Abstract
Background and Objective
The disease burden of sickle cell anemia (SCA) in sub-Saharan African (SSA) countries is substantial, with many children dying without an established diagnosis or proper treatment. The global burden of SCA is increasing each year, making therapeutic intervention a high priority. Hydroxyurea is the only disease-modifying therapy with proven feasibility and efficacy suitable for SSA; however, no one has quantified the health economic implications of its use. Therefore, from the perspective of the health care provider, we estimated the incremental cost-effectiveness of hydroxyurea as a fixed-dose regimen or maximum tolerated dose (MTD) regimen, versus SCA care without hydroxyurea.
Methods
We estimated the cost of providing outpatient treatment at a pediatric sickle cell clinic in Kampala, Uganda. These estimates were used in a discrete-event simulation model to project mean costs (2021 US$), disability-adjusted life years (DALYs), and consumption of blood products per patient (450 mL units), for patients between 9 months and 18 years of age. We calculated cost-effectiveness as the ratio of incremental costs over incremental DALYs averted, discounted at 3% annually. To test the robustness of our findings, and the impact of uncertainty, we conducted probabilistic and one-way sensitivity analyses, scenario analysis, and price threshold analyses.
Results
Hydroxyurea treatment averted an expected 1.37 DALYs and saved US$ 191 per patient if administered at the MTD, compared with SCA care without hydroxyurea. In comparison, hydroxyurea at a fixed dose averted 0.80 DALYs per patient at an incremental cost of US$ 2. The MTD strategy saved 11.2 (95% CI 11.1–11.4) units of blood per patient, compared with 9.1 (95% CI 9.0–9.2) units of blood per patient at the fixed-dose alternative.
Conclusions
Hydroxyurea at MTD is likely to improve quality of life and reduce the consumption of blood products for children with SCA living in Uganda. Compared with a fixed dose regimen, treatment dosing at MTD is likely to be a cost-effective treatment for SCA, using realistic ranges of hydroxyurea costs that are relevant across SSA. Compared with no use of the drug, hydroxyurea could lead to substantial net savings per patient, while reducing the disease morbidity and mortality and increasing quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hydroxyurea—the only disease-modifying drug that has proven safe, efficacious, and feasible for patients with sickle cell anemia in Uganda and similar settings—has a high probability of being cost-effective when considered from a health care provider’s perspective. |
Using hydroxyurea to manage sickle cell anemia can limit the substantial consumption of blood products among patients. |
Despite more investments in monitoring and drug procurements, hydroxyurea may be most cost-effective if provided at the maximum tolerated dose, compared with a fixed lower dose. |
1 Introduction
Sickle cell anemia (SCA) is one of the greatest public health concerns of all hemoglobinopathies [1]. Patients with SCA are homozygous for the hemoglobin (Hb) S mutation, thus producing the abnormal Hb S molecule rather than normal Hb A. During deoxygenation, HbS polymerizes, which damages erythrocytes and leads to hemolytic anemia, acute vaso-occlusive events, high susceptibility to infections, chronic organ damage, and early mortality [1]. Among the most devastating manifestations in childhood is a high rate of stroke, which increases mortality and long-term morbidity, especially in the absence of stroke-prevention measures [1].
Management of SCA has drastically improved in high-income settings such as the USA and various European countries [1, 2], with life expectancy far exceeding that of patients in sub-Saharan Africa (SSA), where, globally, the majority of persons with SCA reside [1]. Here, an estimated 43% of patients die before their tenth birthday [3], as screening and access to comprehensive care are limited [1]. In Uganda, the fundamental care recommended for all patients with SCA currently includes pneumococcal vaccination, penicillin prophylaxis, malaria prophylaxis, and daily folic acid, as well as regular follow-up by a clinician [4]. Although these recommendations correspond with evidence, the consistency and quality of care are generally limited in low-income countries in SSA [1]. Consequently, and in accordance with the World Health Organization’s (WHO) strategy for sickle cell disease in Africa, the situation requires increased efforts to provide comprehensive care that is feasible to implement and accessible for patients [5, 6].
Hydroxyurea has been identified as a potentially transformative therapy for SCA in SSA [7,8,9]. Originally approved in 1967 as an antineoplastic drug, hydroxyurea has notably been used to treat chronic myeloid leukemia and other myeloproliferative disorders [10]. Its approval for treating SCA in the USA followed in 1998 [11]. Hydroxyurea has since become standard of care for SCA in high-income settings [12] and has recently been established as safe, effective, and clinically feasible for use in SSA [7,8,9].
The double-blinded, placebo-controlled NOHARM trial demonstrated the safety and efficacy of hydroxyurea administered at a fixed dose of 20 ± 2.5 mg/kg per day for young children in Uganda [7]. The findings were consistent throughout a follow-up study, which also demonstrated a significant reduction in stroke risk measured as transcranial Doppler (TCD) velocities [13]. Finally, a third trial reported increased clinical benefits when hydroxyurea was escalated to the maximum tolerated dose (MTD) of about 30 mg/kg, which significantly reduced hospitalizations and transfusions by more than half compared with the fixed-dose strategy, without compromising safety [9]. The safety of the MTD strategy was confirmed by the open-label hydroxyurea REACH trial, which also achieved significant reductions in mortality rates across four SSA countries [8].
Hydroxyurea has been listed as an essential medicine by the WHO since 2011[14] and several African countries now recommend hydroxyurea in the management of SCA. Despite this important development for public health in the region, hydroxyurea is not consistently available for all SCA patients through public health services, such as the Mulago Hospital Sickle Cell Clinic (MHSCC) in Kampala, Uganda. Due to the high number of patients, there is currently only enough medicines to supply patients with a portion of their prescribed doses. Thus, patients who need the medicine must buy the drug at retail pharmacies to ensure continuous supplies. Since 84% of patient caregivers at MHSCC have an income of under US$ 1.9 per day [15], hydroxyurea is largely unavailable for most patients unless incorporated into standard benefit packages. However, economic evidence is absent regarding whether hydroxyurea is cost-effective and should be prioritized over alternative interventions to be funded by Uganda’s limited health budget.
