Abstract
Objectives
A drug that improves survival and/or disease progression can create real option value (ROV)—the additional health gain from future innovations enabled by a longer survival. ROV can be a relevant consideration for both clinical and payer decision-makers. We aimed to estimate the ex ante ROV for first-line (1L) alectinib in anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer (NSCLC).
Methods
We developed a Markov model to estimate life-years (LYs) and quality-adjusted life-years (QALYs) gained with 1L alectinib versus 1L crizotinib due to potential future second-line (2L) drugs. Transition probabilities were derived from the phase 3 trial of 1L alectinib and phase 2 trial of 2L brigatinib. We identified drugs being studied in phase 2 and 3 trials in ALK-positive NSCLC at the time of alectinib’s 1L approval and projected the likelihood and timing of their arrival and their potential efficacy based on publicly available data.
Results
The discounted incremental LYs and QALYs for alectinib increased by 12.9% (95% CR − 2.96%, 34.82%; 1.25 vs. 1.11) and 11.2% (95% CR − 2.14%, 29.29%; 1.03 vs. 0.92), respectively, after accounting for ROV. The incremental ROV of alectinib was sensitive to the projected efficacy of future drugs, uptake level, and the hazard ratio of progression-free survival of alectinib (vs. crizotinib).
Conclusions
Ex ante ROV can be a significant value consideration in therapeutic areas with high levels of expected innovation. The potential efficacy of future drugs and incremental survival with alectinib at the projected time of arrival are important considerations in assessing ROV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study applied the current state-of-the-art approach (i.e., pipeline approach) for estimating ex ante clinical ROV. |
We showed that health gains due to increased probability of receiving future drugs for 1L alectinib was 11% of the conventional value in ALK-positive non-small cell lung cancer. |
The most impactful factor for ex ante clinical ROV was the potential efficacy of future drugs, highlighting the importance of accurately projecting future arrivals and their efficacy. |
1 Introduction
Over the last decade, we have witnessed substantial improvement in health from new innovative cancer therapies accompanied by rising costs of cancer care [1,2,3,4]. This has led to several initiatives to assess the value of cancer therapies and, in particular, the contribution of novel elements of value [5,6,7,8]. Real option value (ROV) is one of the novel elements that has gained increased attention in light of rapid medical innovation. ROV refers to the value generated when current drugs improved survival and/or disease progression, thereby increasing the probability of receiving future drugs for which would not be otherwise eligible [9, 10]. Since this concept is related to the survival extension of a medical technology, it is also called “option value of survival”. The term ROV has been used with two additional meanings in healthcare, and thus it is important to differentiate them from the definition in this study [10]. One of the other definitions is the utility of knowing that one may have access to a healthcare service should one need it in the future, which is also called “insurance value” [10, 11]. Also, the term ROV can refer to the value of deferring uncertain and irreversible decisions to a later time, which was originated from financial options theory [10, 12]. In recent years, it has been suggested that accounting for the ROV from survival extension may be relevant for decision-making about life-extending drugs in therapeutic areas with rapid technological advancement [9].
Previous studies in the field of ROV so far have mainly focused on estimating health gains due to the increased probability of receiving future drugs [13,14,15,16,17]. We call this “clinical ROV” as we focused on the health gains while costs of future drugs were not incorporated. Several existing studies have estimated the clinical ROV from an ex post perspective, treating the arrival of future innovations as actual events, and found that the magnitude of clinical ROV was up to 50% of the conventional value [15, 16, 18]. Furthermore, studies have provided real-world evidence of ROV in metastatic melanoma [17, 19]. These findings imply that considering ROV in initial health technology assessment could affect provider and payer evaluations of new products, thereby affecting shared clinical decision-making and formulary placement.
Despite the increasing number of efforts to estimate ROV as part of initial health technology assessment, there is no consensus on whether and how it should be incorporated into CEAs [10]. For purposes of prospective reimbursement, ROV for a medical technology should be estimated using an ex ante approach where ROV is estimated at the time of launch of a current medical technology by treating the arrival of future innovations as uncertain events (as opposed to an ex post approach where the estimation of ROV is based on the actual arrival of innovative drugs) [13,14,15,16,17,18,19,20]. To date, studies have utilized two alternative methodologies for estimating an ex ante indication-specific clinical ROV that can be applied in economic modeling and health technology assessment: (i) the survival trend approach and (ii) the pipeline approach.
