Abstract
Background
Chest low-dose computed tomography (LDCT) is a promising technology for population-based screening because it is non-invasive, relatively inexpensive, associated with low radiation and highly sensitive to lung cancer. To improve the cost-effectiveness of lung cancer screening, simultaneous screening for other diseases could be considered. This systematic review was conducted to analyse studies that published evidence on the cost-effectiveness of chest LDCT screening programs for different diseases.
Methods
Scopus and PubMed were searched for English publications (1 January 2011–22 July 2022) using search terms related to screening, computed tomography and cost-effectiveness. An additional search specifically searched for the cost-effectiveness of screening for lung cancer, chronic obstructive pulmonary disease or cardiovascular disease. Included publications should present a full health economic evaluation of population screening with chest LDCT. The extracted data included the disease screened for, model type, country context of screening, inclusion of comorbidities or incidental findings, incremental costs, incremental effects and the resulting cost-effectiveness ratio amongst others. Reporting quality was assessed using the 2022 Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist.
Results
The search yielded 1799 unique papers, of which 43 were included. Most papers focused on lung cancer screening (n = 40), and three were on coronary calcium scoring. Microsimulation was the most commonly applied modelling type (n = 16), followed by life table analysis (n = 10) and Markov cohort models (n = 10). Studies reflected the healthcare context of the US (n = 15), Canada (n = 4), the UK (n = 3) and 13 other countries. The reported incremental cost-effectiveness ratio ranged from US$10,000 to US$90,000/quality-adjusted life year (QALY) for lung cancer screening compared to no screening and was US$15,900/QALY–US$45,300/QALY for coronary calcium scoring compared to no screening.
Discussion
Almost all health economic evaluations of LDCT screening focused on lung cancer. Literature regarding the health economic benefits of simultaneous LDCT screening for multiple diseases is absent. Most studies suggest LDCT screening is cost-effective for current and former smokers aged 55–74 with a minimum of 30 pack-years of smoking history. Consequently, more evidence on LDCT is needed to support further cost-effectiveness analyses. Preferably evidence on simultaneous screening for multiple diseases is needed, but alternatively, on single-disease screening.
Registration of systematic review
Prospective Register of Ongoing Systematic Reviews registration CRD42021290228 can be accessed https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=290228.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Literature regarding the health economic benefits of simultaneous low-dose computed tomography (LDCT) screening for multiple diseases is absent. |
Evidence on the cost-effectiveness of LDCT screening for cardiovascular disease is very limited and is absent for chronic obstructive pulmonary disease. |
More evidence on LDCT for diseases other than lung cancer is needed to support further cost-effectiveness analyses. |
1 Introduction
Population-based screening could avoid severe consequences of diseases through early detection and early treatment, thereby improving health outcomes and potentially reducing healthcare costs in the long term [1]. However, large-scale screening requires major upfront investments. Given that healthcare budgets are limited, assessment of the long-term impact on health outcomes and costs is required to assist public policy decision-making toward the implementation of screening [2].
Low-dose computed tomography (LDCT) is a promising technology for use in population-based screening programs because it is non-invasive, has a low radiation dose and is relatively inexpensive compared to other imaging modalities [3]. Chest LDCT for lung cancer, specifically, has high sensitivity, has been investigated extensively and is recommended for population-based screening by the US Preventive Services Task Force (USPSTF) [4]. In many European countries, nationwide lung cancer screening is currently being investigated but not yet implemented. Previously, two reviews investigated methodological differences between health economic evaluations of LDCT lung cancer screening [5, 6]. Both concluded that there is no consensus on whether LDCT lung cancer screening is cost-effective and that reported conclusions on cost-effectiveness depend heavily on the screening strategy evaluated (e.g. which population was targeted) as well as methodological decisions (e.g. whether overdiagnosis was included in the analysis).
The European Society of Radiology and European Respiratory Society (ESR/ERS) position statement on lung cancer screening additionally stated that researchers still disagree on what the drivers of a cost-effective lung cancer screening program would be [7]. Drivers could include the target population selected, frequency of screening and the inclusion of smoking cessation programs. According to the National Lung Screening Trial (NLST) data from the US, the main driver is the number of computed tomography (CT) scans, showing that the number of extra CT scans for follow-up after a positive screen should be minimised. Furthermore, the position statement mentions that lung cancer screening might have even more value when broadening screening to also include detection of chronic obstructive pulmonary disease (COPD), cardiovascular disease (CVD) and other smoking-related diseases. This suggests that the cost-effectiveness of lung cancer screening with LDCT may be improved by screening for more diseases on a single scan, thereby increasing screening yield with minor additional costs.
Clinical evidence indeed exists showing that LDCT biomarkers can be used to also detect, amongst others, subclinical COPD and coronary artery disease [8, 9], or even a wider range of cardiovascular, respiratory and oncological diseases [10]. For health economic evidence, an early health economic evaluation, conducted by our group, estimated the potential of screening for lung cancer, COPD and CVD in a multi-disease screening program [11], concluding that there is potential value in simultaneously screening for these diseases.
This review aims to analyse the evidence for the cost-effectiveness of population-based screening programs using chest LDCT across a range of diseases, with a specific focus on full health economic evaluations.
