducted a meta-analysis, all used appropriate/partly appropriate methods for statistical combination of results (item 11), and 60% investigated publication bias (item 15). However, only one review (8%) assessed the impact of RoB on the results (item 12).
3.5 Methodological Quality of Included RCTs
Supplementary Table 3 provides a summary of the PEDro scores. Among the 79 RCTs, the mean rating was 5.1, indicating that the overall collection of studies was of good quality. Of these, 33 studies were rated as excellent, 26 studies were rated as good, and 20 studies were rated as fair. Notably, none of the studies included in the analysis were deemed to be of poor quality.
3.6 Meta-Analysis
3.6.1 Interval Training Versus CON
The summary of meta-analyses is presented in Table 3. Compared with CON, interval training demonstrated significant reductions in total BF% (28 RCTs, WMD of − 1.50%; 95% CI − 2.41% to − 0.58%, p = 0.001, Fig. 3), FM (19 RCTs, WMD of − 0.79 kg; 95% CI − 1.55 to − 0.04 kg, p = 0.03), VAT (7 RCTs, SMD of − 0.26; 95% CI − 0.51 to − 0.01, p = 0.04), AFsubcutaneous (6 RCTs, SMD of − 0.33; 95% CI − 0.64 to − 0.02, p = 0.04), AFandroid (4 RCTs, SMD of − 0.49; 95% CI − 0.90 to − 0.08, p = 0.02), and AFgynoid (4 RCTs, SMD of − 1.26; 95% CI − 2.31 to − 0.21, p = 0.02). No significant between-group difference was observed for other outcome measures. Subgroup analyses indicated that longer duration interventions tended to result in greater reductions in BF%, BM, BMI, and FM. In addition, greater BF% loss was observed in individuals with overweight/obesity. Both HIIT (19 RCTs, WMD of − 1.64%; 95% CI − 2.86% to − 0.42%, p = 0.01) and SIT (12 RCTs, WMD − 1.81%; 95% CI − 2.48% to 0.13%, p = 0.08) showed similar reductions in BF% loss, whereas HIIT tended to favor reductions in BMI (18 RCTs, WMD of − 0.79 kg/m2; 95% CI − 1.52 to − 0.07 kg/m2, p = 0.03) compared with SIT (8 RCTs, WMD of 0.17 kg/m2; 95% CI − 1.13 to 0.80 kg/m2, p = 0.74). Cycling exercise mode (15 RCTs, WMD of − 1.63%; 95% CI − 2.97% to − 0.29%, p = 0.02) and low-volume HIIT (8 RCTs, WMD of − 1.62%; 95% CI − 2.71% to − 0.54%, p = 0.003) appeared to have more pronounced BF% reduction than running/walking/jogging (10 RCTs, WMD of − 0.90%; 95% CI − 2.25% to 0.45%, p = 0.19) and high-volume HIIT (6 RCTs, WMD of − 0.68%; 95% CI − 2.96% to 1.61%, p = 0.56).
3.6.2 Interval Training Versus MICT
The summary of meta-analyses is presented in Table 4. Compared with MICT, interval training demonstrated significantly greater reductions in total BF% (40 RCTs, WMD of − 0.77%; 95% CI − 1.22% to − 0.32%, p = 0.0008, Fig. 4). No significant between-group difference was observed for other outcome measures. Subgroup analyses indicated that both HIIT (25 RCTs, WMD of − 0.62%; 95% CI − 1.12% to − 0.12%, p = 0.01) and SIT (22 RCTs, WMD of − 1.16%; 95% CI − 2.06% to − 0.26%, p = 0.01) resulted in superior BF% loss than MICT (Fig. 4). Long-term interval training interventions (22 RCTs, WMD of − 1.10%; 95% CI − 1.67% to − 0.53%, p = 0.0002) and individuals with overweight/obesity (37 RCTs, WMD of − 0.74%; 95% CI − 1.19 to − 0.30%, p = 0.001) tended to show superior benefits of BF% loss than short-term interval training interventions (24 RCTs, WMD of − 0.38%; 95% CI − 1.22% to 0.46%, p = 0.38) and individuals with normal BMI (4 RCTs, WMD of − 0.45%; 95% CI − 2.87% to 1.97%, p = 0.72). Cycling exercise mode (29 RCTs, WMD of − 0.90%; 95% CI − 1.43% to − 0.36%, p = 0.001) and low-volume HIIT (11 RCTs, WMD − 1.14%; 95% CI − 1.94% to − 0.35%, p = 0.005) also appeared to have more pronounced BF% reduction than running/walking/jogging (14 RCTs, WMD of − 0.66%; 95% CI − 1.71% to 0.38%, p = 0.21) and high-volume HIIT (7 RCTs, WMD of − 0.03%; 95% CI − 0.99% to 0.92%, p = 0.94), when compared with MICT.
4 Discussion
To the best of our knowledge, this is the first umbrella review with large-scale meta-analysis examining the efficacy of interval training, including HIIT and SIT, in improving body composition and adiposity in adults. We identified 16 systematic reviews, reporting the findings of 79 original RCTs, involving 2474 unique participants. The findings of our umbrella review support the widespread efficacy of interval training in improving a range of body composition and adiposity-related outcomes, such as total BF%, FM, VAT, AFsubcutaneous, and AFandroid compared with CON. While the difference appeared modest, our analysis also revealed that both HIIT and SIT resulted in a superior reduction in BF% compared to MICT. This effect was particularly pronounced in individuals with overweight/obesity and in interventions with longer durations, as well as in protocols with cycling as the exercise modality and low HIIT volume (see Fig. 5 for the graphical representation of findings).