Most studies estimating ex ante ROV have used the survival trend approach where future trends in survival are forecasted based on the historical survival trends observed in patient registry data [13, 14, 20]. However, this approach may not be suitable for some situations, for example, where historical trends in survival are not expected to remain the same or when patient registries are not well developed. Alternatively, the pipeline approach can be used by identifying drugs that are currently in clinical development. Specifically, this approach estimates the impact of future innovation by forecasting the success rate, timing of arrival, and potential efficacy of the drugs in the pipeline for the therapy area of interest [20]. The pipeline approach has so far been used in only one study and applied in one disease area that is characterized by rapid disease progression and high mortality. Additional case studies using the pipeline approach in other disease areas with different progression and mortality trajectories will help us to better understand the key drivers and uncertainties when estimating ex ante ROV.
Therefore, we aimed to estimate the ex ante clinical ROV using the pipeline approach for first-line (1L) alectinib compared to crizotinib in anaplastic lymphoma kinase (ALK)-positive NSCLC. We focused on clinical ROV, which is the denominator of the incremental cost-effectiveness ratio (ICER), and did not incorporate costs of future innovative drugs. We chose ALK-positive NSCLC because it is a disease area with continuous innovation and with patients experiencing relatively slow disease progression compared to other advanced cancer types. Alectinib significantly prolonged the PFS of ALK-positive NSCLC patients compared to crizotinib in the first-line setting (25.7 vs. 10.4 months) [21].
2 Methods
2.1 Model Structure
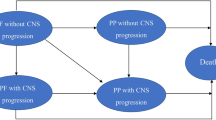
We developed a Markov model with four health states—pre-progression on 1L treatment, progressed with and without using innovative drugs as a second-line (2L) therapy, and dead—to project life-years (LYs) and quality-adjusted life-years (QALYs) gained from 1L alectinib versus crizotinib (standard of care) among treatment-naïve patients with ALK-positive NSCLC, accounting for potential future innovations (Fig. 1 and Appendix 1). The mean age of the model population was assumed to be 55 years per the average patient age reported in the phase 3 study of 1L alectinib versus crizotinib (ALEX trial) [21].
We estimated the incremental LYs and QALYs of 1L alectinib versus crizotinib under the conventional scenario where the arrival of new 2L treatments was not considered (Scenarios A and C in Fig. 2) and the option value scenario where the potential arrival was accounted for (Scenarios B and D in Fig. 2). Under the conventional scenario, patients received brigatinib in the 2L once they progressed on their 1L treatment, as brigatinib was the most efficacious 2L option among the recommended treatments post 1L failure for ALK-positive NSCLC at the time of US Food and Drug Administration (FDA) approval of 1L alectinib (11/2017) [22,23,24]. Under the option value scenario, it was assumed that the next innovative drug arrived ‘T’ months after the date of 1L alectinib approval with the probability of approval of ‘p’, and patients who progressed on their 1L treatment after the arrival of the new drug were eligible to receive the future treatment with an uptake level of q%. Patients who progressed before the arrival of the new 2L drug were assumed to receive brigatinib as a 2L treatment. Expected LYs and QALYs gained were calculated for a lifetime horizon (i.e., for 45 years up to the age of 100 years) and discounted by 3% annually.
Scenarios of comparison; p probability of success, T time to arrival. In scenarios B and D, eligible patients (i.e., those who are newly progressed on first-line (1L) treatment at or after the time of arrival) receive the next innovative drugs as a second-line (2L) treatment with a level of uptake of ‘q’
2.2 Clinical Inputs
2.2.1 Transition Probabilities: Conventional Scenario
Transition probabilities under the conventional scenario were derived from the ALEX trial and the phase 2 study of 2L brigatinib (ALTA trial) (see Appendix 1 for details) [21, 23]. Monthly transition probabilities from the pre-progression to the progressed state without innovative drugs were derived from progression-free survival (PFS) curves for 1L crizotinib and alectinib. We fitted a parametric PFS curve for 1L crizotinib to the Kaplan-Meier (KM) PFS data from the ALEX trial [25]. Then, the hazard ratio (HR) of PFS from the trial was used to construct a PFS curve for 1L alectinib (see Appendix 3 for the analysis without using HR). Monthly transition probabilities from the progressed state to death were derived using the overall survival (OS) curve of 2L brigatinib following 1L crizotinib obtained from the ALTA trial [23]. We fitted a parametric curve to the KM OS data from the ALTA trial and applied it to both of the 1L alectinib and crizotinib arms. The parametric curves for the PFS of 1L crizotinib and OS of 2L brigatinib were fitted with the exponential distribution based on the goodness-of-fit and visual inspection. Our decision on the distribution was supported by a previous study that chose the distribution based on expert opinions (Appendix 2) [25]. We assumed that those patients whose disease is controlled would have a mortality rate similar to that in the general population. The risk of dying directly from the pre-progression state over time was based on the age-specific mortality rate obtained from the US life table for the general population [26].
