Abstract
Background
Respiratory syncytial virus (RSV) is a major cause of acute respiratory infection (ARI), with high morbidity and mortality worldwide. RSV costing and burden estimates can highlight the potential benefits of future vaccination programs and are essential for economic evaluations.
Objective
We aimed to determine RSV healthcare costs across age groups and the overall disease burden of medically attended RSV in Canada.
Methods
We conducted a retrospective case–control study to estimate the attributable healthcare costs per RSV case in Alberta. We used two case definitions to capture diversity in case severity: laboratory-confirmed RSV and ARI attributable to RSV. Matching occurred on five criteria: (1) age, (2) urban/rural status, (3) sex, (4) prematurity and (5) Charlson Comorbidity Index score. We calculated the age-specific burden of medically attended RSV in Canada from 2010 to 2019 by multiplying the weekly age-specific incidence of medically attended ARI with the RSV positivity rate.
Results
Costs per laboratory-confirmed RSV case were (in Canadian dollars [CAD], year 2020 values) $CAD12,713 and 40,028 in the first 30 and 365 days following diagnosis, respectively, whereas a case of ARI potentially attributable to RSV cost $CAD316 and 915, in 30 and 365 days, respectively. Older (aged ≥ 65 years) and younger (aged < 90 days) age groups had the highest case costs. The average medically attended RSV incidence rate across nine seasons was 1743 cases per 100,000 people per year.
Conclusions
RSV is a common and expensive infection at the extremes of life, and the development of immunization programs targeting older and younger ages may be important for the reduction of RSV burden and cost.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Very few studies have evaluated the costs and burden of disease associated with a respiratory syncytial virus (RSV) case, and this is one of the first studies to analyze RSV case costs across multiple years and age groups. |
Estimates of healthcare costs associated with RSV can help researchers and policy makers identify populations at highest risk for costly infection, which can help set immunization program priorities and conduct economic evaluations. |
Our study findings show that, although RSV costing studies have largely focused on infants and children, RSV remains a common and expensive infection throughout life, with older adults having some of the highest costs and burden of RSV disease. |
1 Introduction
Respiratory syncytial virus (RSV) is the most common cause of acute lower respiratory infection in infants and children worldwide. By the age of 2 years, almost all children have had at least one RSV infection, and half have had two [1]. Following initial infection, RSV protection is incomplete, and reinfection occurs throughout an individual’s life. RSV typically manifests as an upper respiratory or an asymptomatic infection in healthy adults; however, infants born at preterm gestation, young infants (< 6 months) and those with comorbidities (e.g., congenital heart disease, chronic lung disease) are at high risk of severe infection, such as bronchiolitis. In the USA and Canada, RSV remains the leading cause of hospitalization for children aged < 1 year. More recently, research identified older adults as a high-risk group for severe RSV infection. A US review estimated that 12% of medically attended acute respiratory infection (ARI) in individuals aged > 50 years was due to RSV [2].
To date, the only way to prevent RSV is through passive immunization with the monoclonal antibody palivizumab, which is used in high-risk children. However, a large number of novel RSV vaccines and long-acting monoclonal antibodies are in clinical trials that target a wide diversity of at-risk groups, including pregnant, pediatric, and older populations [3]. Estimates of healthcare costs associated with RSV can help researchers and policy makers identify populations at highest risk for severe infection and those who cost the most to treat, which can help set immunization program priorities across age groups. Furthermore, identifying trends in RSV costs allows researchers to identify the biggest cost drivers for RSV and how changes in policy and testing may impact RSV costs over time. Context-specific cost and burden data are also needed for appropriate economic evaluation of future RSV immunization programs to truly understand the benefits of immunization programs with the availability of newer products. Moreover, to evaluate the impact of immunization programs, researchers need infectious disease models calibrated to empirical incidence data such as age-specific incidence rates, for which there are no estimates in Canada.
Very few studies have evaluated the costs and burden of disease associated with an RSV case [4,5,6]. Globally, the majority of RSV burden of disease and costing studies have focused on infants or young children and hospitalized cases [7,8,9,10]. These studies can overestimate the average costs associated with medically attended RSV, as the infection is typically more severe in these populations, yet simultaneously underestimate the overall burden of disease by not capturing the impact of RSV in other age groups (e.g., older adults). Estimating RSV burden of disease and associated costs across age groups remains a priority. Moreover, many of the RSV cost estimates are out of date [11, 12], and—given that costs vary by location and structure of health systems—it is important to have up-to-date and local evidence available.
