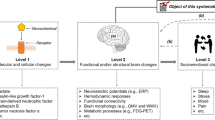
Abstract
Background
Despite the intriguing potential of physical exercise being able to preserve or even restore brain volume (grey matter volume in particular)—a tissue essential for both cognitive and physical function—no reviews have so far synthesized the existing knowledge from randomized controlled trials investigating exercise-induced changes of the brain’s grey matter volume in populations at risk of neurodegeneration. Our objective was to critically review the existing evidence regarding this topic.
Methods
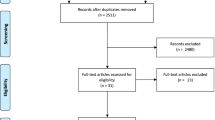
A systematic search was carried out in MEDLINE and EMBASE databases primo April 2020, to identify randomized controlled trials evaluating the effects of aerobic training, resistance training or concurrent training on brain grey volume changes (by MRI) in adult clinical or healthy elderly populations.
Results
A total of 20 articles (from 19 RCTs) evaluating 3–12 months of aerobic, resistance, or concurrent training were identified and included, involving a total of 1662 participants (populations: healthy older adults, older adults with mild cognitive impairment or Alzheimer’s disease, adults with schizophrenia or multiple sclerosis or major depression). While few studies indicated a positive effect—although modest—of physical exercise on certain regions of brain grey matter volume, the majority of study findings were neutral (i.e., no effects/small effect sizes) and quite divergent across populations. Meta-analyses showed that different exercise modalities failed to elicit any substantial effects on whole brain grey volume and hippocampus volume, although with rather large confidence interval width (i.e., variability).
Conclusion
Altogether, the current evidence on the effects of physical exercise on whole/regional grey matter brain volume appear sparse and inconclusive, and does not support that physical exercise is as potent as previously proposed when it comes to affecting brain grey matter volume.



Similar content being viewed by others
Availability of Data and Materials
Data are available from the authors upon reasonable request.
References
Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Invest. 2003;111(1):3–10.
Pini L, et al. Brain atrophy in Alzheimer’s disease and aging. Ageing Res Rev. 2016;30:25–48.
Miller DH, et al. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain. 2002;125(8):1676–95.
Favaretto A, et al. Effects of disease modifying therapies on brain and grey matter atrophy in relapsing remitting multiple sclerosis. Mult Scler Demyelinating Disord. 2018;3(1):1.
Jack CR Jr, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484–9.
Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–48.
Vollmer T, et al. Relationship between brain volume loss and cognitive outcomes among patients with multiple sclerosis: a systematic literature review. Neurolog Sci. 2016;37(2):165–79.
Rocca MA, Comi G, Filippi M. The role of T1-weighted derived measures of neurodegeneration for assessing disability progression in multiple sclerosis. Front Neurol. 2017;8:433.
Seidman LJ, et al. Relationship of prefrontal and temporal lobe MRI measures to neuropsychological performance in chronic schizophrenia. Biol Psychiatry. 1994;35(4):235–46.
van Haren NE, et al. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68(9):871–80.
Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–66.
Eshaghi A, et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol. 2018;83(2):210–22.
Raz N, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–89.
Vinke EJ, et al. Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiol Aging. 2018;71:32–40.
Vollmer T, et al. The natural history of brain volume loss among patients with multiple sclerosis: a systematic literature review and meta-analysis. J Neurol Sci. 2015;357(1–2):8–18.
Kahn RS, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067.
Colcombe SJ, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–80.
Erickson KI, et al. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc. 2019;51(6):1242–51.
Colcombe SJ, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–70.
Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–22.
Kjolhede T, et al. Can resistance training impact MRI outcomes in relapsing–remitting multiple sclerosis? Mult Scler. 2018;24(10):1356–65.
Batouli SAH, Saba V. At least eighty percent of brain grey matter is modifiable by physical activity: a review study. Behav Brain Res. 2017;332(Supplement C):204–17.
Firth J, et al. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. Neuroimage. 2018;166:230–8.
Halloway S, et al. Effects of endurance-focused physical activity interventions on brain health: a systematic review. Biol Res Nurs. 2017;19(1):53–64.