As an off-patent drug [16], hydroxyurea has been demonstrated to be cost-effective in the USA [17, 18], but this has little validity for a low-income African setting, and region-specific economic evidence is therefore needed [9]. We set out to estimate the cost-effectiveness of hydroxyurea provided for children with SCA that receive public healthcare services in Uganda. We assessed the incremental cost, incremental effectiveness, and incremental cost-effectiveness of both SCA care with hydroxyurea at the MTD and fixed dose, compared with SCA care without hydroxyurea. Additionally, because blood transfusions have limited availability [19] but are crucial for treatment of these patients [1], we modeled how the treatment alternatives influence the expected long-term consumption of this vital resource.
2 Methods
2.1 Study Settings and Perspective
We performed a model-based health economic evaluation from the perspective of the health care provider in Kampala, Uganda. We chose this perspective because an analysis based on a societal perspective would require data that are limited in the current setting, and we did not have the opportunity to collect such data at this time. However, estimates based on the health care provider’s perspective is valid information for decision-makers when prioritizing scarce health sector resources to maximize health for patients [20]. We considered health care services in the same setting as the NOHARM-trials, namely the MHSCC—a specialized clinic for children with SCA [7, 9, 13]. We also assumed that all treatment costs, including the cost of hydroxyurea, were born by the health care provider. We reported our findings according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist, which is provided in the electronic supplementary material [21], alongside more details on the study settings.
2.2 Interventions Compared
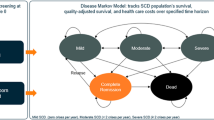
We compared two hydroxyurea arms—MTD and fixed-dose—and a comparator arm without hydroxyurea. We included these two hydroxyurea strategies since they have been compared in clinical trials in the study setting [9]. We assumed that all treatment groups received basic care recommended by MHSCC (Mulago Hospital Sickle Cell Clinic, Kampala, Uganda, unpublished guidelines) and national guidelines [4]. This included prophylactic penicillin, folic acid, and anti-malaria medication (Supplementary Table S6), as well as quarterly (Supplementary Table S5) outpatient follow-up for patient not on hydroxyurea, and a more frequent monitoring regime for patients on hydroxyurea, described in supplementary Table S5. For clinical events, we assumed the same treatment path and standard of care for all groups (Fig. 1 and Supplementary Fig. S1); however, the rates of hospitalizations and event-related blood transfusions (Table 1) were subject to treatment effects (Supplementary Table S3).
Overview of the discrete event simulation model. All arms of the model—sickle cell anemia (SCA) treatment without hydroxyurea, fixed-dose hydroxyurea, and hydroxyurea escalated to the maximum tolerated dose—have the same structure, as illustrated. Boxes with solid lines illustrate health states, while boxes with dashed lines illustrate events. Black arrows indicate an event within a health state, green arrows indicate assignment of a (new) health state after an event, and red arrows indicate death. In accordance with Mulago Hospital guidelines (unpublished), we assume that the clinic provides a secondary stroke prophylaxis with blood transfusions every 3–4 weeks, terminated after 4–6 months for patients that receive hydroxyurea. The model assumes probabilities of surviving three recurrent strokes, each time with an increase in baseline disutility, and that a fourth stroke always will be deadly. OPT outpatient treatment
For patients who suffered a stroke, we assigned chronic blood transfusions received every 3–4 weeks (Table 1), as recommended for secondary stroke prevention at the MHSCC (Mulago Hospital Sickle Cell Clinic, Kampala, Uganda, unpublished guidelines). MHSCC guidelines allow for discontinuation of chronic blood transfusion after 6 months if patients receive hydroxyurea, which we accounted for. To not overestimate the use of blood products, we assumed that 80% (\(\pm 20\%\)) of patients adhered to their stroke prophylaxis and varied this between 50% and 100% in deterministic sensitivity analyses.
2.3 Model Overview
We constructed a discrete-event simulation (DES) model using TreeAge Pro 2021. DES is a flexible technique to simulate sequences of health-related events for individual patients [22]. For each arm, the model predicts costs, disability-adjusted life years (DALYs), and blood consumption. We assigned a most likely starting age of 2.2 years [7], with minimum and maximum starting age of 9 months [12] and 4 years [7], respectively (Table 1). Due to limited available data on disease progression, treatment costs, and intervention effectiveness for adults with SCA in SSA—since most patients have not survived childhood—we ended our base case simulation at age 18 years [23]. However, to account for potential longer-term impacts, we conducted a limited scenario analysis with an extended time horizon. This approach aims to provide a comprehensive understanding of the disease burden, given the current state of knowledge.
Briefly, the model captures life-threatening and non-life-threatening clinical events (Fig. 1). These events may lead to a clinic visit, hospitalization, and/or an event-related blood transfusion (i.e., transfusions other than prophylaxis for secondary stroke). We assumed that life-threatening events always prompt admission to in-patient care. Apart from recurring clinical events, the model accounts for both primary and secondary strokes (Fig. 1); whereas each stroke prompts the transition to a worse health state and a chronic or 6 month long transfusion program. Finally, we assumed that patients may die from strokes and other SCA or non-SCA-related causes (Fig. 1).