2 Methods
2.1 Search Strategy
The systematic review (Prospective Register of Ongoing Systematic Reviews registration CRD42021290228) was conducted by searching in Scopus and PubMed, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. The focus of the search strategy was to identify articles and scientific reports describing full health economic evaluations on CT-related population-screening programs. Search terms were kept broad, to ensure that all relevant literature was included, such as searching for “computed tomography” although the inherent focus of this paper is chest LDCT. In addition, to ensure that important studies were not missed in the search, a second set of search strategies was used to search for the cost-effectiveness of screening for diseases that are known to have screening potential using CT (lung cancer, COPD and CVD) without specifying CT. Only studies from the last 10 years before the start of this systematic review have been included due to significant changes in treatment, especially for lung cancer. The search strategy was reviewed by an information specialist and included articles in English, from 1 January 2011 to 22 July 2022.
Health economic evaluations were identified by including search terms similar to those used by Degeling et al. such as cost-effectiveness analysis, cost-utility analysis, cost-benefit analysis, decision analytical model and economic evaluation [13]. Overall, a variety of terminology was included. Studies that were familiar to the authors before the search as well as the reference lists of systematic reviews identified with the search were used in combination with the final set of publications of these studies to verify that the search strategy identified all relevant papers. Therefore, reviews were included in the search strategy, but not in the final set of included studies. Articles, conference papers and reviews were included, and editorial letters, notes, book chapters, short surveys and special reports were excluded. The search strategies were discussed with an information specialist and can be found in Online Resource Table 1 (see the electronic supplementary material).
2.2 Inclusion and Exclusion
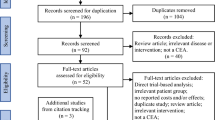
Studies were screened to include full health economic evaluations of population-based screening using chest CT. Full health economic evaluations required the reporting of incremental costs and health outcomes of screening strategies or total costs and health outcomes from which the incremental values could be derived. Population-based screening was defined as screening focusing on target groups that are not limited to a patient group, but consist of a group of individuals at risk of a certain disease. Therefore, studies investigating a screening policy within an already existing patient group were excluded. All abstracts were screened independently by the first reviewer (CB) and a random sample of 10% by a second reviewer (MOW). If it was not clear from the title and abstract if a study should be included, the study was labelled as relevant for full-text assessment. Studies on which the reviewers did not agree were discussed until consensus was reached. The first reviewer screened all studies included in the full-text assessment. The second reviewer screened a random sample of 10% and another 10% that the first reviewer identified as challenging cases. All disagreements within the 20% sample were discussed with a third reviewer (HK) until consensus was reached.
2.3 Data Extraction
Information was extracted from included papers by one reviewer (CB) using Covidence online software for systematic reviews [14], and all unclear extractions were discussed between all reviewers (CB, MOW, HK). Extractions included general information such as the authors, journal name, year, country and aim of the study. Information extracted about screening included diseases screened for, recurrence of screening, starting and stopping age of screening and other disease-specific population descriptions. Modelling decisions were extracted such as model type, study perspective, comparator strategy, time horizon, discount rate, treatments in the care pathway and which co-occurring diseases and incidental findings were included. Evidence extracted as used in the simulation model included screening costs, sensitivity, specificity and participation rates. Finally, results were extracted, including (1) the subgroup(s) within the target population being considered, (2) incremental health outcomes and costs, (3) the incremental cost-effectiveness ratio (ICER) of the most cost-effective strategy, (4) the comparator, (5) which willingness-to-pay (WTP) threshold the results were compared to and (6) the conclusion on whether or not the best-performing strategy was considered cost-effective. The reported ICER values are either the final reported base case value or the most cost-effective result when multiple strategies are compared in the main analysis (not considering results from the sensitivity analysis). Incremental costs and effects were reported as found in the respective studies or calculated as the difference between the total costs and effects of the intervention and no screening or another comparator. If the total incremental costs and effects were reported, it was converted to per person costs and effects to be able to compare outcomes across countries with different population sizes. The cost-effectiveness conclusions were reported as found in the respective studies and, if not reported, the ICERs were compared to the WTP threshold reported. The reported incremental costs associated with the reported ICERs were expressed in 2021 values of the local currency using the local inflation rates and subsequently converted to US dollars using the average exchange rate from November 2021. US dollars have been used as a reporting currency, as it is easily interpretable by readers from different countries.
Heterogeneity and bias in the results from different studies were not evaluated in this paper, due to expected variability in methods used. This paper focuses on providing a narrative synthesis and comparison of studies.
2.4 Quality Assessment
The reporting quality of all papers included in the final set was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [15]. This checklist contains 28 reporting criteria, which were scored with 0, 1 or not applicable, based on which the reporting of studies was classified as high (> 85%), medium (60–85%) or low quality (< 60%).
3 Results
3.1 Search Results
The search strategy yielded 1799 unique studies as shown in the PRISMA flowchart, Online Resource Fig. 1 (see the electronic supplementary material). Screening title and abstract resulted in 62 studies for full-text assessment. During this assessment, studies were excluded if they did not present a full health economic evaluation (n = 7), full texts were not available in English (n = 9) and studies did not report, and did not allow the calculation of, incremental health outcomes and costs of screening (n = 4). The remaining 43 studies were included for data extraction, of which the most important information can be found in Table 1.