2.2.2 Transition Probabilities: Option Value Scenario
In the option value scenario, the benefit from using innovative drugs as a 2L treatment was accounted for by lowering the transition probabilities from the progressed state. We assumed that those transition probabilities were lowered when a new treatment for ALK-positive NSCLC became available [20]. Therefore, we forecasted the probability of approval, timing of arrival, the uptake level, and the potential efficacy of new 2L treatment after alectinib using the information available at the time of launch of 1L alectinib. We reviewed ClinicalTrials.gov for ongoing phase 2 and 3 clinical studies in ALK-positive NSCLC in a second- or later-line (2L+) setting as of November 2017 to identify likely future drugs (Appendix 4). For the identified investigational new drugs, we searched for all efficacy information (e.g., overall response rate (ORR), PFS, or OS) that was available as of November 2017 from published papers, conference abstracts, and manufacturers’ press releases. Drugs with a lower efficacy than the standard of care (i.e., 2L brigatinib) were excluded from the list of candidates. The only efficacy data available as of November 2017 for the likely future drugs was ORR. Using ORR as a surrogate marker for efficacy, we estimated the relative decrease in the mortality from the progressed state in our model due to the use of innovative drugs based on the ratio in ORR between the likely future drugs and brigatinib (Appendix 5).
2.2.3 Projected Likelihood and Timing of Approval and the Uptake Level
The likelihood of approval of the investigational new drugs was estimated based on the published statistics on the success rate of targeted drugs in oncology. Specifically, we calculated the likelihood of FDA approval for any one of the included investigational new molecules using the probability of succeeding phase 2 and phase 3 studies (Appendix 5) [27]. The time of the arrival of the next innovation was estimated using the data on R&D time of existing targeted drugs approved for ALK-positive NSCLC. We estimated the time from the start date of a clinical trial to the corresponding FDA approval date for each of the previously approved ALK inhibitors and used the average time to arrival of them. Assuming that not everyone who was eligible for the new treatment received it, the uptake level of 75% was used. Our assumption was based on the analysis of the nationwide Flatiron Health electronic health record-derived, de-identified database for the probability of receiving 2L treatment among patients with ALK-positive NSCLC who failed 1L therapy with ALK-inhibitors.
2.3 Utility Inputs
Utility values for the health states were obtained from the ALEX trial where the utility was estimated using EQ-5D-3L [21, 25]. Utility for the pre-progression state was not varied by the 1L treatment because the ALEX trial showed that treatment was not a significant predictor of utility. We used the same utility value for the progressed states with and without innovative drugs in the base-case because the identified future likely drugs were in the same class as brigatinib.
2.4 Sensitivity Analysis
We performed one-way sensitivity analysis (OWSA) to identify the key drivers of the incremental ex ante ROV of alectinib versus crizotinib (Fig. 3). We varied model inputs with available measures of parameter uncertainty. For the model parameters without 95% confidence intervals (CI) or standard errors, the range was informed by existing literature (Appendix 6).
We also performed a two-way sensitivity analysis to show the variation of the incremental ex ante ROV when simultaneously varying the estimated time of arrival and efficacy of future innovative drugs. We chose to vary these two parameters because they are unique to the calculation of ROV.
Furthermore, probabilistic sensitivity analysis (PSA) with 5000 iterations of Monte Carlo simulation was performed to calculate the 95% credible interval (CR) of the conventional and ex ante ROV of 1L alectinib. We included all base-case model inputs shown in Table 1 except for age, year, and discount rate in the PSA. The distribution for each model parameter included in the PSA is shown in Table 1.