2.4 Statistical Inputs
We derived baseline event rates primarily from region-specific data that we extrapolated across age groups with data from a high-income setting (Supplementary Tables S1–S2). We incorporated the efficacy of hydroxyurea mainly as rate ratios (Supplementary Table S3), except for strokes, where we used a stepwise approach. The steps cover the following presumptions: First, the main risk factor for strokes in children with SCA is the narrowing of large intracranial arteries [35]. This elevates the blood flow velocities of the affected arteries, which is detectable by TCD ultrasonography [35]. Second, the TCD-detected velocities can be stratified into normal (< 170 cm/s), conditional (170–199 cm/s), or abnormal levels (≥ 200 cm/s)—with conditional and abnormal velocities being associated with the highest risk of stroke [35]. Third, hydroxyurea normalizes TCD velocities and thus reduces the risk of stroke in children with SCA [13, 36]. Therefore, to predict stroke risk in each treatment arm, we combined data on the distribution of TCD categories in SSA [30] (Table 1) and the influence of hydroxyurea on the conversion of risk (Supplementary Table S3) [13, 36], with estimated stroke rates for each risk category (Supplementary Table S2). We provide further descriptions of baseline inputs and hydroxyurea treatment effects in the Supplementary Materials.
2.5 Costs
We collected cost data for outpatient services at the MHSCC in Kampala, Uganda, between November 2019 and January 2020 (Table 1, Supplementary Fig. S1, and Table S4), complemented with unit costs of in-patient care and centralized laboratory testing from the literature (Table 1 and Supplementary Table S5). With an ingredients approach [37], we estimated resources consumed in 2018 and 2019. We valued all local resources in Ugandan shillings, and present costs in 2021 US dollars, using the mean exchange rate for 2021 of 3587 UGX [38]. We provide a full description of the costing methodology in chapter 2 of the electronic supplementary material.
We determined the price of hydroxyurea with an international reference price of US$ 0.24 per 500 mg [27], and we assigned unit costs to MHSCC guidelines and study protocols [9] to establish monitoring costs (Table 1 and Supplementary Table S5). We based the cost of blood transfusions of US$ 0.18 per mL whole blood on estimates from the Uganda Blood Transfusion Services (UBTS) (personal communication with Dr. Dorothy Kyeyune-Byabazaire, Executive Director of the UBTS, October 2021); their estimates comprised the costs of administration, procurement, processing, and distribution of one unit of whole blood. We accounted for the cost of transfusions given during acute events and in secondary stroke prevention (Table 1 and Fig. 1).
2.6 Effectiveness Outcomes
We measured effectiveness as DALYs averted because this outcome is comparable across diseases [39]. The model estimated DALYs as the sum of years lived with disability (YLD) and years of life lost (YLL) [39]. In our base case analysis, YLL comprised years between the age at death and the age of 18 years. In sensitivity analyses, we considered deaths before ages 10 years, up until Uganda’s national life expectancy of 62.85 [40] years as premature, and thus YLL. YLD was estimated using disability weights (Table 1; Supplementary Tables S7–S8), and information about event durations (Table 1). We did not include age weighting [41]. We estimated mean units (450 mL per unit) of blood saved by the health care provider as a secondary outcome.
2.7 Discounting of Future Costs and Health Outcomes
We discounted DALYs and costs at an annual rate of 3%, as this has long been an established practice for cost-effectiveness analyses in global health [42]. Since this discount rate is based on guidelines for a high-income setting, and global consensus for discounting is non-existing, we included alternative discount rates (0–6%) for both costs and DALYs in one-way sensitivity analyses [43]. These alternatives would account for higher discount rates for costs that have been suggested in a low-income setting like Uganda [42]. We did not discount the consumption of blood units [28].
2.8 Cost-Effectiveness Analysis
We considered the cost-effectiveness of interventions by estimating and comparing their incremental cost-effectiveness ratios (ICERs) [37]. When comparing ICERs, we excluded dominated strategies—i.e., strategies that were more costly and less effective than their alternatives [37]. We compared the cost-effectiveness of undominated strategies to thresholds suggested by the WHO, where an ICER within one times the GDP per capita (US$ 858 in 2021) is considered highly cost-effective and an ICER between one and three times the GDP per capita is defined as cost-effective [44]. We did also consider a conservative range of WTP thresholds, from 17% to 22% of GDP per capita, suggested for Uganda by Ochalek et al. [45]. We used GraphPad Prism, version 9.2.0 (San Diego, California USA), to present our outcomes graphically.
2.9 Uncertainty and Sensitivity Analyses
We estimated our outcomes probabilistically in a microsimulation with two nested loops [46]. In the outer loop, model parameters were resampled 1000 times, with 10,000 patient-level iterations in the inner loop per sample. We approximated parameter distributions for the outer loop using the method of moments [46], applying uncertainty ranges from the literature. For parameters where empirical ranges were not available, we generally assumed ranges of ± 20% [46] or, with a few exceptions where wider ranges were considered appropriate (Table 1 and Supplementary Table S2).
We explored the sensitivity to individual parameters by one-way sensitivity analyses [46], determining range widths in the same way as in PSA. For the hydroxyurea drug price, we deemed ± 20% of the international reference price as appropriate—a range that would also cover unit costs for hydroxyurea indicated by the Ugandan Ministry of Health (MoH) [47], with and without adjustments for 10% shipping costs as recommended by Management Sciences for Health in their International Medical Products Price Guide [26]. For blood products, we explored the influence of a lower cost estimate from literature [48] and a ± 20% increase to the base case that the UBTS provided. Additionally, to identify what unit costs of hydroxyurea and blood products that would alter conclusions about cost-effectiveness, we also conducted unit cost threshold analyses.
In an exploratory scenario analysis, we extended the time horizon of the model to 62.85 years of age—the average life expectancy in Uganda [40]—to investigate the potential impact of longer-term disease progression and treatment costs on our results. We did not make any further assumptions about changes in parameters beyond the age of 18 years.