3.2 General Characteristics
The general characteristics of all studies are summarised in Online Resource Table 2 (see the electronic supplementary material). All studies focused on lung cancer screening (n = 40, 93%) apart from three studies on CVD (7%) [16, 17]. One study included used CT calcium scoring but then focused the health economic analysis only on identifying incidental pulmonary nodules to detect lung cancer [18]. This study was therefore considered a lung cancer screening study. Studies focused on the healthcare context of the US (n = 15, 35%), Canada (n = 4, 9%), the UK (n = 3, 7%) and 13 other countries, with some countries investigated more than once. The CHEERS score for reporting quality ranged from 37% to 100%, with 20 studies (47%) classified as high quality, 20 (47%) as medium quality and three (7%) as low quality. Online Resource Fig. 2 displays the proportion of studies that reported each item on the CHEERS checklist. No considerable conclusions could be drawn between the reporting quality of the studies and their methods or conclusions. However, it is striking that no studies included a health economic analysis plan, which is an addition to the 2022 checklist. Studies considered target screening populations from the age of 45 up to 100. The minimum number of pack-years of smoking to be eligible for lung cancer screening ranged from 20 to 40. However, some studies had no specific requirement on the minimum number of pack-years smoked (n = 10), of which two used risk assessment to identify the eligible population. The maximum number of years since smoking cessation for former smokers to be eligible for screening was 10 or 15 years, while some studies had no smoking cessation requirements (n = 19, 44%) and some studies only screened current smokers (n = 4, 9%). Two studies investigated the cost-effectiveness of screening in China and Japan, respectively, including non-smokers due to the lower proportion of lung cancer cases attributed to smoking [19, 20]. Two of the three CVD studies included asymptomatic individuals aged 40–85 with intermediate risk based on the Framingham risk score, and the third included individuals aged 40–70 with a family history coronary artery disease events.
Figure 1 illustrates the screening strategies that were considered to be the most cost-effective and are ordered firstly alphabetically according to the country of the study context followed by the surname of the first author. The vertical lines indicate at which ages screening takes place. From this figure, it is clear that there is a consensus on the age (55–74) and frequency (annually) of screening. However, this consensus is likely partially artificial given that the majority (n = 25, 58%) of studies use NLST data (in combination with national survival data or other, smaller trials) and therefore reflect the NLST inclusion criteria in the definition of their screening strategy. The eligibility criteria of this trial were current and former smokers aged 55–74 with ≥ 30 pack-years smoked or who stopped smoking within 15 years from the start of screening. One study investigating the cost-effectiveness of lung cancer screening separately for men and women concluded that men should be screened annually between the ages of 55 and 80 and women should be screened biennially between the ages of 50 and 80 [21], and another concluded that screening is only cost-effective in men [19].
Visual representation of the age of the target screening population for lung cancer screening. A continuous line represents once-off screening as each black vertical line indicates a screening round. At the end of each study, the smoking requirements are shown. “30;10” indicates > 30 pack-years smoked and < 10 years since cessation. “0;0” indicates that there were no minimum number of pack-years and 0 years since smoking cessation (only current smokers). “NA;NA” indicates that minimum pack-years is not applicable because smoking was not a requirement (in studies that use a risk calculator to identify eligible individuals) and no maximum years since smoking cessation in the eligible population. Whether a screening strategy is classified as cost-effective or not was firstly based on the reported study conclusion or, if no conclusion was presented, by comparing the base-case results in the paper with the provided willingness-to-pay threshold. NLST National Lung Screening Trial. NA not applicable. *Study based (partially) on NLST data
3.3 Modelling Choices
Modelling choices are summarised in Online Resource Table 3 (see the electronic supplementary material). Microsimulation was the most common model type (n = 16, 37%), followed by life table analysis (n = 10, 23%), Markov cohort models (n = 10, 23%), decision trees (n = 4, 9%), discrete event simulation (n = 2, 5%) and multistate risk model (n = 1, 2%). In general, the reasoning behind the choice of model type in the publications was limited. Only ten studies discussed the choice of model type. Most studies reportedly applied a public health or public payer perspective (n = 25, 58%), followed by a societal perspective (n = 10, 23%) and a commercial health insurer perspective (n = 6, 14%) or did not report any perspective (n = 2, 5%). The time horizons applied were lifetime (until death) (n = 23, 53%) or 5, 10, 15, or 20 years (n = 10, 23%). In the other cases (n = 10, 23%), the applied time horizon was not reported explicitly.
The majority of studies did not include incidental findings, co-occurrence of other diseases or comorbidities (n = 35, 81%) in the cost-effectiveness model. One study [22] mentioned that incidental findings with potential clinical implications from the NELSON (Dutch-Belgian Lung cancer screening) trial were reported [23] without significant impact on morbidity and mortality, and therefore did not include such findings in the model. Four studies [24,25,26,27] included only the additional (diagnostic) costs of incidental findings. Only one study [18] included both health effects and costs of incidental findings in their model. This study evaluated the cost-effectiveness of screening for incidental pulmonary nodules in the part of the lung that was screened during cardiac CT calcium scoring [18]. Although this study defined the pulmonary nodules as being incidental because the scan was intended for calcium scoring, only results and outcomes of lung cancer screening were included in the model. In general, the stated reason not to include the effect of incidental findings or comorbidities was a lack of evidence.