2.5 Scenario Analyses
The use of future drugs in the 2L setting is likely to extend the length of PFS during the 2L treatment. Since the utility is generally higher in the PFS than in the progressed state, the extension of the PFS period may improve the utility during the 2L treatment. Since we did not separately model 2L pre-progressed and 2L progressed states, in a scenario analysis we used a higher utility value for the 1L progressed states with using 2L innovative drugs (vs. without innovative drugs) to account for the potential difference in the utility among patients with versus without using 2L future drugs (Appendix 7). We did not include this effect in the main analysis because of paucity of evidence.
We performed two additional scenario analyses using a different distribution to fit PFS or OS curves. In one scenario, we used a Weibull parametric function for PFS curve fit of 1L crizotinib because a previously published cost-effectiveness study showed that using a Weibull function (instead of exponential) for the PFS curve can substantially change the conventional value of alectinib [25]. This finding implied that the choice of distribution could also substantially impact the ROV. In the other scenario, we used a Weibull parametric function for OS curve fit of 2L brigatinib because the shape of the OS parametric curve with a Weibull distribution was notably dissimilar to other distributions, which can potentially lead to different results. We used Engauge Digitizer 12.1 for the curve fitting (http://digitizer.sourceforge.net/), and Microsoft Excel for the model building.
3 Results
3.1 Conventional Value of Alectinib
In the conventional scenario, the LYs for 1L alectinib and crizotinib were 7.85 and 6.75 years, respectively, and the QALYs gained in each arm were 5.91 and 4.98 QALYs, respectively, resulting in incremental LYs and QALYs gained of 1L alectinib versus crizotinib of 1.11 years and 0.92 QALYs, respectively (Table 2).
3.2 Ex ante Real Option Value (ROV) of Alectinib
When the next innovation was accounted for under the option value scenario, the incremental LYs and QALYs increased by 0.14 years and 0.10 QALYs, respectively. Thus, the additional LYs and QALYs under the option value scenario (i.e., the incremental ex ante ROV of alectinib), represented 12.9% and 11.2%, respectively, of the incremental health outcomes of alectinib under the conventional scenario.
3.3 Sensitivity Analysis
In OWSA with the base-case value of time to arrival of the next innovation (i.e., 24 months), the potential efficacy of the next innovative drugs was the biggest driver of the incremental ex ante ROV of alectinib. The ex ante ROV was also affected by the level of uptake and the PFS HR for 1L alectinib versus crizotinib. The results of OWSA with time to arrival of 12 and 36 months, the lower and upper bound of the estimated time to arrival, were consistent with the OWSA with the base-case value of time to arrival (Table 2).
The two-way sensitivity analysis showed that the incremental ex ante ROV of alectinib ranges from − 0.00 to 0.26 QALYs (− 0.4% to 28.1% of the conventional value) when the two key parameters of ex ante ROV—the potential efficacy of the next innovation and the time to arrival—vary simultaneously within their plausible range. While the efficacy of the next innovation had a positive relationship with the ex ante ROV, the estimated time to arrival had an inverted U-shaped relationship with ROV (Fig. 4).
Two-way sensitivity analysis showing the incremental real option value (ROV) of alectinib with the variation in the potential efficacy of the next innovation and the time to arrival; The range for the potential efficacy of the next innovation was determined by ± 30% of the base-case value (0.78). The range for the time to arrival was determined by ± 1 year of the base-case value (24 months). HR hazard ratio, PPS post-progression survival
The PSA showed that the conventional values of 1L alectinib are 1.14 (95% CR 0.57, 1.93) and 0.97 (95% CR 0.44, 1.74) in LYs and QALYs, respectively, which is similar to the base-case results (Table 2). The mean ex ante incremental ROV of 1L alectinib obtained from the PSA was also comparable to the base-case results: 0.13 (95% CR − 0.03, 0.37) in LYs and 0.09 (95% CI − 0.02, 0.26) in QALYs.
3.4 Scenario Analysis
When a higher utility value was used for the progressed state with using innovative drugs compared to without, the incremental ex ante ROV was estimated to be 0.11 QALYs (12.1% of the conventional value), compared to 0.10 QALYs (11.2% of the conventional value) in the base case.
