Abstract
Background
Recent advances in the development of biomarker-directed therapy and immunotherapy, for advanced and metastatic gastric cancers, have the potential to improve survival and quality of life. Much attention has been directed towards second- and later-line treatments, and the landscape here is evolving rapidly. However, uncertainty in relative effectiveness, high costs and uncertainty in cost effectiveness represent challenges for decision makers.
Objective
To identify economic evaluations for the second-line or later-line treatment of advanced and metastatic gastric cancer. Also, to assess key criteria (including model assumptions, inputs and outcomes), reporting completeness and methodological quality to inform future cost-effectiveness evaluations.
Methods
A systematic literature search (from database inception to 5 March 2023) of EconLit via EBSCOhost, Cochrane Library (restricted to National Health Service [NHS] Economic Evaluation Database and Health Technology Assessment [HTA] Database), Embase, MEDLINE and of grey literature was conducted. This aimed to identify systemic treatments that align with National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) Clinical Practice Guidelines. Data were collected on key criteria and on reporting completeness and methodological quality. A narrative synthesis focussed on cost-effectiveness and cost-of-illness studies. Outcomes of interest included total and incremental costs and outcomes (life-years and quality-adjusted life-years), ratios of incremental costs per unit outcome and other summary cost and outcome measures. Also, for cost-effectiveness studies, reporting completeness and the methodological quality were assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) and the Philips Checklist, respectively.
Results
A total of 19 eligible economic evaluations were identified (cost-effectiveness studies [n = 15] and cost-of-illness studies [n = 4]). There was a general lack of consistency in the methodological approaches taken across studies. In the main, the cost-effectiveness studies indicated that the intervention under consideration was more effective and more costly than the comparator(s). However, most interventions were not cost effective. No studies were fully compliant with reporting-completeness and methodological-quality requirements. Given the lack of consistency in the approaches taken across cost-of-illness studies, outcomes could not be directly compared.
Conclusions
To our knowledge, this is the first published systematic literature review that has qualitatively synthesised economic evaluations for advanced and metastatic gastric cancer. There were differences in the approaches taken across the cost-effectiveness studies and the cost-of-illness studies. The conclusions of most of the cost-effectiveness studies were consistent despite identified differences in approaches. In the main, the interventions under consideration were not cost effective, presenting challenges to sustainability and affordability. We highlight a requirement for cost-effectiveness evaluations and for second-line or later-line treatments of advanced and metastatic gastric cancer that consider all relevant comparators and that are compliant with reporting-completeness and methodological-quality requirements. By addressing the methodological gaps identified here, future healthcare decision-making, within the context of this rapidly changing treatment landscape, would be better informed.
Prospero Registration Number
CRD42023405951.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
To our knowledge, this is the first published systematic literature review that has qualitatively synthesised economic evaluations for the treatment of advanced and metastatic gastric cancer (second or later-line). |
This work highlights challenges associated with the cost effectiveness of treatments (second or later-line) for advanced and metastatic gastric cancers. It also highlights the need for robust economic evaluations to inform future reimbursement decisions. |
Given the rapidly changing treatment landscape, our work makes a strong and timely case for robust economic evaluations. This work will be of interest to a wide audience including HTA analysts and academics, pharma, policy makers and healthcare providers. |
1 Introduction
Among all cancers, gastric cancer ranks fifth in terms of incidence globally with over one million cases reported annually [1,2]. The National Cancer Institute in the USA states that the 5-year relative survival rates for localised gastric cancer, regional gastric cancer and metastatic gastric cancer are 75%, 35% and 7% respectively. Gastric cancer is the third most prominent cause of cancer-related deaths globally [3]. While surgical treatment with curative intent is possible in the earlier stages, individuals with advanced or metastatic gastric cancer are typically treated with systemic therapies only.
According to European Society for Medical Oncology (ESMO) Clinical Practice Guidelines 2022, standard first-line treatments include platinum in combination with (+) fluoropyrimidine (platinum + fluoropyrimidine) or irinotecan + fluorouracil (5FU) for those intolerant of platinum compounds. In patients with HER2-overexpression, trastuzumab + chemotherapy is recommended. Additionally, in patients with HER2-negative disease, with PD-L1 combined positive score (CPS) ≥ 5, nivolumab + chemotherapy is recommended [4]. The EMA and FDA [5] have also approved pembrolizumab + trastuzumab + chemotherapy in patients with HER2-positive disease with PD-L1 CPS ≥ 1 [6]. The FDA recently approved pembrolizumab + chemotherapy in the first-line setting for patients with HER2-negative disease regardless of PD-L1 CPS [7].
For second- or later-line treatment, standard-of-care (SoC) chemotherapies include docetaxel monotherapy, paclitaxel monotherapy (or in combination with ramucirumab) or irinotecan monotherapy [8,9]. These offer comparable effectiveness but distinct toxicity profiles [8,9]. For the second-line treatment of metastatic, chemorefractory, gastric cancer, the National Comprehensive Cancer Network (NCCN) suggests pembrolizumab or ramucirumab or various taxanes (as monotherapy or in combination with ramucirumab) [10,11]. The ESMO recommends pembrolizumab for second-line treatment, where MSI-H/dMMR status is positive [4] and also where the tumour mutational burden is high (i.e. greater than or equal to ten mutations per megabase) [11]. ESMO also suggest that treatment with dostarlimab–gxlyg could be considered where MSI-H/dMMR status is positive [11]. Where there is HER2 overexpression, trastuzumab deruxtecan has been associated with benefits versus irinotecan or paclitaxel in patients who have progressed on first-line line trastuzumab-containing regimens and is FDA and EMA approved in this setting [12]. The NCCN suggests entrectinib or larotrectinib [11] where tumours have genetic alterations in NTRK 1/2/3, ROS1 and ALK. The ESMO notes that FOLFIRI (5-FU + leucovorin + irinotecan) is utilised, although evidence is limited [4]. The ESMO suggests that trifluridine–tipiracil be considered for third-line treatment [4].