Throughout the study process, we validated the structure and outcomes of our model in collaboration with clinical experts within our group. This validation process involved rigorous assessment and refinement to ensure that the assumptions and calculations utilized in the model align with the clinical context. The technical properties of the model were validated by inspecting that changes in parameter values were reflected in the output according to theoretical expectations.
3 Results
3.1 Base Case Analysis
In the base case scenario, hydroxyurea administered at MTD averted more DALYs and was less costly than the two alternative interventions and was therefore the dominant strategy (Table 2). Compared with SCA care without hydroxyurea, MTD averted 1.37 DALYs while saving US$ 191 per patient. In comparison, fixed-dose treatment incurred US$ 2 extra cost per patient and averted 0.80 DALYs relative to SCA care with no hydroxyurea. Treatment with MTD averted an expected (95% CI) 11.2 (11.2–11.4) units of blood, while fixed-dose hydroxyurea averted 9.1 (9.0–9.2) units (Fig. 2 and Table 2). The mean time horizon amounted to 15.74 years.
Violin plot showing the frequency distribution of blood consumed in each treatment group over an average time horizon of 15.74 years—i.e., starting the simulation from a likely (minimum to maximum) age of 2.2 (0.75–3.99) years and stopping at an age of 18 years. The width of each plot is proportional to the probability density. Dashed lines represent the medians, and dotted lines represent quartiles. Median units of blood consumed (interquartile range) per patient was 14.3 (12.7–16.5) without hydroxyurea, 5.6 (5.1–6.1) with hydroxyurea at a fixed dose, and 3.5 (3.2–3.8) with hydroxyurea escalated to the maximum tolerated dose
3.2 Probabilistic Sensitivity Analysis
We illustrate the overall uncertainty in our estimates through an incremental cost-effectiveness scatter plot (Fig. 3). For all iterations, the cost-effectiveness pairs of both MTD and fixed dose were within a WTP threshold of one GDP per capita when compared individually with SCA care without hydroxyurea. In 77% of the samples, MTD was both more effective and less costly than no hydroxyurea, compared with 47% for fixed dose (Fig. 3). Conclusions about cost-effectiveness were robust to lower levels of WTP, and the MTD strategy had 95–98% probabilities of being cost-effective at conservative WTP thresholds of 17–22% of GDP, respectively (supplementary Fig. S2) [45].
Incremental cost-effectiveness scatter plots from 1000 samples that compared each hydroxyurea strategy (fixed dose and with escalation to the maximum tolerated dose) to the comparator strategy (sickle cell anemia care without hydroxyurea). Each sample plot represents the ratio of expected incremental US$ over disability-adjusted life years averted per patient. The observations are compared to a willingness to pay (WTP)-threshold equal to Uganda’s gross domestic product per capita [44]
3.3 One-Way Sensitivity Analyses
One-way sensitivity analyses show that the drug price of hydroxyurea was the most influential single parameter, but it did not have potential to increase the ICER of hydroxyurea at MTD, compared with SCA care without hydroxyurea, beyond a WTP threshold of GDP per capita per DALY averted (Fig. 4). The ICER was also sensitive to hydroxyurea monitoring costs, as well as our choice of benchmark age for premature death, but none of these parameters could reverse a conclusion about cost-effectiveness (Fig. 4 and Supplementary Fig. S4). Lastly, no realistic variation of any other parameter had the potential to push the ICER above a WTP threshold of one times GDP per capita per DALY averted.
Tornado diagram of the dominating strategy (hydroxyurea with escalation to maximum tolerated dose) versus the null intervention (sickle cell anemia care without hydroxyurea). The width of each bar represents the changes to the incremental cost-effectiveness ratio (ICER) caused by changes to the parameters listed along the y axis. We provide the uncertainty ranges in parentheses. All negative ICERs represent domination (i.e., superior health effect at a lower cost). EV expected value with base case assumptions, not including probabilistic sensitivity analysis. *Annual cost of monitoring with escalation relative to annual cost of monitoring with fixed-dose intervention, after initial year (drug-escalation period). **Adherence to chronic blood transfusions for patients in secondary stroke prevention. ***Outpatient visits for non-life-threatening events that do not require transfusion and/or hospitalization. TAMM time-averaged mean of the maximum velocities in both cerebral hemispheres
3.4 Price Threshold Analyses
The threshold analyses for drug price showed that hydroxyurea at the MTD was more cost-effective than no hydroxyurea if the cost of hydroxyurea did not exceed US$ 0.36 (Supplementary Table S9). For blood transfusions, the intervention became more cost-effective as the cost of blood increased (Fig. 4) and remained cost-effective at a one times GDP per capita per DALY averted WTP threshold for the entire range of unit price of blood. However, at WTP thresholds of 17% and 22% of the GDP per capita, hydroxyurea at the MTD was cost-effective with blood unit prices of minimum US$ 14 and US$ 4, respectively (supplementary Table S9).
3.5 Alternative Time-Horizon
In the alternative scenario analysis using Uganda’s national life expectancy of 62.85 years [49] as the time horizon, hydroxyurea at MTD remained the most effective intervention (Supplementary Table S10). However, the strategy had changed from being the least costly in the base case scenario with an 18 year time horizon to become the most costly, and consequently was no longer dominant (Supplementary Table S10). Fixed-dose hydroxyurea had a higher probability than MTD of being cost-effective below a US$ 304 threshold, while care without hydroxyurea had the highest probability of being cost-effective at a WTP between US$ 0 and US$ 223 (Supplementary Fig. S5 and Table S10).
4 Discussion
Using a DES model, we showed that providing hydroxyurea for patients that receive SCA care in a low-income SSA setting has a very high probability of being cost-effective. Simultaneously, hydroxyurea can substantially reduce the need for blood transfusions, which are a particularly scarce resource. We found that hydroxyurea given at the MTD was the preferred dosing strategy at all levels of WTP, because it saves resources while averting more DALYs compared with both alternatives.