3.4 Modelling Evidence and Parameters
The parameters used for modelling, including their values, are given in Online Resource Table 4 (see the electronic supplementary material). Only 21 studies (49%) included non-perfect screening participation rates in the base case or the sensitivity analysis. This means that half of the studies assumed 100% participation, which is unrealistic for any screening program, or, less likely, implemented a lower participation rate without reporting it.
The cost of screening varied greatly, from $37 to $1232 after conversion, as some models only included the cost of an LDCT scan and others included additional costs such as invitations to screening, database management and overhead screening costs. All costs are reported in their original currency in Online Resource Table 4. Sensitivity and specificity values for LDCT used in the model were not reported in 14 studies (33%); 18 studies (42%) explicitly stated the sensitivity and specificity values used, of which five reportedly included sensitivity and specificity based on the NLST. Nine Studies (21%) only reported a false positive rate, and two studies (5%) reported the included sensitivity, but not specificity. Five of the studies included sensitivity values depending on the size of the nodule and the stage of disease, respectively [21, 28]. Reported sensitivity values ranged between 43% and 100% and specificity between 62 and 99%.
3.5 Economic Evaluation Results
The results and conclusions of all included studies are summarised in Online Resource Table 5 (see the electronic supplementary material). The reported incremental costs and effects per screened individual were used or were calculated based on the total costs and effects reported. Most of the studies (n = 33, 77%) concluded that the evaluated screening program would be cost-effective. For lung cancer screening, the ICERs ranged from − $4019/LYG to $113,800/LYG and $190,500/quality-adjusted life year (QALY) compared to no screening and six other comparators (2021 US$). The ICERs for cardiac CT calcium scoring were $15,900/QALY, $37,400/QALY and $45,300/QALY compared to no screening (2021 US$). Studies evaluating lung cancer screening compared to no screening are plotted in Fig. 2 for studies reporting LYG and Fig. 3 for studies reporting QALYs. Studies with negative ICERs were excluded from the figures to support readability. The negative ICERs were driven by a decrease in incremental cost of screening. The country indicated on the plot indicates the context of the study. In these figures, the colour indicates whether a study is cost-effective either based on the reported conclusion or, if not reported, based on comparing reported results with the study-specific WTP threshold.
Lung cancer screening incremental cost-effectiveness plane with LYG per person plotting each study, indicating the context country. Whether a screening strategy is classified as cost-effective or not was firstly based on the reported study conclusion or, if no conclusion was presented, by comparing the base-case results in the paper with the provided willingness-to-pay threshold. LYG life years gained
Lung cancer screening incremental cost-effectiveness plane with incremental QALYs per person plotting each study, indicating the context country. Whether a study is classified as cost-effective or not was firstly based on the reported study conclusion or, if no conclusion was presented, the base-case results were compared to the provided willingness-to-pay threshold. QALY quality-adjusted life year
4 Discussion
This systematic review presents and analyses the reported evidence of full health economic evaluations on the cost-effectiveness of screening programs using chest LDCT without limiting the evidence to a specific disease. All but three studies evaluated lung cancer screening; the others evaluated coronary calcium scoring using LDCT for CVD. A limited number of studies included co-morbidities, co-occurrences of diseases or incidental findings in their cost-effectiveness models. One of the studies evaluated screening for incidental detection of pulmonary nodules using full chest CT calcium scoring [18], which is the only study that suggests multi-disease screening even though the cost-effectiveness model only covers the costs and benefits of detecting pulmonary nodules. Thus, while a recent early health economic analysis with limited data suggested that extending LDCT screening for lung cancer with screening for emphysema and CVD may be valuable [11], full health economic evidence of the impact of multi-disease screening with LDCT on cost-effectiveness is absent. Multi-disease modelling is also of increasing interest in the field of blood-based (genomic) biomarkers, as explored in recent studies [29, 30]. Additionally, the effects of incidental findings were rarely modelled, because of a lack of evidence. Increasing attention to COPD and coronary calcium and especially the effect of smoking cessation programs included in lung cancer screening programs, such as the Yorkshire Enhanced Stop Smoking (YESS) study, will likely provide more evidence in this regard [31].
The ERS/SER position statement also suggests broadening screening to include COPD, CVD and other smoking-related diseases, but based on this systematic review, such multi-disease screening programs are not yet reflected in the current health economic evidence. It is expected that the reason for this lack of health economic evidence is very likely due to a lack of clinical evidence for multi-disease screening. Other than the limited evidence on the cost-effectiveness of screening for CVD using LDCT, no health economic evaluation was found investigating the value of COPD screening using LDCT. The current standard of detection of COPD is spirometry, which is cheap, simple and gives an immediate result [32]. Although this explains the absence of health economic evaluations of screening for COPD using LDCT, health economic evidence on the potential value of adding screening for COPD to lung cancer screening is still lacking even though clinical evidence is increasing [33,34,35].