Graphical representation of the efficacy of interval training in reducing body adiposity in apparently healthy adults. CI confidence interval, CON control, HIIT high-intensity interval training, MICT moderate-intensity continuous training, RCTs randomized controlled trials, SIT sprint interval training, WMD weighted mean difference
Several mechanisms that may contribute to the observed fat loss associated with interval training have been documented in the literature [104, 105]. One commonly proposed mechanism is the phenomenon known as excess postexercise oxygen consumption (EPOC). Interval training involves short bursts of intense exercise followed by brief recovery periods. This pattern creates an oxygen debt that the body needs to repay during the recovery period, leading to increased calorie burning and fat oxidation after exercise cessation [106]. The metabolic rate remains slightly elevated in response to exercise intensity, ranging from an hour to several hours with higher intensities [107, 108]. However, given that many interval training protocols involve a low volume of exercise, it is debatable whether EPOC can lead to a greater total energy deficit when compared with MICT, which tends to result in greater energy expenditure during the exercise bout [109]. Thus, hormonal changes induced by interval training may also play a role in fat loss. High-intensity exercise (i.e., above 65% maximal oxygen uptake [VO2max]) stimulates the release of growth hormone and catecholamines (epinephrine and norepinephrine), which elevate tissue lipolysis [110, 111]. Recent evidence suggests that interval training may be particularly effective in reducing adipose tissues in the visceral regions, as the significantly increased catecholamine responses during interval training favor lipolysis via beta-adrenergic receptors located in visceral adipose tissue [112]. Furthermore, exercise may trigger changes in the levels of circulating appetite-related hormones and metabolites, as well as sensations of hunger and satiety [113]. These responses also appear to be dependent on exercise intensity [114], as higher intensity exercise was found to promote appetite suppression [115]. Interval training has been shown to have a favorable impact on appetite-regulating hormones, such as leptin and ghrelin, leading to a decrease in postexercise appetite and potentially lower energy intake [105, 116]. Collectively, EPOC, enhanced catecholamine release that promotes tissue lipolysis, and decreased postexercise appetite provide a scientific basis for the potency of interval training for reducing adiposity.
Regarding the clinical significance of our results, it is acknowledged that there is currently no universally agreed-upon minimal clinically meaningful or cutoff value of BF% reduction in relation to cardiometabolic risk [117]. This value may vary depending on individual factors and the specific guideline being referenced. However, a recent epidemiological study suggested cutoff values of 25.8% for men and 37.1% for women for predicting the cardiovascular risk factors related to obesity [118]. Considering these benchmarks, we recognize that the observed WMD in BF% in our study may appear modest when comparing interval training with CON (− 1.5%; 95% CI − 2.41% to − 0.58%) and MICT (− 0.77%; 95% CI − 1.22% to − 0.32%). These differences are only incrementally higher than the typical biological error of laboratory-standard body composition techniques such as dual-energy X-ray absorptiometry [119]. The relatively small magnitude of improvement raises questions about the clinical significance of our results, despite their statistical significance. Nonetheless, our subgroup analysis revealed greater benefits in longer duration interventions (≥ 12 weeks) and in individuals with overweight/obesity, who are the priority target for public health promotion. Additionally, it is important to note that most included studies in our review controlled for participants’ diets to minimize the confounding effects of diet on body composition parameters. These findings indicate that the impact of interval training on BF% reduction may be amplified in individuals with a relatively high baseline BF% who adhere to an energy-restrictive diet, as typically prescribed for weight management, over a sustained period of engagement.
Another noteworthy finding from the subgroup analysis was that cycling appeared to be more efficacious than running/walking/jogging in reducing BF%. One possible explanation is that while all these modalities were commonly employed in our included studies, cycling is a nonweight-bearing activity that is gentler on the joints. This lower impact nature of cycling may make it a suitable exercise option, particularly for individuals with overweight/obesity or musculoskeletal issues, as it reduces stress on the joints and lowers the risk of injury [2, 120]. This may in turn enable individuals to sustain longer and more intense exercise sessions, leading to more efficient fat loss. Additionally, our subgroup analysis indicates that HIIT protocols with low volume (i.e., < 15 min of high-intensity exercise per session) yielded comparable effects for most body composition outcomes and possibly superior improvements in BF% reduction, as compared with interventions with high-volume protocols. Existing literature suggests that low-volume HIIT has the potential to rapidly enhance cardiometabolic adaptations, including increased mitochondrial biogenesis and improved insulin sensitivity, through enhanced molecular signalling activities [121, 122]. These adaptations are believed to contribute to an improved capacity for fat oxidation, which can enhance metabolic health and facilitate the reduction of body fat, particularly in individuals with metabolic disorders and impaired fatty acid oxidation [123]. However, from a physiological standpoint, the mechanisms proposed for the benefits of low-volume HIIT would also apply to high-volume HIIT. Moreover, high-volume HIIT has the added benefit of higher overall exercise session energy expenditure, which should theoretically lead to greater fat loss if all other factors are equal. The small actual differences observed, while modestly larger than technical/biological error, could also be due to uncontrolled or unaccounted for factors; although, a similar counterintuitive finding has been shown for reduced-volume SIT before [124]. Further research with stronger statistical power is needed to fully elucidate the precise mechanisms contributing to the observed effects of HIIT protocols with varying volumes on body composition outcomes. Another advantage of low-volume protocols is their perceived “time efficiency” [122], which may make it easier for individuals to incorporate them into their routine. However, it is worth noting that these time-saving benefits may not be substantial when considering factors such as warm-up/cool-down periods and rest intervals. Nevertheless, from a practical perspective, our results suggest that low-volume HIIT can serve as a viable exercise alternative or complement to more traditional forms of aerobic exercise regimen, such as high-volume HIIT and MICT, for improving body composition and adiposity.