Two recently reported phase 3 studies have demonstrated an overall survival (OS) benefit to the addition of zolbetuximab, a monoclonal antibody against claudin 18.2, to chemotherapy in the first line setting for patients who are positive for claudin 18.2 overexpression. To date, zolbetuximab has not been approved by either the FDA or EMA; although, it has received approval in Japan [13]. Additional promising biomarker-selected therapies include addition of a monoclonal antibody against FGFR2b to chemotherapy in patients with FGFR2b overexpression, currently under evaluation in phase 3 randomised studies alone and in combination with immunotherapy [14]. As the management of first-line therapy for advanced gastric cancer gains complexity, the treatment of patients in the second line is anticipated to evolve. Refinement of patient selection and optimisation of the sequence of a given biomarker-directed approach will be a key development.
Most cases of gastric cancer are diagnosed at advanced stages [15]. Consequently, patients face poor prognosis [16]. Moreover, even after surgical resection [4] and first line therapy [17], disease progression remains a challenge. It is reported that 25–30% of patients on first-line treatment for advanced gastric cancer will progress at their first evaluation of response [18]. Thus, in recent years, much attention has been directed towards second- and later-line treatments, and the landscape here is evolving rapidly [19]. However, high costs and uncertainty in cost effectiveness of these second-and later-line treatments represent challenges for decision makers [20]. Systematic literature reviews (SLRs) of relative effectiveness of second and third-line treatments for patients with advanced gastric cancer have been identified [21,22,23,24,25]. However, no SLRs of economic evaluations for systemic treatments of second-line and later-line treatments for advanced and metastatic gastric cancers were identified in literature. Such information is of value to decision makers. Therefore, this SLR aims to fill the gap in the literature by identifying economic evaluations for systemic treatments of second-line or later-line regimens, for advanced and metastatic gastric cancers, in accordance with the ESMO and NCCN guidelines. Our goal is to assess key study criteria (including model assumptions, inputs and outcomes), reporting completeness and methodological quality to inform the future development of cost-effectiveness evaluations.
2 Methods
A SLR protocol was devised and registered with PROSPERO [26] , registration number CRD42023405951. The SLR adheres to PRISMA guidelines [27], The ISPOR Good Practices Task Force Report 2021 on the Critical Appraisal of Systematic Reviews with Costs and Cost-Effectiveness Outcomes [28] and national guidelines in Ireland [29].
2.1 Review Question
The review question was informed by the PICOS framework [30], please see Table 1. The population of interest was individuals with locally advanced or metastatic gastric cancer, in the second-line or later-line treatment setting. Eligible interventions were systemic anticancer treatments including all chemotherapies and immunotherapies. Radiotherapy, surgery and combinations of these with systemic therapy were also considered. Comparators could consist of systemic treatments, as well as surgical procedures and supportive care.
2.2 Search Strategy
Electronic searches were conducted for eligible studies published from inception to March 2023 inclusive. Clinical search criteria were informed by a published SLR and network meta-analysis on previously treated advanced gastric cancer [31]. These were supplemented with economic search filters obtained from the Scottish Intercollegiate Guidelines Network (SIGN) 2022 [32] and a relevant published SLR [33]. Additional cancer drug-related search terms were derived from Lordick et al. [4] and Ajani et al. [11]. The resources used were EconLit through EBSCOhost, restricted sections of Cochrane Library (specifically National Health Service [NHS] Economic Evaluation Database and HTA Database), Embase and MEDLINE via Ovid. The complete search string is presented in Supplementary Table 1 of the Electronic Supplementary Material (ESM). Also, a targeted grey-literature search was carried out via The National Institute for Health and Care Excellence (NICE), the Norwegian Institute of Public Health (NIPH), the Scottish Medicines Consortium (SMC) and the Canadian Agency for Drugs and Technologies in Health (CADTH) (Supplementary Table 2 of ESM). Backward and forward citation searching was performed.
2.3 Study Identification, Data Extraction and Quality Assessment
Preliminary screening of title and abstracts of the retrieved studies was undertaken by one reviewer (S.S.). Citation management was facilitated by Covidence® and EndNote® 20 software. Reviewer S.S. was responsible for identifying duplicates. Eligible studies, based on title and abstract screening, were retrieved for full-text review by two independent reviewers (S.S. and L.M.); reasons for exclusions were documented. Disagreements were resolved through dialogue or by involving two researchers (N.C. and D.M.), who acted as arbitrator. Two reviewers (S.S. and L.M.) performed independent data extractions using an established data extraction form in Microsoft Excel®; discrepancies were resolved via discussions. S.S. and L.M. assessed the reporting completeness of the included cost-effectiveness studies via the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [34]. Additionally, S.S. and L.M. systematically assessed the methodological quality of the decision analytical model-based cost-effectiveness studies via the Philips checklist [35]. HTA Agency Evaluation Reports were excluded from the appraisal of reporting-completeness and methodological-quality given that they were all heavily redacted. Further, the cost-of-illness studies were also excluded from appraisal given that no published, relevant checklists were identified. Two reviewers (N.C. and D.M.) worked as a team and were involved in discussions when disagreements arose.
2.4 Data Synthesis
Methodological aspects of the model-based studies were analysed and summarised, including model type, structure, perspective, evidence synthesis, costs and health-related quality of life data. Key results, such as total and incremental costs, total and incremental outcomes and incremental cost-effectiveness ratios (ICERs) were reported.
Eligible studies were categorised as:
-
cost-effectiveness studies (comprising cost-effectiveness analyses [CEAs], cost-utility analyses [CUAs] and cost-minimisation analyses [CMAs]) or
-
cost-of-illness studies.
All costs and cost-related outcomes were adjusted to 2022 levels using the country-specific Consumer Price Index (CPI) for health. If the CPI for health, for a jurisdiction, was unavailable then overall CPI, for that jurisdiction, was used. Subsequently, these values were converted to US dollars based on the Organisation for Economic Cooperation and Development Purchasing Price Parity index [36]. Where the study cost year was not reported, it was assumed to be equivalent to publication year.