Li MY, et al. The effects of aerobic exercise on the structure and function of DMN-related brain regions: a systematic review. Int J Neurosci. 2017;127(7):634–49.
Chen FT, et al. The effect of exercise training on brain structure and function in older adults: a systematic review based on evidence from randomized control trials. J Clin Med. 2020;9(4):914.
Yuan Y, Hunt RH. Systematic reviews: the good, the bad, and the ugly. Am J Gastroenterol. 2009;104(5):1086–92.
Esteban-Cornejo I, et al. Commentary: at least eighty percent of brain grey matter is modifiable by physical activity: a review study. Front Hum Neurosci. 2018. https://doi.org/10.3389/fnhum.2018.00195.
Smart NA, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc. 2015;13(1):9–18.
Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of meta-essentials: a free and simple tool for meta-analysis. Res Synth Methods. 2017;8(4):537–53.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum Associates; 1988.
Mowinckel AM, Vidal-Piñeiro D (2019) Visualisation of brain statistics with R-packages ggseg and ggseg3d. Other statistics. arXiv: 1912.08200 [v1].
Pajonk FG, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–43.
Feys P, et al. Effects of an individual 12-week community-located “start-to-run” program on physical capacity, walking, fatigue, cognitive function, brain volumes, and structures in persons with multiple sclerosis. Mult Scler. 2019;25(1):92–103.
Langeskov-Christensen M, et al. Efficacy of high-intensity aerobic exercise on brain MRI measures in multiple sclerosis. Neurology. 2021;96(2):e203–13.
Morris JK, et al. Aerobic exercise for Alzheimer’s disease: a randomized controlled pilot trial. PLoS ONE. 2017;12(2):e0170547.
Best JR, et al. Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. J Int Neuropsychol Soc. 2015;21(10):745–56.
Scheewe TW, et al. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol. 2013;23(7):675–85.
Maass A, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2015;20(5):585–93.
Kleemeyer MM, et al. Changes in fitness are associated with changes in hippocampal microstructure and hippocampal volume among older adults. Neuroimage. 2016;131:155–61.
Jonasson LS, et al. Aerobic exercise intervention, cognitive performance, and brain structure: results from the physical influences on brain in aging (PHIBRA) study. Front Aging Neurosci. 2017. https://doi.org/10.3389/fnagi.2016.00336.
Niemann C, Godde B, Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front Aging Neurosci. 2014;6:1–24.
Ten Brinke LF, et al. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br J Sports Med. 2015;49(4):248–54.
Tarumi T, et al. Exercise training in amnestic mild cognitive impairment: a one-year randomized controlled trial. J Alzheimers Dis. 2019;71(2):421–33.
Krogh J, et al. The effect of exercise on hippocampal volume and neurotrophines in patients with major depression—a randomized clinical trial. J Affect Disord. 2014;165:24–30.
Gylling AT, et al. The influence of prolonged strength training upon muscle and fat in healthy and chronically diseased older adults. Exp Gerontol. 2020;136:110939.
Suo C, et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol Psychiatry. 2016;21(11):1633–42.
Niemann C, et al. Exercise-induced changes in basal ganglia volume and cognition in older adults. Neurosci. 2014;281:147–63.
Nagamatsu LS, et al. Exercise mode moderates the relationship between mobility and basal ganglia volume in healthy older adults. J Am Geriatr Soc. 2016;64(1):102–8.
Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14(23):2919–37.
Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–72.
Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270(5236):593–8.
Pedersen BK. Physical activity and muscle–brain crosstalk. Nat Rev Endocrinol. 2019;15(7):383–92.
Cheng A, et al. Truncated tyrosine kinase B brain-derived neurotrophic factor receptor directs cortical neural stem cells to a glial cell fate by a novel signaling mechanism. J Neurochem. 2007;100(6):1515–30.
Benraiss A, et al. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21(17):6718–31.
Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–31.