This is the first cost-effectiveness analysis of hydroxyurea in a low-income setting in Africa; however, our findings are consistent with outcomes from wealthier countries. In the USA, increased monitoring costs induced by hydroxyurea were outweighed by reductions in acute care, particularly hospitalizations [17, 18]. In Uganda, since treatment costs are only a fraction of those in high-income countries, the costs for hydroxyurea and monitoring has higher influence (Fig. 4) [17, 18]. This is supported by evidence from Jamaica, where hydroxyurea was found to be a cost-effective although not a cost-saving means to prevent recurring strokes in children with SCA [50]. Here, annual monitoring costs per patient were more than 11 times higher for patients on hydroxyurea compared with the non-hydroxyurea group, which the authors acknowledged as excessive and likely to become lower as monitoring frequencies are reduced, compared with more recent monitoring protocols such as those we used (supplementary Table S5) [50].
If annual monitoring costs are higher with an MTD strategy than with a fixed-dose regimen, the probability of MTD being dominant will be lower (Supplementary Fig. S4). This seems unrealistic since dose escalation efforts are limited to the drug initiation phase (Supplementary Table S5). Nonetheless, decision-makers should consider exploring circumstances in which additional monitoring necessary for full-dose escalation might not be feasible, for example, in rural settings with high costs for transporting laboratory tests. In such situations, where achieving MTD is not feasible, a fixed-dose variant could be recommended as a cost-effective option, although some laboratory monitoring would still be required. At the same time, personnel costs are by far the highest expense in SCA treatment (Supplementary Table S4), which is consistent with findings from Kenya [51]. Additional costs can therefore partially be overcome by task shifting—reallocating tasks typically conducted by specialists to other less costly health workers [52]. Nevertheless, our findings indicate that, at conventional assumptions about the WTP, one can tolerate higher monitoring costs (Supplementary Fig. S4). However, cost-efficient monitoring strategies with simplified dosing protocols, as have been called for [9, 53], should increase the probability that hydroxyurea at MTD is the dominant strategy.
While pragmatic strategies can reduce monitoring costs associated with SCA treatment, the drug pricing is influenced by factors beyond the control of health services. We find that when the price per 500 mg hydroxyurea exceeds US$ 0.36, hydroxyurea is no longer cost-effective, with a WTP of one GDP per capita (Supplementary Table S9). With conservative assumptions about WTP, the results are even more sensitive to drug prices. Although it is not clear what the costs of hydroxyurea procurement, including shipping and other overheads, could become for the provider, the combined costs may exceed what the MoH expects [47]. However, in Tanzania, the drug became substantially cheaper when produced generically in-country, at a cost of US$ 0.09 per 500 mg [27]. With such prices, the net savings per patient should be even larger than in our base case scenario. This underlines previous calls for fair drug pricing and cost-efficient infrastructures for delivering and administering hydroxyurea [5, 27, 53].
We assume that many of the barriers outlined by the WHO regional committee for Africa [5, 6], which Power-Hays and Ware have elaborated on [53], have been overcome. Importantly, we assume that follow-up is consistently provided and adhered to, which arguably appears unrealistic in many non-trial settings at this point [32]. While this challenge is not limited to a lack of specialized services, developing infrastructures to accommodate patients with SCA has been slow [6, 53]. This challenge must be overcome as access to neonatal screening (NBS) is emerging [1]. Although NBS programs in SSA are likely to be cost-effective in all countries with a high burden of SCA, the expansion of screening will increase the number of patients diagnosed with SCA at a higher rate than the general population growth and may substantially increase health care costs [54]. Consequently, hydroxyurea should be a part of a broader strategy for comprehensive management of SCA in a growing population of patients diagnosed with the disease [6, 53].
With more patients with SCA requiring specialized services [1], it is important to consider the potential increased demand for blood transfusions. Our finding that hydroxyurea should substantially reduce blood consumption has important implications for other non-SCA patients who often urgently require blood, such as children with severe anemia, women with postpartum hemorrhage, and people involved in traffic accidents [19]. Moreover, fewer transfusions are both indicators of health improvements for patients with SCA [1], and reduced exposure to the risk of transfusions-transmissible infections [19]. While the Uganda Blood Transfusion Services aim to raise their quality of production toward an international standard, these measures will probably increase the costs of blood, driving prices beyond our assumption [19]. Overall, reducing blood consumption may represent economical and health-related benefits across the health system beyond our estimates.
4.1 Methodological Limitations
There were several methodological limitations to this study. First, to extrapolate age-specific event rates (supplementary Table S1), we had to use data from large cohorts in the USA [55,56,57,58], since longitudinal data from the current region are limited [23]. We therefore used studies before treatment with hydroxyurea was implemented in the USA, to better resemble our study setting. However, variations in the extrapolated event rates did not influence the ICER enough to be included in Fig. 4, and thus would not alter our conclusion. Clinical parameters that had meaningful influence on our results—transfusion and hospitalizations, as well as stroke risk and other life-threatening events, were region specific [8, 30,31,32,33].
Second, by adopting a provider’s perspective, our estimates should have high relevance for priorities within the health care system, but not when considering society as a whole [20]. We acknowledge recommendations to consider costs based on the societal perspective [28]; however, an International Society for Pharmacoeconomics and Outcomes Research (ISPOR) special task force have specified that “a valid and informative CEA could be conducted from the perspective of any [relevant] stakeholders, depending on the purpose of the analysis” [20]. The current findings can be used to prioritize within the health care sector when asking how healthcare, as opposed to society, should be organized to maximize health [20]. It may add value if future studies estimate the economic impact of interventions that target SCA in SSA beyond the health sector [59]. This is particularly important since most patients reside in rural low-income areas [1], where the frequent and severe presentation of SCA—consuming time and funds of patients, their caretakers, and communities—can pose a substantial opportunity cost [15, 60].