When a Weibull function was used to fit the PFS curve of 1L crizotinib, there was a slight decrease in the proportion of patients who remained progression-free at the time of 1L alectinib arrival. Since the decrement was very small (approximately 2.7 percentage points) and almost equal in both arms, the ex ante incremental ROV remained similar to the base-case results: 0.15 LYs and 0.11 QALYs (17.3 and 14.9% of the conventional value).
The use of the Weibull function to fit the OS curve of 2L brigatinib significantly increased the risk of dying from the progressed states in both 1L alectinib and crizotinib arms, shortening the length of stay in the progressed states where patients benefit from future drugs. Due to the significant decrease in health gains from future drugs in both arms, the incremental ROV of alectinib decreased: 0.05 LYs and 0.04 QALYs (4.6% and 4.0%, respectively, of the conventional values).
4 Discussion
We estimated ex ante clinical ROV of 1L alectinib versus crizotinib in ALK-positive NSCLC. We found that the incremental LYs and QALYs of 1L alectinib increased by 12.9% (95% CR − 2.96%, 34.82%) and 11.1% (95% CR − 2.14%, 29.29%), respectively, after the arrival of future innovative drugs for 2L was accounted for. The ex ante ROV was most sensitive to the potential efficacy of future drugs and the uptake level.
In this case study, the most impactful factor for the ex ante ROV was the efficacy of future drugs compared to the standard of care, highlighting the importance of evidence-based and clinically plausible estimation of the potential efficacy of future drugs. Forecasting the efficacy of future drugs can be challenging, as noted in previous studies on ex ante ROV [13, 14, 20]. Although the pipeline approach can overcome some of the limitations of the survival trend approach (e.g., inconsistent survival trend over time and the lack of registry data), it has its own challenges. One of the biggest challenges is the often-limited efficacy data for future drugs at the time of analysis, especially when drugs are in early-phase studies. Given the inherent difficulties of projecting the future, analysts should consider using a wide range of the efficacy estimates and evaluate the size of ROV within that range.
Furthermore, our study results illustrate the potential impact of PFS on ROV by showing that ROV largely depends on the difference in the proportion of the progression-free population at the time of arrival of new future drugs. Previous studies on ex ante ROV either did not consider PFS or patients progressed so rapidly that it was negligible (e.g., metastatic melanoma) [13, 14, 20]. However, accounting for PFS is important in disease areas where progression is slow because patients who progressed long before the arrival of future innovative drugs are unlikely to receive them as subsequent therapy.
In our case study, the time to arrival had an inverted U-shaped relationship with the size of incremental ROV of alectinib. The incremental ROV had the maximum value when the time to arrival was between 20 and 30 months. This is because the difference in PFS between the 1L alectinib arm versus crizotinib arm varied over time. There is a relatively small difference in PFS curves between 1L alectinib and crizotinib at times earlier or later than 20–30 months, hence, a smaller difference in the size of the patient population eligible for future drugs between the two arms.
Lastly, we found that the magnitude of clinical ROV is substantial in ALK-positive NSCLC. This finding was consistent with existing studies on ex ante clinical ROV that found the size of ROV to be in the range of 5–15% of the conventional value [13, 14, 20]. A consistent finding of the non-negligible health gains from future innovations may indicate clinical benefits of innovations may need to be considered by analysts and decisions makers in therapeutic areas of high innovation. Despite the growing body of studies on ROV, however, there remain some issues to be discussed before applying it to a decision-making process. First, with the current state-of-the art methods, the size of the estimated clinical ROV is subject to a high degree of uncertainty because of having to project the future. Therefore, there is a need for improving the methods to mitigate the uncertainty or incorporating the uncertainty into the clinical decision making and value-based pricing. Furthermore, there needs to be a consensus on how the clinical ROV should be used in a decision-making process. Two immediately obvious approaches could be: (a) as a contextual consideration in assessing the clinical benefit, and (b) as a quantitative adjustment to the conventionally measured QALYs in value assessments. Lastly, little has been studied on incorporating the costs of future innovations. In a decision-making setting where costs of care are important, one needs to consider estimating the ROV that incorporates both the clinical and the economic impact of future innovations [18]. Additional studies aiming to develop and apply approaches of projecting future costs are needed.