There is an understandable concern about the practicality and safety of implementing interval training in less fit or previously inactive populations, including some individuals who with overweight/obesity. For instance, a recent commentary has raised doubts about the long-term sustainability of HIIT [125]. The transition from short-term supervised exercise programs to long-term self-directed interventions in research settings has been linked to decreased participation, partly due to the ongoing need for supervision, monitoring, and support. However, this concern does not seem unique to HIIT. A recent systematic review and meta-analysis conducted by Santos et al. [126], which included 188 unique studies with a total of 8928 participants, revealed that in unsupervised, real-world interval training interventions (inclusive of both HIIT and SIT), the average adherence rate (i.e., completion of unsupervised physical activity) was moderate at 63%, which was comparable with the adherence rate of MICT interventions at 68%. Furthermore, the analysis showed that compliance rates (i.e., supervised intervention attendance) to both interval training and MICT were high among insufficiently active adults and adults with a medical condition, with rates of 89% and 92%, respectively. These high compliance rates align with the modest discontinuation rate (~ 15%) reported in the included RCTs within our review that reported dropout rates specifically in interval training programs. Previous studies have demonstrated that interval training performed at high intensities appears to be safe, well tolerated, and achievable, even when applied in clinical populations with low initial fitness levels (e.g., patients with coronary artery disease, heart failure, and various forms of cancer) [14, 127,128,129]. Nevertheless, inactive individuals with cardiovascular risk factors should be encouraged to undergo a medical evaluation before initiating any exercise program [2]. Although current research suggests that interval training is safe for most healthy individuals, it is prudent for fitness and health professionals to perform proper prescreening and deliver all exercise programming in a progressive manner with adequate supervision.
A limitation of this umbrella review is that most of the included systematic reviews were rated as critically low (n = 6) or low (n = 6), based on the AMSTAR-2 quality rating. Specifically, only a small number of reviews referred to a predefined methodology or assessed the impact of RoB on the results. None of the studies provided a list of excluded studies with reasons for exclusions or reported on the sources of funding for the included studies. This underscores the importance of exercising caution when interpreting certain included reviews and highlights the need for well-conducted systematic reviews in this particular field. Nonetheless, our methodological quality assessment of all 78 included RCTs indicated relatively high PEDro scores, with most RCTs rated as excellent (41%) or good (33%). This suggests that our meta-analysis is expected to contribute to a strong and reliable evidence base on interval training and its effects on body composition and adiposity. Furthermore, it is noted that the terms HIIT and SIT were defined somewhat inconsistently across studies. For instance, Bartlett et al. [29] initially described their protocols as HIIT, involving repeated high-intensity sprints lasting between 15 and 60 s at an intensity exceeding 90% HRmax. However, considering the recognized time delay in achieving a steady-state HR (typically exceeding 1 min), any protocol utilizing short (e.g., ≤ 1-min) intervals and relying solely on HR% should be subjected to scrutiny when distinguishing between SIT and HIIT. Lastly, it should be noted that the target population of this umbrella review and meta-analysis was apparently healthy adults without acute or chronic diseases. Therefore, caution should be taken when generalizing the results to other populations, such as children and adolescents, as well as different clinical populations (e.g., persons with type 2 diabetes, metabolic syndrome, or hypertension).
5 Conclusions
This novel umbrella review with large-scale meta-analysis provides robust evidence supporting the efficacy of interval training, including both HIIT and SIT, in reducing adiposity in adults. Interval training demonstrated significant but modestly greater reductions in total BF% compared with traditional MICT and nonactive control groups. These benefits appeared to be more prominent in individuals with overweight/obesity and longer duration interventions (≥ 12 weeks), as well as in protocols employing cycling as a modality and using low-volume HIIT (i.e., < 15 min of high-intensity exercise per session). Our findings can help address the existing limitations in PA guidelines regarding the recommendation of interval training as a viable exercise strategy for improving body composition and adiposity. Further research and implementation efforts are warranted to optimize the integration of interval training into comprehensive obesity prevention and management programs and to evaluate the impact of different interval training interventions on obesity-related comorbidities.
References
WHO. World Health Organization Physical Activity Fact Sheet; 2016.
ACSM. ACSM's Guidelines for Exercise Testing and Prescription (11th ed.): Wolters Kluwer; 2022.
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62.
Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6(10):e1077–e86.
You YW, Li WK, Liu JX, Li XT, Fu YY, Ma XD. Bibliometric review to explore emerging high-intensity interval training in health promotion: a new century picture. Front Public Health. 2021;23:9.
Thompson W. Worldwide survey of fitness trends for 2023. ACSM’s Health Fitness J. 2022;27(1):9–18.
MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595(9):2915–30.
Coates AM, Joyner MJ, Little JP, Jones AM, Gibala MJ. A perspective on high-intensity interval training for performance and health. Sports Med. 2023.
Weston KS, Wisloff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014;48(16):1227–34.
Com-mittee PAGA. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC; 2018.
CMO U. UK Chief Medical Officers' Physical Activity Guidelines 2019.
Metcalfe RS, Atef H, Mackintosh K, McNarry M, Ryde G, Hill DM, et al. Time-efficient and computer-guided sprint interval exercise training for improving health in the workplace: a randomised mixed-methods feasibility study in office-based employees. BMC Public Health. 2020;20(1):313.
Vollaard NBJ, Metcalfe RS. Research into the health benefits of sprint interval training should focus on protocols with fewer and shorter sprints. Sports Med. 2017;47(12):2443–51.
Wewege M, van den Berg R, Ward RE, Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev. 2017;18(6):635–46.