There was substantial variation in study outcomes caused by, firstly, the different healthcare contexts in which the analyses are conducted, secondly, the different modelling methods used and, thirdly, the difference in input and assumptions made, among others. A microsimulation with a public health perspective, 3% discount rate and a lifetime horizon was most commonly used. Analyses were conducted in the healthcare contexts of 15 different countries, which are not easily transferable between healthcare contexts due to differences in clinical guidelines and cost of healthcare procedures. Only some studies included screening costs broader than only the LDCT scan, such as invitation costs and costs to store and secure personal data, which are unavoidable costs. Moreover, only a few studies acknowledged that a screening participation rate of 100% is not achievable. It is assumed perfect participation is unrealistic given the limited evidence on real-world participation. For example, screening participation of eligible smokers in the US was 7.3%, while at least 40% participation would be required for a cost-effective screening program [36]. Since only two studies were found on the health economic evaluation for diseases other than lung cancer, results from this study overlap with results of previous systematic reviews focusing purely on health economic evaluations of LDCT lung cancer screening [5, 6]. This review confirms the conclusions of these previous reviews that studies are heterogenous in the healthcare setting, the screening intervals and the methods used for analysis. In contrast with these previous reviews, this review highlights the large overlap in target screening population between different health economic evaluations although the most cost-effective target screening population is not the same across all health economic evaluations. This review does not have a large focus on screening and modelling consequences such as overdiagnosis, lead-time bias and length bias and, rather, adds to the literature by highlighting the importance of incorporating screening participation rates in cost-effectiveness studies and by providing a more up-to-date review and an insightful comparison of the different target populations considered, and, most importantly, based on the aim of reviewing literature for screening for any disease, this review highlights the gap in literature for health economic evaluations of multi-disease screening using CT.
In contrast to the large variation in health economic outcomes, researchers largely agree on the target screening population for LDCT lung cancer screening. However, this agreement could partially be artificial due to the limited availability of data to use in health economic models evaluating lung cancer screening. Most of the studies used data from the NLST trial and therefore the large agreement of the target screening population also corresponds with the NLST criteria of current and former smokers aged 55–74 with a minimum of 30 pack-years of smoking history [24]. Some studies aimed to identify the target population in which screening is most cost-effective within the NLST criteria, concluding that more strict criteria did not result in improved cost-effectiveness. Even studies without NLST data as input decided upon the NLST screening criteria as the target population.
The limitations of this current review are, first, that the CHEERS checklist is primarily intended as a reporting checklist and was not developed to assess the methodological quality of evaluations. However, as reporting quality is likely correlated with the quality of the evaluation itself, it has been used in multiple similar reviews to gain some insight into the evaluation quality. Second, the search was limited to the last 10 years because of a rapid change in lung cancer treatment during recent years and to therefore make results comparable. Applying a longer search period could have resulted in the inclusion of more studies. Third, we searched PubMed and Scopus and included studies in English. Including more databases could have provided more inclusions. In addition, including all languages would have extended our results, as we found a few non-English studies. Finally, this review does have a risk of bias, as firstly, not all health economic evaluations performed might have been published, especially when results show an intervention to be unfavourable.
5 Conclusion
With limited care resources and the increasing technical capabilities to detect multiple different diseases on a single CT scan, exploring the potential benefits of multi-disease screening strategies is increasingly valuable. This directly applies to LDCT given its low cost, low radiation, non-invasive nature and broad range of applications. However, this review shows that health economic evidence for multi-disease screening using CT does not yet exist. Further research on multi-disease screening using LDCT should focus on gathering additional clinical evidence, only then, estimating the health economic impact of multi-disease screening will be feasible. In such an analysis, it is important to consider the complexity of competing risks and heterogeneity of diseases within the target population. In particular, it would also be important to investigate how the optimal target screening population may shift when moving from lung cancer screening to multi-disease screening.
References
WHO. Screening programmes: a short guide. WHO Press. 2020;1:1–70.
Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2015.
Mohamed Hoesein F. Low-dose computed tomography instead of radiography in suspected pneumonia. Breathe. 2019;15:81<P – 83. https://doi.org/10.1183/20734735.0319-2018.
United States Preventitive Services Task Force (2021) U.S. Preventive Services Task Force Issues Draft Recommendation Statement on Screening for Lung Cancer. Washington, D.C.
Peters JL, Snowsill TM, Griffin E, et al. Variation in model-based economic evaluations of low-dose computed tomography screening for lung cancer: a methodological review. Value Heal. 2022;25:656–65. https://doi.org/10.1016/j.jval.2021.11.1352.
Raymakers AJN, Mayo J, Lam S, et al. Cost-effectiveness analyses of lung cancer screening strategies using low-dose computed tomography: a systematic review. Appl Health Econ Health Policy. 2016;14:409–18. https://doi.org/10.1007/s40258-016-0226-5.
Kauczor H-U, Baird A-M, Blum TG, et al. ESR/ERS statement paper on lung cancer screening. Eur Respir J. 2020;55:1900506. https://doi.org/10.1183/13993003.00506-2019.
Xia C, Rook M, Pelgrim GJ, et al. Early imaging biomarkers of lung cancer, COPD and coronary artery disease in the general population: rationale and design of the ImaLife (Imaging in Lifelines) Study. Eur J Epidemiol. 2019. https://doi.org/10.1007/s10654-019-00519-0.
Du Y, Li Q, Sidorenkov G, et al. Computed tomography screening for early lung cancer, COPD and cardiovascular Disease in Shanghai: rationale and design of a population-based comparative study. Acad Radiol. 2020;28:36–45. https://doi.org/10.1016/j.acra.2020.01.020.