This study is not without limitations. First, by the nature of forecasting the future, the base-case estimates are subject to high uncertainty. Future studies need to be conducted to adapt or implement good practices (e.g., data-driven and expert elicitations) for forecasting the model parameters and characterizing their uncertainty.
Also, we assumed that patients who progress before the arrival of future drugs were not eligible for the 2L future innovative drugs, although there is a chance that they receive future drugs as a third- or later-line of therapy. Nonetheless, we expect this effect to be smaller than from 2L ROV because there would be a smaller proportion of patients who survive to the end of 2L treatment and are still physically fit enough to receive additional lines of systematic therapy.
Additionally, although we assumed that those who progressed earlier than the date of innovation would remain on brigatinib as their 2L therapy, there is a chance that patients switch to a new drug. We did not, however, incorporate the chance of switching because future drugs in the pipeline were in the same class as brigatinib, meaning that the new drugs and brigatinib are similar in terms of their mechanism and toxicity or response profiles.
Another limitation is that patient and societal preferences for ROV likely change over the course of disease and are heterogenous across patients.
Furthermore, the reduction in the risk of mortality in the progressed state when receiving innovative drugs was estimated based on the ORR data. Acknowledging that ORR is not always correlated well with PFS and OS, our sensitivity analyses used a wide range for the relative efficacy.
Moreover, we assumed that the general age-specific mortality rate can approximate the pre-progression mortality. It is possible, however, that patients with ALK-positive NSCLC have higher mortality even though their disease has not progressed. If so, our assumption can lead to an overestimate of the number of patients who would receive future drugs.
One of our scenario analyses with a higher patient utility for the 2L innovative drugs than 2L brigatinib exhibited might have generated an overly optimistic estimate of ROV. Although innovative drugs are expected to extend the length of PFS during the 2L therapy, this is highly uncertain.
Lastly, we did not incorporate the costs of future innovative drugs into the ROV estimation because we aimed to measure ROV in terms of health gains. Analysts should forecast the costs of future innovative drugs if interested in estimating ROV as a change in ICER.
5 Conclusion
We estimated the ex ante clinical ROV of 1L alectinib versus crizotinib in ALK-positive NSCLC. We found that this ex ante ROV accounted for more than 10% of the conventional value for patients who started the 1L treatment around the time 1L alectinib was launched. The ex ante ROV was sensitive to the potential efficacy of future drugs. This study suggests the need for incorporating ROV into the initial assessment of a therapy in disease areas experiencing rapid innovation and contributes to the development of a general framework for prioritizing and estimating ROV in diverse disease areas.
References
Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;9:1300. https://doi.org/10.3389/fphar.2018.01300.
Tran G, Zafar SY. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Ann Transl Med. 2018;6(9):166. https://doi.org/10.21037/atm.2018.03.28.
Wilking N, Lopes G, Meier K, Simoens S, van Harten W, Vulto A. Can we continue to afford access to cancer treatment. Eur Oncol Haematol. 2017;13(2):114–9.
Glode AE, May MB. Rising cost of cancer pharmaceuticals: cost issues and interventions to control costs. Pharmacotherapy. 2017;37(1):85–93. https://doi.org/10.1002/phar.1867.
Cherny N, Dafni U, Bogaerts J, Latino N, Pentheroudakis G, Douillard J-Y, et al. ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol. 2017;28(10):2340–66.
American Society of Clinical Oncology, Value Framework Net Health Benefit Worksheet: Advanced Disease Setting. 2020
Drug Abacus, Drug Pricing Lab, Memorial Sloan Kettering Cancer Center. 2020
Lakdawalla DN, Doshi JA, Garrison LP Jr, Phelps CE, Basu A, Danzon PM. Defining elements of value in health care-a health economics approach: an ISPOR Special Task Force Report [3]. Value Health. 2018;21(2):131–9. https://doi.org/10.1016/j.jval.2017.12.007.
Garrison LP Jr, Kamal-Bahl S, Towse A. Toward a broader concept of value: identifying and defining elements for an expanded cost-effectiveness analysis. Value Health. 2017;20(2):213–6.
Fornaro G, Federici C, Rognoni C, Ciani O. Broadening the concept of value: a scoping review on the option value of medical technologies. Value Health. 2021;24(7):1045–58. https://doi.org/10.1016/j.jval.2020.12.018.