Wu ZJ, Wang ZY, Gao HE, Zhou XF, Li FH. Impact of high-intensity interval training on cardiorespiratory fitness, body composition, physical fitness, and metabolic parameters in older adults: a meta-analysis of randomized controlled trials. Exp Gerontol. 2021;15(150): 111345.
Chang YH, Yang HY, Shun SC. Effect of exercise intervention dosage on reducing visceral adipose tissue: a systematic review and network meta-analysis of randomized controlled trials. Int J Obes (Lond). 2021;45(5):982–97.
Sultana RN, Sabag A, Keating SE, Johnson NA. The effect of low-volume high-intensity interval training on body composition and cardiorespiratory fitness: a systematic review and meta-analysis. Sports Med. 2019;49(11):1687–721.
Rugbeer N, Constantinou D, Torres G. Comparison of high-intensity training versus moderate-intensity continuous training on cardiorespiratory fitness and body fat percentage in persons with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. J Phys Act Health. 2021;18(5):610–23.
Martland R, Mondelli V, Gaughran F, Stubbs B. Can high-intensity interval training improve physical and mental health outcomes? A meta-review of 33 systematic reviews across the lifespan. J Sport Sci. 2020;38(4):430–69.
Martinez-Calderon J. Overviews of systematic reviews in sports and exercise medicine: what are they and why are they important? Brit J Sport Med. 2023;57(16):1005–+.
Gates M, Gates A, Pieper D, Fernandes RM, Tricco AC, Moher D, et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ. 2022;9(378): e070849.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;21(358): j4008.
Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368–75.
Higgins JT, J. Cochrane handbook for systematic reviews of interventions version 6.4; 2023.
Ahmadizad S, Avansar AS, Ebrahim K, Avandi M, Ghasemikaram M. The effects of short-term high-intensity interval training vs. moderate-intensity continuous training on plasma levels of nesfatin-1 and inflammatory markers. Horm Mol Biol Clin Investig. 2015;21(3):165–73.
Arad AD, DiMenna FJ, Thomas N, Tamis-Holland J, Weil R, Geliebter A, et al. High-intensity interval training without weight loss improves exercise but not basal or insulin-induced metabolism in overweight/obese African American women. J Appl Physiol. 2015;119(4):352–62.
Astorino TA, Schubert MM, Palumbo E, Stirling D, McMillan DW. Effect of two doses of interval training on maximal fat oxidation in sedentary women. Med Sci Sports Exerc. 2013;45(10):1878–86.
Ballin M, Lundberg E, Sorlen N, Nordstrom P, Hult A, Nordstrom A. Effects of interval training on visceral adipose tissue in centrally obese 70-year-old individuals: a randomized controlled trial. J Am Geriatr Soc. 2019;67(8):1625–31.
Bartlett DB, Shepherd SO, Wilson OJ, Adlan AM, Wagenmakers AJM, Shaw CS, et al. Neutrophil and monocyte bactericidal responses to 10 weeks of low-volume high-intensity interval or moderate-intensity continuous training in sedentary adults. Oxid Med Cell Longev. 2017;2017:8148742.
Ben Abderrahman A, Zouhal H, Chamari K, Thevenet D, de Mullenheim PY, Gastinger S, et al. Effects of recovery mode (active vs. passive) on performance during a short high-intensity interval training program: a longitudinal study. Eur J Appl Physiol. 2013;113(6):1373–83.
Bouri S, M. Z, Peeri M, Azarbayjani A, Ahangarpour A. The effect of physical activity on adiponectin and osteocalcin in overweight young females. Int Med J. 2015;22(1):43–6.
Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, MacDonald MJ, Mcgee SL, et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol-Lond. 2008;586(1):151–60.
Cheema BS, Davies TB, Stewart M, Papalia S, Atlantis E. The feasibility and effectiveness of high-intensity boxing training versus moderate-intensity brisk walking in adults with abdominal obesity: a pilot study. Bmc Sports Sci Med R. 2015;7(1).
Cocks M, Shaw CS, Shepherd SO, Fisher JP, Ranasinghe A, Barker TA, et al. Sprint interval and moderate-intensity continuous training have equal benefits on aerobic capacity, insulin sensitivity, muscle capillarisation and endothelial eNOS/NAD(P)Hoxidase protein ratio in obese men. J Physiol-Lond. 2016;594(8):2307–21.
Cocks M, Shaw CS, Shepherd SO, Fisher JP, Ranasinghe AM, Barker TA, et al. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J Physiol. 2013;591(3):641–56.
Cooper JHF, Collins BEG, Adams DR, Robergs RA, Donges CE. Limited effects of endurance or interval training on visceral adipose tissue and systemic inflammation in sedentary middle-aged men. J Obes. 2016;2016.
Dunham C, Harms CA. Effects of high-intensity interval training on pulmonary function. Eur J Appl Physiol. 2012;112(8):3061–8.
Edge J, Bishop D, Goodman C. The effects of training intensity on muscle buffer capacity in females. Eur J Appl Physiol. 2006;96(1):97–105.
Eimarieskandari R, Zilaeibouri S, Zilaeibouri M, Ahangarpour A. Comparing two modes of exercise training with different intensity on body composition in obese young girls. Ovidius Univ Ann Ser Phys Educ Sport. 2012;12(2).
Elmer DJ, Laird RH, Barberio MD, Pascoe DD. Inflammatory, lipid, and body composition responses to interval training or moderate aerobic training. Eur J Appl Physiol. 2016;116(3):601–9.
Eskelinen JJ, Heinonen I, Loyttyniemi E, Saunavaara V, Kirjavainen A, Virtanen KA, et al. Muscle-specific glucose and free fatty acid uptake after sprint interval and moderate-intensity training in healthy middle-aged men. J Appl Physiol (1985). 2015;118(9):1172–80.