Finamore P, Tanese L, Longo F, et al. The additional value of lung cancer screening program in identifying unrecognized diseases. BMC Pulm Med. 2022;22:48. https://doi.org/10.1186/s12890-022-01826-1.
Behr CM, Koffijberg H, Degeling K, et al. Can we increase efficiency of CT lung cancer screening by combining with CVD and COPD screening? Results of an early economic evaluation. Eur Radiol. 2022. https://doi.org/10.1007/s00330-021-08422-7.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Degeling K, Koffijberg H, IJzerman MJ. A systematic review and checklist presenting the main challenges for health economic modeling in personalized medicine: towards implementing patient-level models. Expert Rev Pharmacoecon Outcomes Res. 2017;17:17–25. https://doi.org/10.1080/14737167.2017.1273110.
Veritas Health Innovation Covidence systematic review software. In: Verit. Heal. Innov. Melbourne, Aust. https://www.covidence.org/. Accessed Jan 2021.
Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Explanation and Elaboration: A Report of the ISPOR CHEERS II Good Practices Task Force. Value Heal. 2022;25:10–31. https://doi.org/10.1016/j.jval.2021.10.008.
Van Kempen BJH, Spronk S, Koller MT, et al. Comparative effectiveness and cost-effectiveness of computed tomography screening for coronary artery calcium in asymptomatic individuals. J Am Coll Cardiol. 2011;58:1690–701. https://doi.org/10.1016/j.jacc.2011.05.056.
van Kempen BJH, Ferket BS, Steyerberg EW, et al. Comparing the cost-effectiveness of four novel risk markers for screening asymptomatic individuals to prevent cardiovascular disease (CVD) in the US population. Int J Cardiol. 2016;203:422–31. https://doi.org/10.1016/j.ijcard.2015.10.171.
Jiang B, Linden PA, Gupta A, et al. Conventional Computed Tomographic Calcium Scoring vs full chest CTCS for lung cancer screening: a cost-effectiveness analysis. BMC Pulm Med. 2020;20:187. https://doi.org/10.1186/s12890-020-01221-8.
Du Y, Li Y, Sidorenkov G, et al. Cost-effectiveness of lung cancer screening by low-dose CT in China: a micro-simulation study. J Natl Cancer Cent. 2022;2:18–24. https://doi.org/10.1016/j.jncc.2021.11.002.
Kowada A. Cost-effectiveness and health impact of lung cancer screening with low-dose computed tomography for never smokers in Japan and the United States: a modelling study. BMC Pulm Med. 2022. https://doi.org/10.1186/s12890-021-01805-y.
Du Y, Sidorenkov G, Heuvelmans MA, et al. Cost-effectiveness of lung cancer screening with low-dose computed tomography in heavy smokers: a microsimulation modelling study. Eur J Cancer. 2020;135:121–9. https://doi.org/10.1016/j.ejca.2020.05.004.
ten Haaf K, Tammemägi MC, Bondy SJ, et al. Performance and cost-effectiveness of computed tomography lung cancer screening scenarios in a population-based setting: a microsimulation modeling analysis in Ontario. Canada PLOS Med. 2017;14:e1002225. https://doi.org/10.1371/journal.pmed.1002225.
Van De Wiel JCM, Wang Y, Xu DM, et al. Neglectable benefit of searching for incidental findings in the Dutch-Belgian lung cancer screening trial (NELSON) using low-dose multidetector CT. Eur Radiol. 2007;17:1474–82. https://doi.org/10.1007/s00330-006-0532-7.
Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N ENGL J Med. 2014;371:1793–802. https://doi.org/10.1056/nejmoa1312547.
Jaine R, Kvizhinadze G, Nair N, Blakely T. Cost-effectiveness of a low-dose computed tomography screening programme for lung cancer in New Zealand. Lung Cancer. 2018;124:233–40. https://doi.org/10.1016/j.lungcan.2018.08.004.
McLeod M, Sandiford P, Kvizhinadze G, et al. Impact of low-dose CT screening for lung cancer on ethnic health inequities in New Zealand: a cost-effectiveness analysis. BMJ Open. 2020. https://doi.org/10.1136/bmjopen-2020-037145.
Venkataraman P, Kawakami H, Huynh Q, et al. Cost-effectiveness of coronary artery calcium scoring in people with a family history of coronary disease. JACC Cardiovasc Imaging. 2021;14:1206–17. https://doi.org/10.1016/j.jcmg.2020.11.008.
Hofer F, Kauczor H-U, Stargardt T. Cost-utility analysis of a potential lung cancer screening program for a high-risk population in Germany: A modelling approach. Lung Cancer. 2018;124:189–98. https://doi.org/10.1016/j.lungcan.2018.07.036.
Tafazzoli A, Ramsey SD, Shaul A, et al. The potential value-based price of a multi-cancer early detection genomic blood test to complement current single cancer screening in the USA. Pharmacoeconomics. 2022;40:1107–17. https://doi.org/10.1007/s40273-022-01181-3.
Etzioni R, Gulati R, Weiss NS. multicancer early detection: learning from the past to meet the future. JNCI J Natl Cancer Inst. 2022;114:168. https://doi.org/10.1093/jnci/djab168.