3 Results
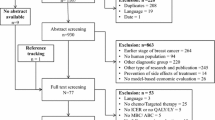
Initially, 816 studies were identified, and 74 duplicates were removed. Title and abstract screening resulted in the elimination of 658. The full text of 85 studies was assessed; 19 were eligible for review (including one obtained through hand searching). For PRISMA flow diagram, please see Fig. 1.
The data extracted from the cost-effectiveness studies and the cost-of illness studies are summarised in Table 2 and in Table 3, respectively, and in Sects. 3.1 to 3.4 inclusive.
3.1 Study Jurisdiction
The 19 eligible studies were conducted in the UK (n = 5) [40,41,43,58,61], USA (n = 3) [50,55,64], Canada (n = 1) [37], Sweden (n = 1) [60], Japan (n = 3) [51,53,63], China (n = 3) [39,52,54], Mexico (n = 1) [65], Columbia (n = 1) [66] and Greece (n = 1) [42].
3.2 Study Type, Population, Interventions and Comparators
The 19 studies included 15 cost-effectiveness studies (comprising eight CUAs [39,40,41,42,43,50,52,58], one CEA [53] and six that reported both CUA and CEA outcomes [37,51,54,55,60,61]; no CMAs were identified) and four cost-of-illness studies [63,64,65,66].
3.2.1 Cost-Effectiveness Studies (n = 15)
Five studies evaluated the cost effectiveness of trifluridine–tipiracil [37,39,40,41,42]. Six evaluated various second-line treatments (ramucirumab, paclitaxel, ramucirumab + paclitaxel, irinotecan, docetaxel, FOLFIRI, best supportive care [BSC]) [43,50,51,52,53,54]. Two studies examined the cost effectiveness of biomarker-guided treatment [55,58]. One evaluated the cost effectiveness of locoregional treatment of peritoneal carcinomatosis from gastric cancer [60]. One evaluated costs and outcomes associated with stage-directed treatment for gastric cancer [61].
3.2.2 Cost-of-Illness Studies (n = 4)
Four studies estimated resource use and financial costs. In three, the target population was described as having advanced or metastatic gastric cancer; in one, the population was described as having stomach cancer [63].
3.3 Study Design and Structural Assumptions
The 19 studies were published from 2013 to 2022 inclusive. Ten were funded independently [37,39,40,41,43,51,53,54,58,60], six received pharmaceutical-industry sponsorship [42,55,63,64,65,66] and a declaration of funding was not reported in three [50,52,61].
3.3.1 Model Structure
Six studies used Markov modelling [39,50,51,52,54,55]. Two conducted Kaplan–Meier (KM) survival analysis directly (without extrapolations) to estimate outcomes and weighted these by utilities to calculate quality-adjusted life years (QALYs) [60,61]. Six used partitioned-survival models [37,40,41,42,43,58]. In one study, the modelling approach used was not described [53] . One of the cost-of-illness studies incorporated a KM OS analysis [64].
3.3.2 Perspective
The health-payer perspective was taken in 14 studies. One study incorporated indirect and direct costs but did not specify a societal perspective [63]. In four, the perspective was not reported. [53,54,60,66].
3.3.3 Discount Rate
Eleven studies (including three cost-of-illness studies) did not report discounting [37,39,40,41,52,53,60,61,64,65,66]; one justified this by a short time-horizon [60]. The remaining eight studies applied the same rate to costs and outcomes; rates ranged from 2 to 3.5%.
3.3.4 Costs
Nine studies reported costs in US dollars [39,50,52,54,55,60,64,65,66], four in British-pound sterling [40,41,43,58], three in Japanese Yen [51,53,63], one in Canadian dollars [37], and two in Euros [42,61]. The cost-year, across 12 studies, ranged from 2008 to 2021; seven studies did not report the cost-year [39,40,41,43,53,58,60].
3.3.4.1 Cost-Effectiveness Studies (n = 15)
In 12 studies, drug-acquisition costs were based on publicly-available drug prices, 1 used confidential discounted prices [41] and 2 used both publicly-available and confidential prices [40,54]. Administration and monitoring costs were included in 11 and 1 excluded these costs [50], and in 3, the approach taken was not reported [39,41,53]. Twelve studies incorporated adverse events costs; three did not [51,60,61]. There was much variation in the approaches taken to include subsequent-treatment costs.
3.3.4.2 Cost-of-Illness Studies (n = 4)
Costs were estimated from Medicare data [64], national reimbursement data [63] or from publicly-available unit costs [65,66]. Indirect costs were calculated in one study.
3.3.5 Time Horizon
3.3.5.1 Cost-Effectiveness Studies (n = 15)
Stated time horizons varied from 3 years to lifetime; although horizons were not always clearly specified. Four studies justified the chosen time horizon [37,51,55,58]. Two explored the time horizon as a driver of uncertainty [40,58]. Where time horizon was stated as lifetime, the duration ranged from 2 [50] to 30 years [58].
3.3.5.2 Cost-of-Illness Studies (n = 4)
In two studies, costs incurred from diagnosis to the end of the data collection were included [64,65]. Novick et al. [66] conducted a retrospective data analysis of costs incurred for individuals who had been diagnosed from 2009 to 2016. Haga et al. [63] estimated annual costs of treating stomach cancer.
3.3.6 Model-Cycle Length
Model-cycle length was reported in 11 of the 15 cost-effectiveness studies; length varied from 1 week [40,42,43,58] to 1 month [39,51,52,55]. The most common lengths were 1 month (n = 4) and 1 week (n = 4). A half-cycle correction was applied in four studies; other studies did not report on this. Cycle length did not constitute a relevant criterion in cost-of-illness studies.
3.3.7 Model Health States
A total of 12 of the 15 cost-effectiveness studies used a conventional, three health-state cancer model (comprising health states of progression-free survival, progressive disease and death). In the remaining three cost-effectiveness studies, health states were not reported [53,60,61]. Health states did not constitute a relevant criterion in cost-of-illness studies.