Therefore, the objectives of this study were to
-
1.
determine the case costs associated with a medically attended RSV infection across age groups and by risk factors and
-
2.
determine the overall disease burden of medically attended RSV.
2 Methods
2.1 Setting and Data Sources
Our study took place in Alberta, a Canadian province with approximately 4.3 million residents, the vast majority of whom (> 99%) are registered with the province’s universal publicly funded health insurance plan. Each member of the provincial health insurance system is captured in the Population Registry, which includes general demographic information (e.g., date of birth, sex) and information on the date and reason for insurance cancellations (e.g., emigrated from Alberta or died). Each individual in our databases had a unique lifetime identifier, which allowed us to deterministically link between various health administrative datasets.
In Alberta, all interactions with the health system are captured in one or more health administrative datasets, including hospitalization and ambulatory care/emergency department (ED) visits (Morbidity and Ambulatory Care Abstract Reporting System [MACARS]) as well as physician visits (Physician Claim Database). Healthcare costs are also captured in these databases. Individual-level data on laboratory-confirmed RSV cases in Alberta were obtained from the Alberta Precision Laboratory databases, which capture the results of most respiratory tests performed in Alberta. Specifically, Albertans with a respiratory illness who are seen by a health professional can be tested with a respiratory laboratory panel that includes an RSV test. In recent years, RSV testing in Alberta has been primarily conducted on inpatients and in EDs, with limited testing in outpatient settings. Moreover, we obtained data on the national positivity rate for RSV (i.e., of all the tests for RSV, the percent that are positive) per week from the Public Health Agency of Canada [13].
In MACARS, healthcare costs associated with inpatient visits are estimated using Resource Intensity Weight (RIW) methodology, as described by the Canadian Institute of Health Information [14], which multiplies the individual case RIW based on age and case mix group by the average provincial cost per weighted case. Similarly, the costs associated with ambulatory care and ED visits are calculated using RIW based on age and the Comprehensive Ambulatory Classification System [15], which classifies individuals who received ambulatory care (i.e., ED visits, day surgeries) by a number of data elements, including diagnosis, interventions, mode of visit, and visit disposition, which determine their RIW [15, 16]. To estimate physician costs, we used the Physician Claims Database, which captures all physician billing claims in the province, including those from outpatient and inpatient visits. We did not estimate laboratory or prescription and over-the-counter drug costs, as these costs are not captured in the health administrative datasets.
2.2 Study Population and Design
2.2.1 Objective 1: Respiratory Syncytial Virus (RSV) Case Cost
We used a retrospective case–control design to estimate the attributable healthcare costs per RSV case in Alberta. We measured costs from the healthcare perspective and did not consider societal or personal costs associated with RSV infection. We assumed healthcare costs attributable to RSV equaled the mean differences in all-cause healthcare costs between cases and controls. We chose all-cause healthcare because very little is known about the long-term effects of RSV on health costs and outcomes, and an RSV case’s contacts with the healthcare system are often not captured under an RSV diagnostic code. The index date for cases was the date of their first interaction with the health system for an RSV-associated event or the order date for their laboratory test; matched controls were given the same index date.
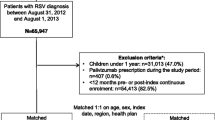
We used two different case definitions to identify RSV in the health administrative data. Case definition one (i.e., laboratory-confirmed RSV) was defined as any individual who had a laboratory-positive RSV test. The date the RSV laboratory test was ordered was considered the index date for these cases. We enrolled laboratory-confirmed cases from 1 September 2014 to 31 August 2018, with costing follow-up occurring until 31 August 2019. The purpose of this case definition was to capture RSV cases with a high level of specificity. However, children and hospitalized cases are tested at a much higher rate than other populations in Alberta and generally have more serious outcomes, so this case definition likely overestimates the cost per case.