Murray RL, Brain K, Britton J, et al. Yorkshire Enhanced Stop Smoking (YESS) study: a protocol for a randomised controlled trial to evaluate the effect of adding a personalised smoking cessation intervention to a lung cancer screening programme. BMJ Open. 2020;10:e037086. https://doi.org/10.1136/bmjopen-2020-037086.
van Schayck OC, Invernizzi G, Román M, et al. Early detection of chronic obstructive pulmonary disease (COPD): the role of spirometry as a diagnostic tool in primary care. Nat Publ Gr. 2003;12:90–3. https://doi.org/10.1038/pcrj.2003.54.
Yang X, Wisselink HJ, Vliegenthart R, et al. Association between chest CT–defined emphysema and lung cancer: a systematic review and meta-analysis. Radiology. 2022;304:322–30. https://doi.org/10.1148/radiol.212904.
Henschke CI, Yip R, Boffetta P, et al. CT screening for lung cancer: Importance of emphysema for never smokers and smokers. Lung Cancer. 2015;88:42–7. https://doi.org/10.1016/j.lungcan.2015.01.014.
Sanchez-Salcedo P, Wilson DO, De-Torres JP, et al. Improving Selection Criteria for Lung Cancer Screening. Potential Role Emphysema. 2015. https://doi.org/10.1164/rccm.201410-1848OC.
van Meerbeeck JP, Franck C. Lung cancer screening in Europe: where are we in 2021? Transl Lung Cancer Res. 2021;10:2407–17. https://doi.org/10.21037/tlcr-20-890.
Black WC. Computed tomography screening for lung cancer in the National Lung Screening Trial a cost-effectiveness analysis. J Thorac Imaging. 2015;30:79–87. https://doi.org/10.1097/RTI.0000000000000136.
Cadham CJ, Cao P, Jayasekera J, et al. Cost-effectiveness of smoking cessation interventions in the lung cancer screening setting: a simulation study. JNCI J Natl Cancer Inst. 2021;113:1065–73. https://doi.org/10.1093/jnci/djab002.
Cressman S, Peacock SJ, Tammemägi MC, et al. The cost-effectiveness of high-risk lung cancer screening and drivers of program efficiency. J Thorac Oncol. 2017;12:1210–22. https://doi.org/10.1016/j.jtho.2017.04.021.
Criss S, Cao P, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019. https://doi.org/10.7326/M19-0322.
Diaz M, Garcia M, Vidal C, et al. Health and economic impact at a population level of both primary and secondary preventive lung cancer interventions: a model-based cost-effectiveness analysis. Lung Cancer. 2021;159:153–61. https://doi.org/10.1016/j.lungcan.2021.06.027.
Esmaeili MH, Seyednejad F, Mahboub-Ahari A, et al. Cost-effectiveness analysis of lung cancer screening with low-dose computed tomography in an Iranian high-risk population. J Med Screen. 2021;28:494–501. https://doi.org/10.1177/09691413211018253.
Evans WK, Flanagan WM, Miller AB, et al. Implementing low-dose computed tomography screening for lung cancer in Canada: Implications of alternative at-risk populations, screening frequency, and duration. Curr Oncol. 2016;23:e179–87. https://doi.org/10.3747/co.23.2988.
Field JK, Duffy SW, Baldwin DR, et al. The UK Lung Cancer Screening Trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Helath Technol Assess. 2016. https://doi.org/10.3310/hta20400.
Fitzgerald NR, Flanagan WM, Evans WK, Miller AB. Eligibility for low-dose computerized tomography screening among asbestos-exposed individuals. Scand J Work Environ Health. 2015;41:407–12. https://doi.org/10.5271/sjweh.3496.
Goffin JR, Flanagan WM, Miller AB, et al. Cost-effectiveness of lung cancer screening in Canada. JAMA Oncol. 2015;1:807–13. https://doi.org/10.1001/jamaoncol.2015.2472.
Goffin JR, Flanagan WM, Miller AB, et al. Biennial lung cancer screening in Canada with smoking cessation—outcomes and cost-effectiveness. Lung Cancer. 2016;101:98–103. https://doi.org/10.1016/j.lungcan.2016.09.013.
Gómez-Carballo N, Fernández-Soberón S, Rejas-Gutiérrez J. Cost-effectiveness analysis of a lung cancer screening programme in Spain. Eur J Cancer Prev. 2022;31:235–44.
Hinde S, Crilly T, Balata H, et al. The cost-effectiveness of the Manchester ‘lung health checks’, a community-based lung cancer low-dose CT screening pilot. Lung Cancer. 2018;126:119–24. https://doi.org/10.1016/j.lungcan.2018.10.029.
Kanarkiewicz M, Szczęsny TJ, Krysiński J, et al. Cost-effectiveness analysis of lung cancer screening with low-dose computerised tomography of the chest in Poland. Współczesna Onkol. 2015;6:480–6. https://doi.org/10.5114/wo.2015.56656.
Al Khayat MNMT, Eijsink JFH, Postma MJ, et al. Cost-effectiveness of screening smokers and ex-smokers for lung cancer in the Netherlands in different age groups. Eur J Heal Econ. 2022. https://doi.org/10.1007/S10198-021-01422-W.
Kumar V, Cohen JT, van Klaveren D, et al. Risk-targeted lung cancer screening. Ann Intern Med. 2018;168:161. https://doi.org/10.7326/M17-1401.
McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6:1841–8. https://doi.org/10.1097/JTO.0B013E31822E59B3.