Gyrd-Hansen D. Using the stated preference technique for eliciting valuations: the role of the payment vehicle. Pharmacoeconomics. 2013;31(10):853–61.
Palmer S, Smith PC. Incorporating option values into the economic evaluation of health care technologies. J Health Econ. 2000;19(5):755–66.
Sanchez Y, Penrod JR, Qiu XL, Romley J, Thornton Snider J, Philipson T. The option value of innovative treatments in the context of chronic myeloid leukemia. Am J Manag Care. 2012;18(11 Suppl):S265–71.
Snider JT, Batt K, Wu Y, Tebeka MG, Seabury S. The option value of innovative treatments for non-small cell lung cancer and renal cell carcinoma. Am J Manag Care. 2017;23(10):E340.
Snider JT, Romley JA, Vogt WB, Philipson TJ. The option value of innovation. Forum for health economics and policy. Berlin: De Gruyter; 2012.
Philipson TJ, Becker G, Goldman D, Murphy KM. Terminal care and the value of life near its end: National Bureau of Economic Research; 2010.
Lee W, Li M, Wong WB, To TM, Garrison LP, Veenstra DL. Modeling the ex post real option value in metastatic melanoma using real-world data. Value Health. 2021. https://doi.org/10.1016/j.jval.2021.07.004.
Snider JT, Seabury S, Tebeka MG, Wu Y, Batt K. The option value of innovative treatments for metastatic melanoma. Forum for Health Economics and Policy. Berlin: De Gruyter; 2018.
Wong WB, To TM, Li M, Lee W, Veenstra DL, Garrison LP Jr. Real-world evidence for option value in metastatic melanoma. J Manag Care Spec Pharm. 2021. https://doi.org/10.18553/jmcp.2021.21192.
Li M, Basu A, Bennette C, Veenstra D, Garrison LP Jr. How does option value affect the potential cost-effectiveness of a treatment? The case of ipilimumab for metastatic melanoma. Value Health. 2019;22(7):777–84. https://doi.org/10.1016/j.jval.2019.02.002.
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–38. https://doi.org/10.1056/NEJMoa1704795.
Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, et al. NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Cancer Netw. 2018;16(7):807–21. https://doi.org/10.6004/jnccn.2018.0062.
Kim DW, Tiseo M, Ahn MJ, Reckamp KL, Hansen KH, Kim SW, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490–8. https://doi.org/10.1200/JCO.2016.71.5904.
Crino L, Ahn MJ, De Marinis F, Groen HJ, Wakelee H, Hida T, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol. 2016;34(24):2866–73. https://doi.org/10.1200/JCO.2015.65.5936.
Carlson JJ, Suh K, Orfanos P, Wong W. Cost effectiveness of alectinib vs. crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Pharmacoeconomics. 2018;36(4):495–504. https://doi.org/10.1007/s40273-018-0625-6.
U.S. Department of Health and Human Services; United States Life Tables 2017, National Vital Statistics Reports. 2017;68.
Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20(2):273–86.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Woojung Lee received consulting fees from Genentech during the conduct of the study. William Wong is an employee of Genentech, Inc. and reports ownership of Roche stock. Stacey Kowal is an employee of Genentech, Inc. and reports ownership of Roche stock. Louis Garrison received consulting fees from Genentech during the conduct of the study. David Veenstra received consulting fees from Genentech during the conduct of the study. Meng Li has no conflict of interest to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Funding
This study was funded by Genentech, Inc.
Data availability
Not applicable.
Code availability
Not applicable.
Author contributions
Conceptualization: WW, DV, LG, ML. Methodology: WL, WW, SK, LG, DV, ML. Formal analysis and investigation: WL. Writing—original draft preparation: WL. Writing—review and editing: WW, SK, LG, DV, ML. Funding acquisition: DV and LG. Supervision: WW, SK, LG, DV, ML.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lee, W., Wong, W.B., Kowal, S. et al. Modeling the Ex Ante Clinical Real Option Value in an Innovative Therapeutic Area: ALK-Positive Non-Small-Cell Lung Cancer. PharmacoEconomics 40, 623–631 (2022). https://doi.org/10.1007/s40273-022-01147-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-022-01147-5