Fedewa MV, Hathaway ED, Higgins S, Forehand RL, Schmidt MD, Evans EM. Moderate, but not vigorous, intensity exercise training reduces C-reactive protein. Acta Cardiol. 2018;73(3):283–90.
Gahreman D, Heydari M, Boutcher Y, Freund J, Boutcher S. The effect of green tea ingestion and interval sprinting exercise on the body composition of overweight males: a randomized trial. Nutrients. 2016;8(8).
Garcia-Pinillos F, Laredo-Aguilera JA, Munoz-Jimenez M, Latorre-Roman PA. Effects of 12-week concurrent high-intensity interval strength and endurance training program on physical performance in healthy older people. J Strength Cond Res. 2019;33(5):1445–52.
Gerosa-Neto J, Panissa VLG, Monteiro PA, Inoue DS, Ribeiro JPJ, Figueiredo C, et al. High- or moderate-intensity training promotes change in cardiorespiratory fitness, but not visceral fat, in obese men: a randomised trial of equal energy expenditure exercise. Respir Physiol Neurobiol. 2019;266:150–5.
Gillen JB, Martin BJ, MacInnis MJ, Skelly LE, Tarnopolsky MA, Gibala MJ. Twelve weeks of sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLoS ONE. 2016;11(4): e0154075.
Gormley SE, Swain DP, High R, Spina RJ, Dowling EA, Kotipalli US, et al. Effect of intensity of aerobic training on VO2max. Med Sci Sports Exerc. 2008;40(7):1336–43.
Gripp F, Nava RC, Cassilhas RC, Esteves EA, Magalhaes COD, Dias-Peixoto MF, et al. HIIT is superior than MICT on cardiometabolic health during training and detraining. Eur J Appl Physiol. 2021;121(1):159–72.
Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39(4):665–71.
Heydari M, Boutcher SH. Rating of perceived exertion after 12 weeks of high-intensity, intermittent sprinting. Percept Mot Skills. 2013;116(1):340–51.
Heydari M, Boutcher YN, Boutcher SH. The effects of high-intensity intermittent exercise training on cardiovascular response to mental and physical challenge. Int J Psychophysiol. 2013;87(2):141–6.
Heydari M, Boutcher YN, Boutcher SH. High-intensity intermittent exercise and cardiovascular and autonomic function. Clin Auton Res. 2013;23(1):57–65.
Heydari M, Freund J, Boutcher SH. The effect of high-intensity intermittent exercise on body composition of overweight young males. J Obes. 2012;2012: 480467.
Higgins S, Fedewa MV, Hathaway ED, Schmidt MD, Evans EM. Sprint interval and moderate-intensity cycling training differentially affect adiposity and aerobic capacity in overweight young-adult women. Appl Physiol Nutr Metab. 2016;41(11):1177–83.
Hornbuckle LM, McKenzie MJ, Whitt-Glover MC. Effects of high-intensity interval training on cardiometabolic risk in overweight and obese African-American women: a pilot study. Ethnic Health. 2018;23(7):752–66.
Hwang CL, Yoo JK, Kim HK, Hwang MH, Handberg EM, Petersen JW, et al. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp Gerontol. 2016;82:112–9.
Jabbour G, Mauriege P, Joanisse D, Iancu HD. Effect of supramaximal exercise training on metabolic outcomes in obese adults. J Sports Sci. 2017;35(20):1975–81.
Jimenez-Garcia JD, Martinez-Amat A, De la Torre-Cruz MJ, Fabrega-Cuadros R, Cruz-Diaz D, Aibar-Almazan A, et al. Suspension training HIIT improves gait speed, strength and quality of life in older adults. Int J Sports Med. 2019;40(2):116–24.
Keating SE, Machan EA, O’Connor HT, Gerofi JA, Sainsbury A, Caterson ID, et al. Continuous exercise but not high intensity interval training improves fat distribution in overweight adults. J Obes. 2014;2014: 834865.
Kong Z, Fan X, Sun S, Song L, Shi Q, Nie J. Comparison of high-intensity interval training and moderate-to-vigorous continuous training for cardiometabolic health and exercise enjoyment in obese young women: a randomized controlled trial. PLoS ONE. 2016;11(7): e0158589.
Kong Z, Sun S, Liu M, Shi Q. Short-term high-intensity interval training on body composition and blood glucose in overweight and obese young women. J Diabetes Res. 2016;2016:4073618.
Lunt H, Draper N, Marshall HC, Logan FJ, Hamlin MJ, Shearman JP, et al. High intensity interval training in a real world setting: a randomized controlled feasibility study in overweight inactive adults, measuring change in maximal oxygen uptake. PLoS ONE. 2014;9(1): e83256.
Macpherson RE, Hazell TJ, Olver TD, Paterson DH, Lemon PW. Run sprint interval training improves aerobic performance but not maximal cardiac output. Med Sci Sports Exerc. 2011;43(1):115–22.
Mader U, Roth P, Furrer R, Brechet JP, Boutellier U. Influence of continuous and discontinuous training protocols on subcutaneous adipose tissue and plasma substrates. Int J Sports Med. 2001;22(5):344–9.
Malin SK, Heiston EM, Gilbertson NM, Eichner NZM. Short-term interval exercise suppresses acylated ghrelin and hunger during caloric restriction in women with obesity. Physiol Behav. 2020;1(223): 112978.
Matsuo T, Saotome K, Seino S, Eto M, Shimojo N, Matsushita A, et al. Low-volume, high-intensity, aerobic interval exercise for sedentary adults: VO(2)max, cardiac mass, and heart rate recovery. Eur J Appl Physiol. 2014;114(9):1963–72.