Third, our choice of time horizon of 18 years for the base case analysis ignores outcomes in the longer term, i.e., life-time horizon. However, given the limited data on disease progression and treatment of adults with SCA in SSA, we believe that our findings represent a conservative estimate of hydroxyurea’s impact on health outcomes and costs for the young population with SCA. We did, however, conduct a scenario analysis with a longer time horizon of 62.85 years, which assumes constant input parameters beyond the age of 18 years in the absence of better evidence. This analysis should be interpreted with caution, as it does not build on valid statistics but on a crude assumption about continuous costs and effectiveness of all treatment alternatives. Moreover, factors such as changes in treatment guidelines and health and technological advances may impact the future outcomes in the next couple of decades.
Finally, we considered costs at the clinic level at a national referral hospital—at the level where new strategies for SCA are likely to be implemented first—and believe these have high internal validity. Since we based inpatient care costs on literature information, these are potentially less valid for our specific context. A costing study that covers a broader range of SCA clinics might have further strengthened the economic evidence, and future research should consider this in association with a budget impact study. Moreover, a budget impact study should also account for the scale-up of comprehensive services, including hydroxyurea, for SCA, and should estimate the economic consequences of providing SCA care within lower-level health care facilities outside the urban areas. While our findings suggest that the cost-effectiveness of hydroxyurea is robust for relatively large variations in costs, it is not clear what the combined investments of a scale-up would entail.
5 Conclusions
Provided at the MTD through a feasible dose escalation strategy, hydroxyurea has a very high probability of being cost-effective in a low-income SSA setting such as Uganda. The cost-effectiveness is sensitive to the cost of hydroxyurea, but within realistic ranges of prices, hydroxyurea as routine care is likely to be cost saving and to improve quality of life through decreased illness, pain crises, transfusions, and hospitalizations. Simultaneously, the implementation of hydroxyurea will reduce expected need for blood transfusions, which allows a vital but limited resource to be more readily available for patients in need of transfusion.
References
Ware RE, de Montalembert M, Tshilolo L, et al. Sickle cell disease. The Lancet. 2017;390:311–23. https://doi.org/10.1016/S0140-6736(17)30193-9.
de Montalembert M, Tshilolo L, Allali S. Sickle cell disease: a comprehensive program of care from birth. Hematol Am Soc Hematol Educ Program. 2019;2019:490–5. https://doi.org/10.1182/hematology.2019000053.
Ranque B, Kitenge R, Ndiaye DD, et al. Estimating the risk of child mortality attributable to sickle cell anaemia in sub-Saharan Africa: a retrospective, multicentre, case-control study. Lancet Haematol. 2022;9:e208–16. https://doi.org/10.1016/S2352-3026(22)00004-7.
Ministry of Health Uganda. Uganda Clinical Guidelines 2016. Kampala, Uganda: 2016. http://library.health.go.ug/sites/default/files/resources/Uganda%20Clinical%20Guidelines%202016_FINAL.pdf. Accessed 21 Jun 2018.
Regional Committee for Africa, 60. Sickle-cell disease: a strategy for the WHO African Region. 2011. https://apps.who.int/iris/handle/10665/1682. Accessed 26 Feb 2020.
World Health Organization RC for A. Progress in the implementation of the African region sickle-cell strategy 2010–2020. World Health Organization; 2020. https://doi.org/10.1016/j.ssci.2020.104773%0A.
Opoka RO, Ndugwa CM, Latham TS, et al. Novel use Of Hydroxyurea in an African Region with Malaria (NOHARM): a trial for children with sickle cell anemia. Blood. 2017;130:2585–93. https://doi.org/10.1182/blood-2017-06-788935.
Tshilolo L, Tomlinson G, Williams TN, et al. Hydroxyurea for children with sickle cell anemia in Sub-Saharan Africa. N Engl J Med. 2019;380:121–31. https://doi.org/10.1056/NEJMoa1813598.
John CC, Opoka RO, Latham TS, et al. Hydroxyurea dose escalation for sickle cell anemia in Sub-Saharan Africa. N Engl J Med. 2020;382:2524–33. https://doi.org/10.1056/NEJMoa2000146.
Bristol-Myers Squibb Company. Droxia® (Hydroxyurea).
Wong TE, Brandow AM, Lim W, et al. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850–7. https://doi.org/10.1182/blood-2014-08-435768.
Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 Evidence-Based Report by Expert Panel Members. JAMA. 2014;312:1033–48. https://doi.org/10.1001/jama.2014.10517.
Opoka RO, Hume HA, Latham TS, et al. Hydroxyurea to lower TCD velocities and prevent primary stroke: the Uganda NOHARM sickle cell anemia cohort. Haematologica. 2020;105:E272–5. https://doi.org/10.3324/haematol.2019.231407.
World Health Organization. The Selection and Use of Essential Medicines. Report of the WHO Expert Committee, March 2011 (including the 17th WHO Model List of Essential Medicines and the 3rd WHO Model List of Essential Medicines for Children). 2012. https://list.essentialmeds.org/files/trs/uM2drf3qopwZIceChtyH0tqRGk7c86aLZRZYZimX.pdf. Accessed 24 Apr 2023.
Kambasu DM, Rujumba J, Lekuya HM, et al. Health-related quality of life of adolescents with sickle cell disease in sub-Saharan Africa: a cross-sectional study. BMC Hematol. 2019;19:9. https://doi.org/10.1186/s12878-019-0141-8.
Center for Drug Evaluation and Research. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm. Accessed 8 May 2023.