Pyenson BS, Sander MS, Jiang Y, et al. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff. 2012;31:770–9. https://doi.org/10.1377/hlthaff.2011.0814.
Pyenson BS, Henschke CI, Yankelevitz DF, et al. Offering lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Heal Drug Benefits. 2014;7:272–81.
Shmueli A, Fraifeld S, Peretz T, et al. Cost-effectiveness of baseline low-dose computed tomography screening for lung cancer: the Israeli experience. Value Heal. 2013;16:922–31. https://doi.org/10.1016/j.jval.2013.05.007.
Snowsill T, Yang H, Griffin E, et al. Low-dose computed tomography for lung cancer screening in high-risk populations: a systematic review and economic evaluation. Health Technol Assess (Rockv). 2018;22:1–276. https://doi.org/10.3310/hta22690.
Sun C, Zhang X, Guo S, et al. Determining cost-effectiveness of lung cancer screening in urban Chinese populations using a state-transition Markov model. BMJ Open. 2021;11:e046742. https://doi.org/10.1136/bmjopen-2020-046742.
Tabata H, Akita T, Matsuura A, et al. Cost-effectiveness of the introduction of low-dose CT screening in Japanese smokers aged 55 to 74 years old. Hiroshima J Med Sci. 2014;63:13–22.
Tomonaga Y, ten Haaf K, Frauenfelder T, et al. Cost-effectiveness of low-dose CT screening for lung cancer in a European country with high prevalence of smoking—a modelling study. Lung Cancer. 2018;121:61–9. https://doi.org/10.1016/j.lungcan.2018.05.008.
Toumazis I, Tsai EB, Erdogan SA, et al. Cost-effectiveness analysis of lung cancer screening accounting for the effect of indeterminate findings. JNCI Cancer Spectr. 2019. https://doi.org/10.1093/jncics/pkz035.
Treskova M, Aumann I, Golpon H, et al. Trade-off between benefits, harms and economic efficiency of low-dose CT lung cancer screening: a microsimulation analysis of nodule management strategies in a population-based setting. BMC Med. 2017;15:162. https://doi.org/10.1186/s12916-017-0924-3.
Veronesi G, Navone N, Novellis P, et al. Favorable incremental cost-effectiveness ratio for lung cancer screening in Italy. Lung Cancer. 2020;143:73–9. https://doi.org/10.1016/j.lungcan.2020.03.015.
Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS ONE. 2013. https://doi.org/10.1371/JOURNAL.PONE.0071379.
Wade S, Weber M, Caruana M, et al. Estimating the cost-effectiveness of lung cancer screening with low-dose computed tomography for high-risk smokers in Australia. J Thorac Oncol. 2018;13:1094–105. https://doi.org/10.1016/j.jtho.2018.04.006.
Yang S-C, Lai W-W, Lin C-C, et al. Cost-effectiveness of implementing computed tomography screening for lung cancer in Taiwan. Lung Cancer. 2017;108:183–91. https://doi.org/10.1016/j.lungcan.2017.04.001.
Zhao Z, Wang Y, Wu W, et al. Cost-effectiveness of low-dose computed tomography with a plasma-based biomarker for lung cancer screening in China. JAMA Netw Open. 2022;5:e2213634–e2213634. https://doi.org/10.1001/jamanetworkopen.2022.13634.
Author information
Authors and Affiliations
Contributions
CMB conducted the systematic review and wrote the first draft of the paper. MJOW performed a sample of the abstract screening and the full-text screening as the second reviewer. HK acted as third reviewer by resolving conflicts regarding reviewing after both screening rounds. MJI, RV and HK advised on the goal and implementation of the review and drawing conclusions from the results. All authors reviewed and contributed to the manuscript.
Corresponding author
Ethics declarations
Consent to participate
No consent was obtained, as there were no participants in this study.
Consent to publish
No human research participants were included in this study; therefore, no consent to publish was obtained.
Data availability
The data gathered and analysed in this study are included in the article as well as the supplementary materials.
Funding
This study is funded by The Netherlands Organisation for Health Research and Development (ZonMw) (IMDI program, dossier number: 10-10400-98-008, B3Care project). The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The University of Twente funded the open access publication of this article.
Conflict of interest
R. Vliegenthart is supported by an institutional research grant from Siemens Healthineers and reports speaker’s fees from Siemens Healthineers and Bayer. In addition, M.J. IJzerman has held advisory board roles with respect to Illumina, and his institution (University of Melbourne) receives unrestricted research funding from Illumina. H. Koffijberg, C.M. Behr and M.J. Oude Wolcherink declare that they have no conflict of interest.
Author contributions
CMB conducted the systematic review and wrote the first draft of the paper. MJOW performed a sample of the abstract screening and the full-text screening as the second reviewer. HK acted as third reviewer by resolving conflicts regarding reviewing after both screening rounds. MJI, RV and HK advised on the goal and implementation of the review and drawing conclusions from the results. All authors reviewed and contributed to the manuscript.
Ethics approval
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Behr, C.M., Oude Wolcherink, M.J., IJzerman, M.J. et al. Population-Based Screening Using Low-Dose Chest Computed Tomography: A Systematic Review of Health Economic Evaluations. PharmacoEconomics 41, 395–411 (2023). https://doi.org/10.1007/s40273-022-01238-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-022-01238-3