Mirghani SJ, Yousefi MS. The effect of interval recovery periods during HIIT on liver enzymes and lipid profile in overweight women. Sci Sport. 2015;30(3):147–54.
Moreira MM, de Souza HPC, Schwingel PA, de Sá CKC, Zoppi CC. Effects of aerobic and anaerobic exercise on cardiac risk variables in overweight adults. Arq Bras Cardiol. 2008;91(4):200–6.
Nalcakan GR. The effects of sprint interval vs. continuous endurance training on physiological and metabolic adaptations in young healthy adults. J Hum Kinet. 2014;44:97–109.
Nemoto K, Gen-no H, Masuki S, Okazaki K, Nose H. Effects of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clin Proc. 2007;82(7):803–11.
Nie J, Zhang H, Kong Z, George K, Little JP, Tong TK, et al. Impact of high-intensity interval training and moderate-intensity continuous training on resting and postexercise cardiac troponin T concentration. Exp Physiol. 2018;103(3):370–80.
Nybo L, Sundstrup E, Jakobsen MD, Mohr M, Hornstrup T, Simonsen L, et al. High-intensity training versus traditional exercise interventions for promoting health. Med Sci Sport Exer. 2010;42(10):1951–8.
Panissa V, Alves I, Salermo G, Franchini E, Takito M. Can short-term high-intensity intermittent training reduce adiposity? Sport Sci Health. 2016;12:99–104.
Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ, MacDonald MJ. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am J Physiol-Reg I. 2008;295(1):R236–42.
Ram A, Marcos L, Jones M, Morey R, Hakansson S, Clark T, et al. The effect of high-intensity interval training and moderate-intensity continuous training on aerobic fitness and body composition in males with overweight or obesity: a randomized trial. Obes Med. 2020;17.
Ramirez-Velez R, Tordecilla-Sanders A, Tellez LA, Camelo-Prieto D, Hernández-Quiñonez PA, Correa-Bautista JE, et al. Similar cardiometabolic effects of high- and moderate-intensity training among apparently healthy inactive adults: a randomized clinical trial (vol 15, 118, 2017). J Transl Med. 2017;13:15.
Rebold MJ, Kobak MS, Otterstetter R. The influence of a tabata interval training program using an aquatic underwater treadmill on various performance variables. J Strength Cond Res. 2013;27(12):3419–25.
Reljic D, Wittmann F, Fischer JE. Effects of low-volume high-intensity interval training in a community setting: a pilot study. Eur J Appl Physiol. 2018;118(6):1153–67.
Rowley TW, Espinoza JL, Akers JD, Wenos DL, Edwards ES. Effects of run sprint interval training on healthy, inactive, overweight/obese women: a pilot study. Facets. 2017;31:2.
Sandvei M, Jeppesen PB, Stoen L, Litleskare S, Johansen E, Stensrud T, et al. Sprint interval running increases insulin sensitivity in young healthy subjects. Arch Physiol Biochem. 2012;118(3):139–47.
Sasaki H, Morishima T, Hasegawa Y, Mori A, Ijichi T, Kurihara T, et al. 4 weeks of high-intensity interval training does not alter the exercise-induced growth hormone response in sedentary men. Springerplus. 2014;3:336.
Sawyer BJ, Tucker WJ, Bhammar DM, Ryder JR, Sweazea KL, Gaesser GA. Effects of high-intensity interval training and moderate-intensity continuous training on endothelial function and cardiometabolic risk markers in obese adults. J Appl Physiol (1985). 2016;121(1):279–88.
Schubert MM, Clarke HE, Seay RF, Spain KK. Impact of 4 weeks of interval training on resting metabolic rate, fitness, and health-related outcomes. Appl Physiol Nutr Metab. 2017;42(10):1073–81.
Sculthorpe NF, Herbert P, Grace F. One session of high-intensity interval training (HIIT) every 5 days, improves muscle power but not static balance in lifelong sedentary ageing men: A randomized controlled trial. Medicine (Baltimore). 2017;96(6): e6040.
Shepherd SO, Cocks M, Tipton KD, Ranasinghe AM, Barker TA, Burniston JG, et al. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J Physiol. 2013;591(3):657–75.
Shepherd SO, Wilson OJ, Taylor AS, Thogersen-Ntoumani C, Adlan AM, Wagenmakers AJ, et al. Low-volume high-intensity interval training in a gym setting improves cardio-metabolic and psychological health. PLoS ONE. 2015;10(9): e0139056.
Sijie T, Hainai Y, Fengying Y, Jianxiong W. High intensity interval exercise training in overweight young women. J Sports Med Phys Fitness. 2012;52(3):255–62.
Sim AY, Wallman KE, Fairchild TJ, Guelfi KJ. Effects of high-intensity intermittent exercise training on appetite regulation. Med Sci Sports Exerc. 2015;47(11):2441–9.
Skleryk JR, Karagounis LG, Hawley JA, Sharman MJ, Laursen PB, Watson G. Two weeks of reduced-volume sprint interval or traditional exercise training does not improve metabolic functioning in sedentary obese men. Diabetes Obes Metab. 2013;15(12):1146–53.
Smith-Ryan AE, Trexler ET, Wingfield HL, Blue MNM. Effects of high-intensity interval training on cardiometabolic risk factors in overweight/obese women. J Sport Sci. 2016;34(21):2038–46.
Tong TK, Zhang HF, Shi HR, Liu Y, Ai JW, Nie JL, et al. Comparing time efficiency of sprint vs. high-intensity interval training in reducing abdominal visceral fat in obese young women: a randomized, controlled trial. Front Physiol. 2018;9.
Trapp EG, Chisholm DJ, Freund J, Boutcher SH. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes. 2008;32(4):684–91.