Moore RD, Charache S, Terrin ML, et al. Cost-effectiveness of hydroxyurea in sickle cell anemia. Am J Hematol. 2000;64:26–31.
Wang WC, Oyeku SO, Luo Z, et al. Hydroxyurea is associated with lower costs of care of young children with sickle cell anemia. Pediatrics. 2013;132:677–83. https://doi.org/10.1542/peds.2013-0333.
Kyeyune-Byabazaire D, Hume HA. Towards a safe and sufficient blood supply in Sub-Saharan Africa. ISBT Sci Ser. 2019;14:104–13. https://doi.org/10.1111/voxs.12468.
Garrison LP, Pauly MV, Willke RJ, et al. An overview of value, perspective, and decision context—a health economics approach: an ISPOR special task force report [2]. Value Health. 2018;21:124–30. https://doi.org/10.1016/j.jval.2017.12.006.
Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Pharmacoeconomics. 2022;40:601–9. https://doi.org/10.1007/s40273-021-01112-8.
Caro JJ, Möller J, Getsios D. Discrete event simulation: The preferred technique for health economic evaluations? Value Health. 2010;13:1056–60. https://doi.org/10.1111/j.1524-4733.2010.00775.x.
Wonkam A, Makani J. Sickle cell disease in Africa: an urgent need for longitudinal cohort studies. Lancet Glob Health. 2019;7:e1310–1. https://doi.org/10.1016/S2214-109X(19)30364-X.
National Medical Stores Uganda. National Medical Stores order forms. https://www.nms.go.ug/index.php/client-services/publications/category/16-order-forms. Accessed 3 Mar 2020.
Institute for Health Metrics Evaluation (IHME). Health Service Provision in Uganda: Assessing Facility Capacity, Costs of Care, and Patient Perspectives. Seattle, WA: IHME 2014. https://www.healthdata.org/sites/default/files/files/policy_report/2014/ABCE/Uganda/ABCE_Uganda_full_report_2014.pdf. Accessed 1 Feb 2021.
MSH (Management Sciences for Health). International Medical products price guide. 2015th ed. Medford: MSH; 2016.
Costa E, Tibalinda P, Sterzi E, et al. Making hydroxyurea affordable for sickle cell disease in Tanzania is essential (HASTE): How to meet major health needs at a reasonable cost. Am J Hematol. 2021;96:E2-5. https://doi.org/10.1002/ajh.26007.
Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103. https://doi.org/10.1001/jama.2016.12195.
Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Disability Weights. Seattle, WA: Institute for Health Metrics and Evaluation (IHME) 2020. http://ghdx.healthdata.org/record/ihme-data/gbd-2019-disability-weights. Accessed 25 Nov 2021.
Noubiap JJ, Mengnjo MK, Nicastro N, et al. Neurologic complications of sickle cell disease in Africa. Neurology. 2017;89:1516–24. https://doi.org/10.1212/WNL.0000000000004537.
Kassim AA, Galadanci NA, Pruthi S, et al. How i treat and manage strokes in sickle cell disease. Blood. 2015;125:3401–10. https://doi.org/10.1182/blood-2014-09-551564.
Opoka RO, Phillip K, Carman AS, et al. The Influence of regular follow-up and comprehensive care on the clinical outcome of pediatric sickle cell anemia at a specialized sickle cell clinic in Kampala, Uganda [Preprint].
Macharia AW, Mochamah G, Uyoga S, et al. The clinical epidemiology of sickle cell anemia In Africa. Am J Hematol. 2018;93:363–70. https://doi.org/10.1002/ajh.24986.
Ministry of Finance, Planning and Economic Development Uganda. Ministerial Policy Statement FY 2019/20-Vote 161: Mulago Hospital Complex. Kampala, Uganda: 2020. https://budget.go.ug/sites/default/files/Sector%20Spending%20Agency%20Budgets%20and%20Performance/VoteMPS_161_MulagoHospitalComplex.pdf. Accessed 1 Feb 2020.
Adams RJ, Brambilla DJ, Granger S, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood. 2004;103:3689–94. https://doi.org/10.1182/blood-2003-08.
Lagunju I, Brown BJJ, Oyinlade AOO, et al. Annual stroke incidence in Nigerian children with sickle cell disease and elevated TCD velocities treated with hydroxyurea. Pediatr Blood Cancer. 2019;66: e27252. https://doi.org/10.1002/pbc.27252.
Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford University Press; 2015.
Exchange Rates UK. US Dollar to Ugandan Shilling Spot Exchange Rates for 2021. https://www.exchangerates.org.uk/USD-UGX-spot-exchange-rates-history-2021.html. Accessed 1 Nov 2022.
Edejer TT, Baltussen R, Adam T, et al. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: World Health Organization 2003. https://www.who.int/choice/publications/p_2003_generalised_cea.pdf. Accessed 11 Mar 2021.
World Bank. Life expectancy at birth, total (years). https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=UG. Accessed 7 May 2023.
Murray CJL, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. The Lancet. 2012;380:2063–6. https://doi.org/10.1016/S0140-6736(12)61899-6.
Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan. 2019. https://doi.org/10.1093/heapol/czz127.
Bertram MY, Lauer JA, Stenberg K, et al. Methods for the economic evaluation of health care interventions for priority setting in the health system: an update from WHO CHOICE. Int J Health Policy Manag. 2021. https://doi.org/10.34172/ijhpm.2020.244.
Commission on Macroeconomics and Health. Macroeconomics and Health: Investing in Health for Economic Development. Geneva: World Health Organization 2001. http://apps.who.int/iris/bitstream/handle/10665/42435/924154550X.pdf?sequence=1. Accessed 17 Feb 2022.
Ochalek J, Lomas J, Claxton K. Estimating health opportunity costs in low-income and middle-income countries: a novel approach and evidence from cross-country data. BMJ Glob Health. 2018. https://doi.org/10.1136/bmjgh-2018-000964.