3.3.8 Data Sources and Extrapolation of Survival Data
3.3.8.1 Cost-Effectiveness Studies (n = 15)
Three studies acquired clinical inputs by SLR and evidence synthesis [43,50,58]; among them, one conducted a network meta-analysis [43]. The remaining 13 lacked evidence of a systematic approach; 2 utilised a targeted literature review [53,55]. No study reported a quality assessment of the clinical evidence. Six studies considered one comparator; of these, five used evidence from the phase III TAGS trial (trifluridine–tipiracil versus placebo) [37,39,40,41,42] and one from the phase III RAINBOW trial (ramucirumab + paclitaxel versus placebo + paclitaxel) [54]. In the remainder (n = 4), varied approaches were taken (the approach was unclear in one [51] and three used real-world data [52,60,61]).
In 14 studies, progression-free survival (PFS) and OS clinical-trial data were the source of treatment effects. Some also incorporated objective response rate [43], disease control rate [37], time to treatment discontinuation [40] and time to off treatment [58]. In six, trial survival data were extrapolated to the model time horizon; standard-parametric extrapolations [67] were employed [41,43,51,58] [40] [42]. For two studies, the extrapolation method entailed converting median PFS and OS rates into probabilities based on a constant hazard rate assumption [50] [39]. In another, piecewise linear approximations of the OS and PFS KM curves were fit and extrapolated; although, limited details of this method were provided [55]. Three studies did not report extrapolation of trial data [53,54,60], while one extrapolated trial data but did not report the method used [37]. Only four studies reported that they assessed the appropriateness of chosen parametric modelling curves or investigated alternative approaches [37,40,43,58]. This criterion was not relevant to the cost-of-illness studies.
3.3.8.2 Cost-of-Illness Studies (n = 4)
Data were sourced from real-world evidence. One study used the Surveillance, Epidemiology, and End Results (SEER)-Medicare database [64], one used government office statistics [63], and two used retrospective-observational data [65,66].
3.3.9 Measurement and Valuation of Health-Related Quality of Life
Fourteen studies reported CUA outcomes. Utilities were sourced directly from clinical trials in three [43,51,58], from trials with subsequent mapping in three [40,41,42], from the literature in four [37,55,60,61] and from published economic evaluations in three [39,52,54]. One study [50] sourced utilities from both a trial and published economic evaluations. Additionally, 1 study adopted a treatment-specific utility approach [61], 11 studies modelled utility based on progression status [37,39,40,41,42,50,51,52,54,55,58], 1 included both approaches [43] and the approach taken in 1 was unclear [60]. Five studies applied disutilities for adverse events in base case analyses [37,40,42,43,55]; another included disutilities in scenario analyses [58]. The value set used to generate the utilities was reported in one study [37]. All other studies did not report the value set. This criterion was not relevant to cost-of-illness studies.
3.4 Study Outcomes
Outcome data (including incremental and total costs and incremental and total QALYs and ICERs) were captured.
3.4.1 Cost-Effectiveness Studies (n = 15)
3.4.1.1 Trifluridine–tipiracil (n = 5)
The cost effectiveness of trifluridine–tipiracil versus BSC was investigated for the treatment of metastatic gastric cancer (previously treated with at least two chemotherapy regimens in the metastatic setting) in five studies [37,39,40,41,42]. In all, the target population was informed by TAGS [38]. All were from the perspective of the respective health-state payers. The resultant ICERs ranged from $39,442/QALY (confidential-price discount included) [41] to $1,115,565/QALY [39] .
Trifluridine–tipiracil was deemed not cost-effective, at the submitted price, in Canada [37] and the USA [39]. It was deemed cost effective in Greece [42]. It was accepted for use, with conditions, in Scotland [40] and for use, at a confidential discounted price, with conditions, under the Cancer Drugs Fund in England and Wales [41].
3.4.1.2 Second-Line Treatment Comparisons (Including Ramucirumab, Various SoC Chemotherapies and BSC) (n = 6)
Six studies evaluated various second-line treatments. NICE, UK concluded that ramucirumab was not cost effective (versus BSC) and that ramucirumab + paclitaxel was not cost effective versus various SoC chemotherapies (irinotecan, docetaxel, paclitaxel and FOLFIRI) or BSC [43].
Lam et al. evaluated ramucirumab, paclitaxel, ramucirumab + paclitaxel, irinotecan, docetaxel and BSC within one multi-technology evaluation (from a US third-party payer perspective). Irinotecan was considered to be the only cost-effective intervention [50].
Saito et al. concluded that ramucirumab + paclitaxel was not cost effective versus paclitaxel from the Japanese health-payer perspective [51]. Also, from the Japanese health-payer perspective, Kimura et al. concluded that ramucirumab + paclitaxel was a less cost effective option than either paclitaxel or irinotecan [53]. Li et al. concluded that ramucirumab + paclitaxel was cost effective (versus placebo + paclitaxel) in 48.3% and 50.9% of probabilistic-analysis iterations at willingness-to-pay thresholds estimated for both mainland China (threshold of $26,022 per QALY) and Beijing city (threshold of $56,263 per QALY) respectively. The thresholds were informed by per capita gross domestic product which differ between mainland China and Beijing city [54].
Zhou et al. [52] concluded that second-line chemotherapy was cost effective, versus BSC, in adults (70 years and over) with advanced or metastatic gastric cancer or advanced gastro-oesophageal junction adenocarcinoma from the health-payer perspective in China.
3.4.1.3 Biomarker-Guided Treatment (n = 2)
Lauren et al. evaluated the cost effectiveness of biomarker-guided, second-line treatment in adults with unresectable recurrent or metastatic gastric cancer. The target population included 40% who expressed PD-L1 CPS > 1% and 10% who had a positive MSI-H/dMMR status. Eight treatment strategies were compared in a multi-technology assessment. Four involved treatment irrespective of biomarker status (pembrolizumab, ramucirumab, ramucirumab + paclitaxel or BSC). In the remaining four, patients with positive biomarker status (PD-L1 (CPS > 1% or MSI-H/dMMR) received pembrolizumab, and those with microsatellite stability (MSS) or PD-L1 negative status received either paclitaxel or ramucirumab + paclitaxel. The authors concluded that biomarker-based treatments improved survival but were not cost-effective at current prices from US payer perspective. The only cost-effective strategy was paclitaxel treatment when given to all patients regardless of biomarker status [55].