Tsekouras YE, Magkos F, Kellas Y, Basioukas KN, Kavouras SA, Sidossis LS. High-intensity interval aerobic training reduces hepatic very low-density lipoprotein-triglyceride secretion rate in men. Am J Physiol-Endoc M. 2008;295(4):E851–8.
Umamaheswari D, Dhanalakshmi Y, Karthik S, John A, Sultana R. Effect of exercise intensity on body composition in overweight and obese individuals. Indian J Physiol Pharmacol. 2017;61(1):58–64.
Vella CA, Taylor K, Drummer D. High-intensity interval and moderate-intensity continuous training elicit similar enjoyment and adherence levels in overweight and obese adults. Eur J Sport Sci. 2017;17(9):1203–11.
Wallman K, Plant LA, Rakimov B, Maiorana AJ. The effects of two modes of exercise on aerobic fitness and fat mass in an overweight population. Res Sports Med. 2009;17(3):156–70.
Yamagishi T, Babraj J. Effects of reduced-volume of sprint interval training and the time course of physiological and performance adaptations. Scand J Med Sci Sports. 2017;27(12):1662–72.
Zhang HF, Tong TK, Kong ZW, Shi QD, Liu Y, Nie JL. Exercise training-induced visceral fat loss in obese women: the role of training intensity and modality. Scand J Med Sci Spor. 2021;31(1):30–43.
Zhang HF, Tong TK, Qiu WF, Wang JJ, Nie JL, He YX. Effect of high-intensity interval training protocol on abdominal fat reduction in overweight Chinese women: a randomized controlled trial. Kinesiology. 2015;47(1):57–66.
Zhang HF, Tong TK, Qiu WF, Zhang X, Zhou S, Liu Y, et al. Comparable effects of high-intensity interval training and prolonged continuous exercise training on abdominal visceral fat reduction in obese young women. J Diabetes Res. 2017;2017.
Poon ETC, Little JP, Sit CHP, Wong SHS. The effect of low-volume high-intensity interval training on cardiometabolic health and psychological responses in overweight/obese middle-aged men. J Sport Sci. 2020;38(17):1997–2004.
Poon ETC, Siu PMF, Wongpipit W, Gibala M, Wong SHS. Alternating high-intensity interval training and continuous training is efficacious in improving cardiometabolic health in obese middle-aged men. J Exerc Sci Fit. 2022;20(1):40–7.
Schjerve IE, Tyldum GA, Tjonna AE, Stolen T, Loennechen JP, Hansen HEM, et al. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci. 2008;115(9–10):283–93.
Alzar-Teruel M, Aibar-Almazán A, Hita-Contreras F, Carcelén-Fraile MD, Martínez-Amat A, Jiménez-García JD, et al. High-intensity interval training among middle-aged and older adults for body composition and muscle strength: a systematic review. Front Public Health. 2022;29:10.
Williams CB, Zelt JG, Castellani LN, Little JP, Jung ME, Wright DC, et al. Changes in mechanisms proposed to mediate fat loss following an acute bout of high-intensity interval and endurance exercise. Appl Physiol Nutr Metab. 2013;38(12):1236–44.
Gore CJ, Withers RT. The effect of exercise intensity and duration on the oxygen deficit and excess post-exercise oxygen consumption. Eur J Appl Physiol Occup Physiol. 1990;60(3):169–74.
Tucker WJ, Angadi SS, Gaesser GA. Excess postexercise oxygen consumption after high-intensity and sprint interval exercise, and continuous steady-state exercise. J Strength Cond Res. 2016;30(11):3090–7.
Sevits KJ, Melanson EL, Swibas T, Binns SE, Klochak AL, Lonac MC, et al. Total daily energy expenditure is increased following a single bout of sprint interval training. Physiol Rep. 2013;1(5).
Panissa VLG, Fukuda DH, Staibano V, Marques M, Franchini E. Magnitude and duration of excess of post-exercise oxygen consumption between high-intensity interval and moderate-intensity continuous exercise: a systematic review. Obes Rev. 2021;22(1).
Trapp EG, Chisholm DJ, Boutcher SH. Metabolic response of trained and untrained women during high-intensity intermittent cycle exercise. Am J Physiol Regul Integr Comp Physiol. 2007;293(6):R2370–5.
Pritzlaff CJ, Wideman L, Blumer J, Jensen M, Abbott RD, Gaesser GA, et al. Catecholamine release, growth hormone secretion, and energy expenditure during exercise vs. recovery in men. J Appl Physiol (1985). 2000;89(3):937–46.
Maillard F, Pereira B, Boisseau N. Effect of high-intensity interval training on total, abdominal and visceral fat mass: a meta-analysis. Sports Med. 2018;48(2):269–88.
Schubert MM, Sabapathy S, Leveritt M, Desbrow B. Acute exercise and hormones related to appetite regulation: a meta-analysis. Sports Med. 2014;44(3):387–403.
Hazell TJ, Islam H, Townsend LK, Schmale MS, Copeland JL. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: potential mechanisms. Appetite. 2016;1(98):80–8.
Melzer K, Kayser B, Saris WH, Pichard C. Effects of physical activity on food intake. Clin Nutr. 2005;24(6):885–95.
Deighton K, Barry R, Connon CE, Stensel DJ. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur J Appl Physiol. 2013;113(5):1147–56.
Ho-Pham LT, Campbell LV, Nguyen TV. More on body fat cutoff points. Mayo Clin Proc. 2011;86(6):584; author reply -5.
Macek P, Biskup M, Terek-Derszniak M, Stachura M, Krol H, Gozdz S, et al. Optimal body fat percentage cut-off values in predicting the obesity-related cardiovascular risk factors: a cross-sectional cohort study. Diabetes Metab Syndr Obes. 2020;13:1587–97.