Lin CY, et al. Brain-derived neurotrophic factor increases vascular endothelial growth factor expression and enhances angiogenesis in human chondrosarcoma cells. Biochem Pharmacol. 2014;91(4):522–33.
Dinoff A, et al. The Effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): a meta-analysis. PLoS ONE. 2016;11(9):e0163037.
Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64.
Marinus N, et al. The impact of different types of exercise training on peripheral blood brain-derived neurotrophic factor concentrations in older adults: a meta-analysis. Sports Med. 2019;49:1529–46.
Jørgensen MLK, et al. Plasma brain-derived neurotrophic factor (BDNF) and sphingosine-1-phosphat (S1P) are NOT the main mediators of neuroprotection induced by resistance training in persons with multiple sclerosis—a randomized controlled trial. Mult Scler Relat Disord. 2019;31:106–11.
Gejl AK, et al. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-45976-5.
Polacchini A, et al. A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Sci Rep. 2015. https://doi.org/10.1038/srep1798910.1038/srep17989.
Liu Y, et al. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab Invest. 2019;99(7):943–57.
Seo DY, et al. Exercise and neuroinflammation in health and disease. Int Neurourol J. 2019;23(Suppl 2):S82-92.
Spielman LJ, Little JP, Klegeris A. Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain Res Bull. 2016;125:19–29.
Gomez-Rubio P, Trapero I. The effects of exercise on IL-6 levels and cognitive performance in patients with schizophrenia. Diseases. 2019;7(1):11.
Negaresh R, et al. Effects of exercise training on cytokines and adipokines in multiple sclerosis: a systematic review. Mult Scler Relat Disord. 2018;24:91–100.
Monteiro-Junior RS, et al. Effect of exercise on inflammatory profile of older persons: systematic review and meta-analyses. J Phys Act Health. 2018;15(1):64–71.
Liberman K, et al. The effects of exercise on muscle strength, body composition, physical functioning and the inflammatory profile of older adults: a systematic review. Curr Opin Clin Nutr Metab Care. 2017;20(1):30–53.
Zivadinov R, et al. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology. 2008;71(2):136–44.
Vidal-Jordana A, et al. Early brain pseudoatrophy while on natalizumab therapy is due to white matter volume changes. Mult Scler. 2013;19(9):1175–81.
Mosconi L, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29(5):676–92.
Branger P, et al. The effect of disease-modifying drugs on brain atrophy in relapsing–remitting multiple sclerosis: a meta-analysis. PLoS ONE. 2016;11(3):e0149685.
Tsivgoulis G, et al. The effect of disease modifying therapies on disease progression in patients with relapsing–remitting multiple sclerosis: a systematic review and meta-analysis. PLoS ONE. 2015;10(12):e0144538.
Kelly ME, et al. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16(1):12–31.
Northey JM, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52(3):154–60.
Gharakhanlou R, et al. Exercise training and cognitive performance in persons with multiple sclerosis: a systematic review and multilevel meta-analysis of clinical trials. Mult Scler. 2020. https://doi.org/10.1177/1352458520917935.
Herold F, et al. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—a systematic review. Eur Rev Aging Phys Act. 2019;16:10.
Author information
Authors and Affiliations
Contributions
LGH, DLH, SFE, and UD contributed to the conception of the study and to the development of the search strategy. LGH and DLH conducted the systematic search and completed the acquisition of data. LGH and DLH performed the data analysis. LGH took the lead in writing the manuscript. All the authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Funding
No external funding was provided for this study.
Conflict of interest
The authors (LGH, DLH, SFE, UD) declare that they have no conflicts of interest relevant to the content of this article.
Ethical approval
The manuscript does not contain patient data, and ethical approval was not required.
Rights and permissions
About this article
Cite this article
Hvid, L.G., Harwood, D.L., Eskildsen, S.F. et al. A Critical Systematic Review of Current Evidence on the Effects of Physical Exercise on Whole/Regional Grey Matter Brain Volume in Populations at Risk of Neurodegeneration. Sports Med 51, 1651–1671 (2021). https://doi.org/10.1007/s40279-021-01453-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-021-01453-6