NICE, UK evaluated the cost effectiveness of entrectinib (versus SoC) for the treatment of solid tumours that express NTRK gene fusion (regardless of tumour site). The NICE Expert Review Group noted that the modelled population in the manufacturer’s submission was not generalisable to the NHS clinical practice. Reported ICERs were above the standard payer threshold and therefore entrectinib was not considered to be cost-effective. However, entrectinib was accepted for use at a confidential discounted price, with conditions, under the Cancer Drugs Fund [58].
3.4.1.4 Systemic Chemotherapy Followed By Cytoreductive Surgery and Intraperitoneal Chemotherapy (n = 1)
Hultman et al. evaluated systemic chemotherapy followed by cytoreductive surgery and intraperitoneal chemotherapy, versus systemic chemotherapy only, in patients with peritoneal carcinomatosis from gastric cancer. The intervention was associated with improved OS and higher costs (in the treatment and post-treatment phases). The main cost drivers were neoadjuvant chemotherapy, surgical procedure and intensive care during the treatment phase. The intervention was not cost effective [60].
3.4.1.5 Stages of Gastric Cancer
Powel et al. [61] investigated the costs and outcomes associated with stage-directed treatment with curative intent (defined as surgery with a selective approach taken to the use of neoadjuvant chemotherapy) and treatment with non-curative intent (BSC) in individuals with gastric adenocarcinoma. BSC included therapeutic endoscopy, single fraction palliative radiotherapy for symptomatic anaemia and palliative chemotherapy for patients with appropriate performance status, as well as no treatment. The authors concluded that, across stages 1 to 4, treatment with curative intent improved overall survival and was cost effective at UK nationally accepted willingness-to-pay thresholds. Reported ICERs increased in line with increasing disease stage from stage 1 ($11,569 per QALY) to stage 4 ($35,628 per QALY).
3.4.2 Cost-of-Illness Studies (n = 4)
From a retrospective analysis of Medicare data, Karve et al. concluded that the mean per-patient cost of treatment of gastric cancer over the entire follow-up period from first-observed, gastric-cancer diagnosis was $88,749 (SD ± $70,966). Additional cancer-directed treatment, after the first-line of chemotherapy, was associated with a further mean per-patient cost of $31,605 and a longer median survival (272 days versus 72 days) [64]. In a retrospective observational study in Mexico, the mean per-patient cost was $1498 per month for first-line treatment and $1451 per month for second-line treatment [65]. In Colombia, based on data for 145 patients, it was concluded that the mean per-patient cost for second-line chemotherapy was $25,856 [66]. Haga et al. [63] conducted a retrospective analysis using data from government office statistics in Japan. They calculated direct, morbidity and mortality costs, estimating the total economic costs for gastric cancer, in Japan, for years 1996, 2002 and 2008 to be $13.2 billion, $12.6 billion and $11.4 billion, respectively. Additionally, they predicted costs for 2014 and 2020 to be $12.1 billion and $12.5 billion, respectively. Fixed-model analysis predicated a 9.8% increase from 2008 to 2020; mixed-model analyses predicted a continuous decrease in total costs.
3.5 Reporting and Quality Assessment
Reporting completeness was assessed using the 28-item CHEERS Statement Checklist [68]. Cost-of-illness studies are not eligible for assessment under CHEERS; these (n = 4) were excluded. Also, evaluation reports from HTA agencies frequently include redacted sections (e.g. confidential discounts and associated ICERs); thus, these (n = 5) were excluded.
The assessment is described in Fig. 2; the y axis describes the 28 items considered. The x axis describes the number of studies that comply (green) or do not comply (red) with each item. As illustrated, no studies were compliant with all 28 items. Amongst all studies, compliance with certain items was high. For example, the study title, the background and objective of the study, along with the comparators under consideration, were adequately described in all ten. Health outcomes chosen were adequately described in nine. Amongst all studies, compliance with certain items was low. For example, across all studies, the health-economics analysis plan, engagement with stakeholders, the measurement and valuation of preference-based outcomes and the characterisation of heterogeneity and uncertainty were, in the main, poorly described.
The y axis describes the CHEERS statement 28 items under consideration. The x axis describes the number of studies that comply (green) or do not comply (red) with each item
An assessment of methodological quality was conducted utilising the 23-item Philips checklist [35]. Cost-of-illness studies are not eligible for appraisal under Philips (n = 4); Evaluation Reports from HTA agencies (n = 5) were excluded given their reporting limitations. One cost-effectiveness study was excluded, as no model was described [60]. Nine studies were eligible for assessment.
In Fig. 3, the y axis describes the 23 items under consideration. The x axis describes the number of studies that comply (green) or do not comply (red) with each item or where reporting is unclear (amber).
As illustrated, no studies were compliant with all 23 items. Amongst all studies, compliance with certain items was high. For example, the objective, perspective and rationale for structure of the model were adequately described in eight studies. The model type, time horizon and disease pathways were adequately described in seven. Across all studies, compliance with certain items was low; data modelling methodology, baseline data, treatment effects, assessment of uncertainty and structural uncertainty were, in the main, poorly described.
The y axis describes the Philips statement 23 items under consideration. The x axis describes the number of studies that comply (green) or do not comply (red) or where reporting is unclear (amber) with each item
4 Discussion
This SLR identified 19 economic evaluations (published from inception to 2022 inclusive) on treatments for the second- or later-line treatment of advanced and metastatic gastric cancer. Fifteen studies investigated cost effectiveness (CEAs and CUAs), and four were cost-of-illness studies.
In 17 studies, the target population comprised individuals regardless of biomarker status; in 2, the intervention under consideration was investigated in a target population with known biomarker status. The source of funding was declared in 16; 10 were independently funded, and 6 had received pharmaceutical sponsorship. The health-payer perspective was taken in the majority of studies. Discount rates were reported for costs and outcomes in nine; rates varied from 2 to 3.5%. The time horizon across studies varied from 3 years to lifetime. Approaches taken to source efficacy data, to extrapolate survival data and to capture costs varied across studies.