Rose GL, Farley MJ, Slater GJ, Ward LC, Skinner TL, Keating SE, et al. How body composition techniques measure up for reliability across the age-span. Am J Clin Nutr. 2021;114(1):281–94.
Gallo-Villegas J, Restrepo D, Perez L, Castro-Valencia LA, Narvaez-Sanchez R, Osorio J, et al. Safety of high-intensity, low-volume interval training or continuous aerobic training in adults with metabolic syndrome. J Patient Saf. 2022;18(4):295–301.
Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(5):1077–84.
Sabag A, Little JP, Johnson NA. Low-volume high-intensity interval training for cardiometabolic health. J Physiol. 2022;600(5):1013–26.
Atakan MM, Guzel Y, Shrestha N, Kosar SN, Grgic J, Astorino TA, et al. Effects of high-intensity interval training (HIIT) and sprint interval training (SIT) on fat oxidation during exercise: a systematic review and meta-analysis. Br J Sports Med. 2022.
Vollaard NBJ, Metcalfe RS, Williams S. Effect of number of sprints in an sit session on change in V O2max: a meta-analysis. Med Sci Sports Exerc. 2017;49(6):1147–56.
Ekkekakis P, Biddle SJH. Extraordinary claims in the literature on high-intensity interval training (HIIT): IV. Is HIIT associated with higher long-term exercise adherence? Psychol Sport Exerc. 2023;64.
Santos A, Braaten K, MacPherson M, Vasconcellos D, Vis-Dunbar M, Lonsdale C, et al. Rates of compliance and adherence to high-intensity interval training: a systematic review and meta-analyses. Int J Behav Nutr Phys Act. 2023;20(1):134.
Hussain SR, Macaluso A, Pearson SJ. High-intensity interval training versus moderate-intensity continuous training in the prevention/management of cardiovascular disease. Cardiol Rev. 2016;24(6):273–81.
Rognmo O, Moholdt T, Bakken H, Hole T, Molstad P, Myhr NE, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012;126(12):1436–40.
Herranz-Gomez A, Cuenca-Martinez F, Suso-Marti L, Varangot-Reille C, Calatayud J, Blanco-Diaz M, et al. Effectiveness of HIIT in patients with cancer or cancer survivors: An umbrella and mapping review with meta-meta-analysis. Scand J Med Sci Sports. 2022;32(11):1522–49.
Andreato LV, Esteves JV, Coimbra DR, Moraes AJP, de Carvalho T. The influence of high-intensity interval training on anthropometric variables of adults with overweight or obesity: a systematic review and network meta-analysis. Obes Rev. 2019;20(1):142–55.
Batacan RB Jr, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. 2017;51(6):494–503.
Depiazzi JE, Forbes RA, Gibson N, Smith NL, Wilson AC, Boyd RN, et al. The effect of aquatic high-intensity interval training on aerobic performance, strength and body composition in a non-athletic population: systematic review and meta-analysis. Clin Rehabil. 2019;33(2):157–70.
Guo Z, Cai J, Wu Z, Gong W. Effect of high-intensity interval training combined with fasting in the treatment of overweight and obese adults: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(8).
Hwang CL, Wu YT, Chou CH. Effect of aerobic interval training on exercise capacity and metabolic risk factors in people with cardiometabolic disorders: a meta-analysis. J Cardiopulm Rehabil Prev. 2011;31(6):378–85.
Keating SE, Johnson NA, Mielke GI, Coombes JS. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18(8):943–64.
Serrablo-Torrejon I, Lopez-Valenciano A, Ayuso M, Horton E, Mayo X, Medina-Gomez G, et al. High intensity interval training exercise-induced physiological changes and their potential influence on metabolic syndrome clinical biomarkers: a meta-analysis. BMC Endocr Disord. 2020;20(1):167.
Steele J, Plotkin D, Van Every D, Rosa A, Zambrano H, Mendelovits B, et al. Slow and steady, or hard and fast? A systematic review and meta-analysis of studies comparing body composition changes between interval training and moderate intensity continuous training. Sports (Basel). 2021;9(11).
Wang R, Zhang X, Ren H, Zhou H, Yuan Y, Chai Y, et al. Effects of different exercise types on visceral fat in young individuals with obesity aged 6–24 years old: a systematic review and meta-analysis. Front Physiol. 2022;13: 987804.
Wang SY, Zhou HY, Zhao CT, He H. Effect of exercise training on body composition and inflammatory cytokine levels in overweight and obese individuals: a systematic review and network meta-analysis. Front Immunol. 2022;23:13.
Acknowledgements
The authors of this study express their sincere gratitude to contacted authors for taking the time to respond to data requests in such a kind and prompt manner. We thank Professor Martin Gibala for his invaluable advice during the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
All authors have no conflicts of interest to disclose.
Availability of data and material
The datasets analyzed in this review are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
E.P., J.L., S.W., and R.H. conceived the idea for the review. E.P., J.L., and R.H. conducted the search, study selection, data extraction, and quality assessment. E.P., J.L., and R.H. drafted the initial manuscript. E.P., J.L., and R.H. contributed to writing the manuscript. All authors reviewed and approved the final manuscript.
Additional information
Systematic Review Registration Number This study was registered in the International Prospective Register of Systematic Review (PROSPERO) database (registration number: CRD42023490819).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Poon, E.TC., Li, HY., Little, J.P. et al. Efficacy of Interval Training in Improving Body Composition and Adiposity in Apparently Healthy Adults: An Umbrella Review with Meta-Analysis. Sports Med (2024). https://doi.org/10.1007/s40279-024-02070-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s40279-024-02070-9