Briggs A, Schulpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Monitor. Govt to slash price of sickle cell drug by half. 2022. https://www.monitor.co.ug/uganda/news/national/govt-to-slash-price-of-sickle-cell-drug-by-half-3854714. Accessed 28 Oct 2022.
Tull K. Blood costs in Zimbabwe K4D Helpdesk Report 246. Brighton: Institute of Development Studies; 2017.
World Bank. GDP per capita (current US$). https://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 28 Oct 2022.
Cunningham-Myrie C, Abdulkadri A, Waugh A, et al. Hydroxyurea use in prevention of stroke recurrence in children with sickle cell disease in a developing country: a cost effectiveness analysis. Pediatr Blood Cancer. 2015;62:1862–4. https://doi.org/10.1002/pbc.25563.
Amendah DD, Mukamah G, Komba A, et al. Routine paediatric sickle cell disease (SCD) outpatient care in a rural Kenyan hospital: utilization and costs. PLoS ONE. 2013;8: e61130. https://doi.org/10.1371/journal.pone.0061130.
Leong SL, Teoh SL, Fun WH, et al. Task shifting in primary care to tackle healthcare worker shortages: an umbrella review. Eur J Gen Pract. 2021;27:198–210. https://doi.org/10.1080/13814788.2021.1954616.
Power-Hays A, Ware RE. Effective use of hydroxyurea for sickle cell anemia in low-resource countries. Curr Opin Hematol. 2020;27:172–80. https://doi.org/10.1097/MOH.0000000000000582.
Kuznik A, Habib AG, Munube D, et al. Newborn screening and prophylactic interventions for sickle cell disease in 47 countries in sub-Saharan Africa: a cost-effectiveness analysis. BMC Health Serv Res. 2016;16:304. https://doi.org/10.1186/s12913-016-1572-6.
Karnon J, Zeuner D, Ades AE, et al. The effects of neonatal screening for sickle cell disorders on lifetime treatment costs and early deaths avoided: a modelling approach. J Public Health Med. 2000;22:500–11. https://doi.org/10.1093/pubmed/22.4.500.
Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Blood. 1995;86:776–83. https://doi.org/10.1182/blood.V86.2.776.bloodjournal862776.
Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. N Engl J Med. 1991;325:11–6. https://doi.org/10.1056/NEJM199107043250103.
Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. Blood. 1994;84:643–9. https://doi.org/10.1182/blood.V84.2.643.643.
Jiao B, Basu A, Roth J, et al. The use of cost-effectiveness analysis in sickle cell disease: a critical review of the literature. Pharmacoeconomics. 2021;39:1225–41. https://doi.org/10.1007/S40273-021-01072-Z.
Olatunya OS, Ogundare EO, Fadare JO, et al. The financial burden of sickle cell disease on households in Ekiti, Southwest Nigeria. Clin Outcomes Res CEOR. 2015;7:545–53. https://doi.org/10.2147/CEOR.S86599.
Acknowledgments
We thank the staff at the Mulago Hospital Sickle Cell Clinic, Global Health Uganda, and the NOHARM research team for accommodating our stay at Mulago Hospital and sharing their expert opinions during the costing study. We thank Isaac Birungi, Study coordinator for Global Health Uganda, for help with validation of our cost analysis at Mulago Hospital Sickle Cell Clinic. We also thank Dr. Angela Rollins for critically reviewing the manuscript draft. Finally, we thank Dr. Dorothy Kyeyune-Byabazaire, Executive Director of the Ugandan Blood Transfusion Services, for providing us with estimates on blood transfusion costs. Everyone acknowledged in this paper gave their written permission to be named.
Funding
Open access funding provided by University of Bergen (incl Haukeland University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The Norwegian Research Council through The Medical Student Research Program at The Faculty of Medicine at the University of Bergen funded DT, while other authors contributed time in their academic positions. The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Competing Interests
Dr. Ware discloses research donations from the Bristol-Myers Squibb Foundation, Addmedica, and Hemex Health, and to have received fees as a medical advisor for Nova Laboratories and as chair for the data and safety monitoring board of one clinical trial by Novartis and one by Editas. Dr. John Discloses research donations from the Doris Duke Charitable Foundation (ICRA 2016156).
Ethics Approval
The Makerere University College of Health Sciences School of Medicine Research Ethics Committee (SOMREC), Uganda, approved this study. We based analyses on anonymized and published data on health outcomes, and cost data at facility level based on a review of treatment guidelines, expert interviews, hospital records such as stock cards, and publicly available price information.
Data Availability
Data collected for this study—the costing tool with inputs required to calculate the cost of outpatient treatment—will be made available upon reasonable request sent to the corresponding author from the time of publication. No patient-level data was included in this data set.
Author Contributions
ROO, CCJ, and BR conceptualized the study idea, while DT and BR designed the study. BR, ROO, and CN reviewed the study protocol drafted by DT. DT constructed the decision model, conducted formal analyses, and interpreted the data, supervised by BR. DT collected the cost data, supervised by ROO and BR, with the on-site consultancies from CN, RK, and HH. REW, HH, CCJ, ROO, and CN validated the decision model and its inputs, suggested data sources, and provided expert opinions. DT and BR had access to underlying primary data. DT and BR wrote the first draft of the paper. All authors reviewed and approved the manuscript before submission.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Teigen, D., Opoka, R.O., Kasirye, P. et al. Cost-Effectiveness of Hydroxyurea for Sickle Cell Anemia in a Low-Income African Setting: A Model-Based Evaluation of Two Dosing Regimens. PharmacoEconomics 41, 1603–1615 (2023). https://doi.org/10.1007/s40273-023-01294-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01294-3