The majority of cost-effectiveness studies used conventional, three health-state cancer models. However, there were key differences in structural assumptions underpinning the models, including the model type, the modelled time horizon and cycle length and the application of discounting, also, in the estimation of resource use, costs and utilities. These differences make it challenging to directly compare the cost-effectiveness outputs across studies. However, in the main, the interventions under consideration were concluded to be more effective and more costly than the comparator(s) considered. Generally, they were deemed not to be cost effective according to the applied decision rules. This presents challenges to affordability and sustainability of healthcare provision.
The same interventions were evaluated across a number of cost-effectiveness studies. There were large variations seen, across these studies, in the ICERs, the predicted costs, and the QALYs. For example, the ICERs, reported for trifluridine–tipiracil versus BSC, across studies ranged from $39,442/QALY to $1,115,565/QALY; cost-effectiveness conclusions, across these studies, were not consistent. The associated variations in total costs seen is likely explained by different healthcare resource use and costs considered (including the consideration given to subsequent treatments) and the use of confidential price discounts by some. Also, likely to be key are the differing approaches taken to modelling, time horizons and applied discount rates. Variations in QALY gains are at least partially explained by the use of utility values derived by different methods, differing time horizons and alternative approaches to survival extrapolations. Taken together, these variations highlight the sensitivity of cost-effectiveness results to modelling assumptions and input parameters and demonstrate the need for extensive sensitivity analysis as part of a robust economic analysis.
In two studies, the cost-effectiveness of biomarker-guided treatment was evaluated; one of these evaluations pertained to tumours regardless of site. Both evaluations indicated that the although the use of biomarker-guided treatments might improve outcomes, the associated high costs were not aligned with cost effectiveness according to the applied decision rules.
A number of interventions under consideration included surgery. It is not possible to directly compare the costs and outcomes across these studies given the differing target populations, the disparate interventions and comparators considered.
None of the cost-effectiveness studies were found to have perfect compliance with CHEERS. The CHEERS statement checklist is an assessment of reporting quality, not methodological quality; failure to follow all the CHEERS requirements does not indicate that the study is of poor methodological quality. The poor reporting, however, did make it difficult to assess the methodological quality, of the cost-effectiveness studies, using the Philips checklist. This methodological quality assessment did highlight key deficiencies across all studies. For example, all of the studies failed to meet the standards required with respect to the key criteria including the modelling approaches taken and the assessment of uncertainty.
Further, we note that not all relevant comparators (as stated in the ESMO [4] and NCCN [11] guidelines) were considered across the located studies. For example, dostarlimab–gxlyg is recommended in the NCCN guidelines for the treatment of tumours that express MSI-H/dMMR. Also, trastuzumab–deruxtecan is a second-line treatment option, recommended in the ESMO and NCCN guidelines, for the treatment of individuals with HER2 overexpression-positive adenocarcinoma who had previously received trastuzumab-based therapy. Neither of these interventions were considered in any of the located studies.
This SLR identified four cost-of-illness studies. These all differed with regards to the target populations considered, the sources of resource-utilisation and cost data used, the approaches taken to estimate and predict costs, the perspectives taken and the time horizons considered. Thus, it is not possible to directly compare the estimated costs of care across these studies.
There are a number of limitations to this work. Our SLR only evaluated economic evaluations that consisted of systemic treatments recommended by ESMO [4] and NCCN [11] guidelines. Treatments outside of these guidelines were, thus, not considered. Also, the SLR was restricted to English language and, necessarily, to only those publications with full text. A notable limitation is that only second- and later-line treatments were included. Inclusion of first-line and early-stage treatments would have provided a broader overview of the treatment pathway of gastric cancer. However, to gain a comprehensive insight into the cost-effectiveness of treatment strategies over the entire disease trajectory, whole disease modelling, as discussed by Tappenden et al. [69] would be valuable. Such models simulate the whole disease and treatment pathway within a single model, capturing relevant impacts of upstream and downstream technologies elsewhere in the pathway upon the intervention under consideration. In the context of the increasing complexity of the treatment pathway of gastric cancer, such an approach would allow decision makers to make more informed decisions. No such models were identified in this SLR.
5 Conclusions
To our knowledge, this is the first published SLR that has qualitatively synthesised economic evaluations for advanced and metastatic gastric cancer. Despite differences in model structures and assumptions, the conclusions of most of the cost-effectiveness studies were consistent. In the main, the interventions under consideration were deemed not to be cost effective; this presents challenges to sustainability and affordability. In the context of a rapidly evolving treatment landscape for patients with advanced and metastatic gastric cancer, we highlight a requirement for a cost-effectiveness evaluation that considers all relevant comparators and is compliant with reporting-completeness, methodological-quality requirements. By addressing the methodological gaps identified here, future healthcare decision-making would be better informed.
Data availability
All data supporting the findings of this study are available within the paper and its supplementary information.
References
Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–48.
National Cancer Institute. Stomach cancer survival rates and prognosis. United States 2023. https://www.cancer.gov/types/stomach/survival#:~:text=Stomach%20Cancer%20Statistics&text=The%205%2Dyear%20relative%20survival%20rates%20for%20different%20stages%20of,nearby%20lymph%20nodes%20or%20organs. Accessed 21 Apr 2023.
Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up☆. Ann Oncol. 2022;33(10):1005–20.
U.S. Food & drug administration. FDA grants accelerated approval to pembrolizumab for HER2-positive gastric cancer 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-her2-positive-gastric-cancer?utm_medium=email&utm_source=govdelivery. Accessed 27 Apr 2023.
Janjigian YY, Kawazoe A, Bai Y, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402(10418):2197–208.
Rha SY, Oh D-Y, Yañez P, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(11):1181–95.
Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31(35):4438–44.
Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8(8):CD004064.
Rogers JE, Ajani JA. Recent advances in the management of gastric adenocarcinoma patients. Fac Rev. 2023;12:2.
Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(2):167–92.
Shitara K, Bang Y-J, Iwasa S, Sugimoto N, Ryu M-H, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–30.
Astellas. U.S. FDA acknowledges Astellas' resubmission of biologics license application for Zolbetuximab and sets new action date. [Press release]. https://www.astellas.com/en/news/29186. Accessed 31 May 2024.
Wainberg ZA, Enzinger PC, Kang Y-K, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022;23(11):1430–40.
Ji L, Liu Z, Zhou B, Cai Y, An F, Wang L, et al. Community-based pilot study of a screening program for gastric cancer in a Chinese population. Cancer Prev Res (Phila). 2020;13(1):73–82.
Kumar V, Soni P, Garg M, Kamholz S, Chandra AB. Emerging therapies in the management of advanced-stage gastric cancer. Front Pharmacol. 2018;9:404.
Lee J, Lim T, Uhm JE, Park KW, Park SH, Lee SC, et al. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18(5):886–91.
Cotes Sanchís A, Gallego J, Hernandez R, Arrazubi V, Custodio A, Cano JM, et al. Second-line treatment in advanced gastric cancer: data from the Spanish AGAMENON registry. PLoS One. 2020;15(7): e0235848.
Yildirim S, Özveren A. Second-line chemotherapy in gastric cancer: a retrospective study. Indian J Surg Oncol. 2023;14(2):423–7.
Lloyd-Williams H, Hughes DA. A systematic review of economic evaluations of advanced therapy medicinal products. Br J Clin Pharmacol. 2021;87(6):2428–43.
Catenacci DV, Chao J, Muro K, Al-Batran SE, Klempner SJ, Wainberg ZA, et al. Toward a treatment sequencing strategy: a systematic review of treatment regimens in advanced gastric cancer/gastroesophageal junction adenocarcinoma. Oncologist. 2021;26(10):e1704–29.
Huang M, Li J, Yu X, Xu Q, Zhang X, Dai X, et al. Comparison of efficacy and safety of third-line treatments for advanced gastric cancer: a systematic review with bayesian network meta-analysis. Front Oncol. 2021;11: 734323.
Zheng Y, Zhu XQ, Ren XG. Third-line chemotherapy in advanced gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(24): e6884.
Kim JH, Zang DY, Jang HJ, Kim HS. A Bayesian network meta-analysis on systemic therapy for previously treated gastric cancer. Crit Rev Oncol Hematol. 2021;167: 103505.
Abderhalden LA, Wu P, Amonkar MM, Lang BM, Shah S, Jin F, et al. Clinical outcomes for previously treated patients with advanced gastric or gastroesophageal junction cancer: a systematic literature review and meta-analysis. J Gastrointest Cancer. 2023;54(4):1031–45.
Sharma S, Carey N, McConnell D, McCullagh L. Systematic Review of economic evaluations of systemic treatments for advanced and metastatic gastric cancer. PROSPERO CRD42023405951 2023. [Protocol]. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023405951. Accessed 19 Jan 2023.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Mandrik OL, Severens JH, Bardach A, et al. Critical appraisal of systematic reviews with costs and cost-effectiveness outcomes: an ISPOR good practices task force report. Value Health. 2021;24(4):463–72.
Health Information and Quality Authority (HIQA). Guidelines for the Retrieval and Interpretation of Economic Evaluations of Health Technologies in Ireland Ireland. 2014. https://www.hiqa.ie/sites/default/files/2017-01/Guidelines-Retrieval-and-Interpretation-of-Econ-Lit.pdf. Accessed 14 Apr 2023.
Amir-Behghadami M, Janati A. Population, intervention, comparison, outcomes and study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. 2020;37(6):387.
Cheng J, Cai M, Shuai X, et al. First-line systemic therapy for advanced gastric cancer: a systematic review and network meta-analysis. Ther Adv Med Oncol. 2019;11:1758835919877726.
Scottish Intercollegiate Guidelines Network. Search filters economic studies. Edinburgh. 2022.
Gorry C, McCullagh L, Barry M. Economic evaluation of systemic treatments for advanced melanoma: a systematic review. Value Health. 2020;23(1):52–60.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Pharmacoeconomics. 2022;40(6):601–9.
Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics. 2006;24(4):355–71.
Organisation for Economic Co-operation and Development. Purchasing power parities (PPP). 2023. https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm. Accessed 06 July 2023.
Canada Drug and Health Technology Agency (CADTH). pan-Canadian Oncology Drug Review Final Economic Guidance Report: Trifluridine-Tipiracil (Lonsurf) for Gastric Cancer. 2020. https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10197TrifluridineTipiracilGC_inEGR_NOREDACT-ABBREV_EC24Mar2020_final.pdf. Accessed 16 May 2023.
Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–48.
Zhou K, Zhou J, Zhang M, Liao W, Li Q. Cost-effectiveness of trifluridine/tipiracil (TAS102) for heavily pretreated metastatic gastric cancer. Clin Transl Oncol. 2020;22(3):337–43.
Scottish Medicines Consortium. SMC2329: trifluridine/tipiracil 15mg/6.14mg and 20mg/8.19mg film-coated tablets (Lonsurf®) 2021. [Medicines advice]. https://www.scottishmedicines.org.uk/media/6026/trifluridinetipiracil-lonsurf-final-may-2021-for-website.pdf. Accessed 17 May 2023.
National Institute for Health and Care Excellence (NICE). Trifluridine–tipiracil for treating metastatic gastric cancer or gastrooesophageal junction adenocarcinoma after 2 or more treatments (TA852) 2022. [Technology appraisal guidance]. www.nice.org.uk/guidance/ta852. Accessed 18 May 2023.
Gourzoulidis G, Koulentaki M, Koumarianou A, et al. Cost-effectiveness of trifluridine/tipiracil as a third-line treatment of metastatic gastric cancer, including adenocarcinoma of the gastrohesophageal junction, among patients previously treated in Greece. Expert Rev Pharmacoecon Outcomes Res. 2022;22(2):259–69.
National Institute for Health and Care Excellence (NICE). Ramucirumab for treating advanced gastric cancer or gastro–oesophageal junction adenocarcinoma previously treated with chemotherapy (TA378) 2016 [Technology appraisal guidance]. www.nice.org.uk/guidance/ta378. Accessed 24 May 2023.
Sym SJ, Hong J, Park J, Cho EK, Lee JH, Park YH, et al. A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemother Pharmacol. 2013;71:481–8.
Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15(1):78–86.
Wilke H, Muro K, Van Cutsem E, Oh S-C, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35.
Roy A, Park S, Cunningham D, Kang Y-K, Chao Y, Chen L-T, et al. A randomized phase II study of PEP02 (MM-398), irinotecan or docetaxel as a second-line therapy in patients with locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma. Ann Oncol. 2013;24(6):1567–73.
Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer—a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011;47(15):2306–14.
Fuchs C, Tomasek J, Yong C, Dumitru F, Passalacqua R, Goswami C, et al. REGARD Trial Investigators Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–9.
Lam SW, Wai M, Lau JE, et al. Cost-effectiveness analysis of second-line chemotherapy agents for advanced gastric cancer. Pharmacother J Hum Pharmacol Drug Ther. 2017;37(1):94–103.
Saito S, Muneoka Y, Ishikawa T, Akazawa K. Cost-effectiveness of paclitaxel + ramucirumab combination therapy for advanced gastric cancer progressing after first-line chemotherapy in japan. Clin Ther. 2017;39(12):2380–8.
Zhou K, Wen F, Zhang P, et al. Efficacy and cost-effectiveness of second-line chemotherapy in elderly patients with advanced gastric cancer. Clin Transl Oncol. 2017;19(9):1117–24.
Kimura M, Usami E, Teramachi H, Yoshimura T. Cost-effectiveness and safety of ramucirumab plus paclitaxel chemotherapy in the treatment of advanced and recurrent gastric cancer. J Oncol Pharm Pract. 2018;24(6):403–11.
Li S, Peng L, Tan C, et al. Cost-Effectiveness of ramucirumab plus paclitaxel as a second-line therapy for advanced gastric or gastro-oesophageal cancer in China. PLoS One. 2020;15(5): e0232240.
Lauren B, Ostvar S, Silver E, et al. Cost-effectiveness analysis of biomarker-guided treatment for metastatic gastric cancer in the second-line setting. J Oncol. 2020;2020:2198960.
Fuchs CS, Doi T, Jang RW-J, Muro K, Satoh T, Machado M, et al. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. Am Soc Clin Oncol. 2017;35:4003.
Shitara K, Özgüroğlu M, Bang Y-J, Di Bartolomeo M, Mandalà M, Ryu M-H, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33.
National Institute for Health and Care Excellence (NICE). Entrectinib for treating NTRK fusion-positive solid tumours (ID1512). 2020. [Technology appraisal guidance]. https://www.nice.org.uk/guidance/ta644. Accessed 01 June 2023.
Demetri G, Paz-Ares L, Farago A, Liu S, Chawla S, Tosi D, et al. Efficacy and safety of entrectinib in patients with NTRK fusion-positive (NTRK-fp) tumors: pooled analysis of STARTRK-2, STARTRK-1 and ALKA-372-001. Ann Oncol. 2018;29:viii713.
Hultman B, Lundkvist J, Glimelius B, Nygren P, Mahteme H. Costs and clinical outcome of neoadjuvant systemic chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from gastric cancer. Acta Oncol (Stockholm, Sweden). 2012;51(1):112–21.
Powell AG, Wheat JR, Eley C, et al. Economic cost-utility analysis of stage-directed gastric cancer treatment. BJS Open. 2021;5(6):zrab129.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Haga K, Matsumoto K, Kitazawa T, et al. Cost of illness of the stomach cancer in Japan—a time trend and future projections. BMC Health Serv Res. 2013;13:283.
Karve S, Lorenzo M, Liepa AM, et al. Treatment patterns, costs, and survival among medicare-enrolled elderly patients diagnosed with advanced stage gastric cancer: analysis of a linked population-based cancer registry and administrative claims database. J Gastric Cancer. 2015;15(2):87–104.
Quintana M, Toriz JA, Novick D, et al. Resources and costs associated with the treatment of advanced and metastatic gastric cancer in the Mexican public sector: a patient chart review. Pharmacoecon Open. 2018;2(2):191–201.
Novick D, Leonardi F, Lee Kay Pen D, et al. Retrospective analysis of patients with advanced or metastatic gastric cancer in Colombia. J Med Econ. 2019;22(9):891–900.
Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials-extrapolation with patient-level data. Report by the Decision Support Unit. 2011. https://www.ncbi.nlm.nih.gov/books/NBK395885/pdf/Bookshelf_NBK395885.pdf. Accessed 03 Aug 2023.
Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BJOG. 2022;129(3):336–44.
Tappenden P, Chilcott J, Brennan A, Squires H, Stevenson M. Whole disease modeling to inform resource allocation decisions in cancer: a methodological framework. Value Health. 2012;15(8):1127–36.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article. Laura McCullagh is an Editorial Board Member of PharmacoEconomics. She was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Funding
Open Access funding provided by the IReL Consortium. This project is funded by Enterprise Ireland, through a Disruptive Technologies Innovation Fund (DTIF) Award (grant code: DT 2020 0265A).
Author contributions
All authors contributed to the study concept and design: N.C., D.M., L.M., S.S., J.O., M.L. The first draft of the manuscript was written by S.S. and L.M., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualisation: L.M., N.C., D.M., J.O. and M.L. Methodology: L.M., N.C., D.M. and S.S. Funding acquisition: J.O. and M.L. Supervision: L.M., N.C. and D.M.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sharma, S., Carey, N., McConnell, D. et al. Systematic Review of Economic Evaluations of Systemic Treatments for Advanced and Metastatic Gastric Cancer. PharmacoEconomics (2024). https://doi.org/10.1007/s40273-024-01413-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s40273-024-01413-8