Therefore, our second case definition, “ARI attributed to RSV,” sought to capture possible RSV cases (i.e., not laboratory confirmed) by defining a case as a medically attended visit for ARI where the most responsible diagnosis could be attributed to an RSV infection. “Most responsible diagnosis” was defined as the first diagnosis classification by the Canadian International Classification of Diseases, Ninth (ICD-9) or Tenth Revision (ICD-10-CA) codes. Relevant ICD codes were based on a review of the literature [17, 18] and the expert opinion of two infectious disease physicians (see Appendix A in the electronic supplementary material [ESM]). Moreover, for case definition two, the event had to occur during RSV season (1 November–30 April) [19, 20]. Although a wide variety of infections can cause ARI, the methods to treat them, and therefore the costs, are generally independent of the causative agent, particularly for nonhospitalized cases. Since case definition two identified many cases (>3,000,000), we used the simple random sampling function in SAS© to select 10% of the population for the final costing analysis. The index date for these cases was the date of their first medically attended ARI event. For ARI attributed to RSV, we captured costs from 1 November 2010 through 30 April 2018, with costing follow-up until 30 April 2019.
Controls for both case definitions were drawn from Alberta’s publicly funded health insurance plan over the year of analysis (i.e., did not emigrate or die within 1 year of the index date). For laboratory-confirmed RSV, each case was matched 1:5 to a non-RSV control; we selected a high ratio to account for variability in control costs. For ARI attributed to RSV, the cases were matched 1:1 to a non-ARI control. In both analyses, controls were defined as no RSV or ARI code within the year of analysis. Controls were randomly selected from all possible matches. For both case definitions, matching of cases to controls was based on five criteria: (1) age in months for cases aged < 1 year and age in years for cases aged ≥ 1 year, (2) urban/rural status (living in urban [≥ 25,000 population] vs. rural [< 25,000 population] areas according to postal code and census data), (3) sex, (4) prematurity (gestational age < 36 weeks) for cases aged < 1 year, and (5) Charlson Comorbidity Index (CCI) score [21, 22] for cases aged ≥ 1 year. Matching was done using a macro program coded in SAS©. The CCI is a measure of the number and intensity of an individual’s comorbidities; a higher number signifies more comorbidities. Previous research has demonstrated that CCI score is a reliable predictor of costs [21]. Moreover, by using the same index date for cases and their controls, we accounted for time of year and RSV season [19, 20]. Individuals could be an RSV case and/or control more than once (since they could acquire RSV multiple times); however, to allow for full cost follow-up, they had to have at least 365 days following their initial identification as a case or control prior to being considered a new case/control. Each laboratory-confirmed RSV case had at least one exact match, but 127 did not have five exact matches. They were retained in the analysis to capture the costs associated with these populations, as they were generally part of smaller demographic groups (e.g., children who were premature).
2.2.2 Objective 2: RSV Burden
To estimate the burden of RSV, we calculated the incidence of medically attended RSV from 1 September 2010 to 31 August 2019 (nine complete RSV seasons) in Alberta. We multiplied the weekly age-specific incidence of medically attended ARI by the national RSV laboratory test positivity rate. ARI was defined as a physician visit with a relevant ICD-9 code or a hospitalization or ED visit with a relevant ICD-10 code (Appendix A in the ESM). However, for the RSV burden analysis, the case did not have to occur during the RSV season, and a case was considered incident if the individual did not have an ARI-relevant ICD code in the preceding 30 days. We did not include any date limits so we could capture RSV cases throughout the calendar year and observe the seasonality of RSV infections. To determine the percentage of total RSV tests that were positive for RSV infection each week from 2010 to 2019, we used aggregated national data from the Public Health Agency of Canada [13].
2.3 Data Analysis
2.3.1 Objective 1: RSV Case Cost
We tracked both case and control costs using the phase-of-care method [23], where costs were calculated over two different phases: (1) acute infection (30 days’ follow-up) and (2) continuing care (365 days’ follow-up). We selected 30 days to capture the majority of costs associated with an acute RSV infection, and our hospitalization data showed length of stays ranging from 0 to 22 days for 99% of cases. A continuing care phase of 365 days is consistent with other studies, allowing us to account for the long-term morbidity associated with RSV [24, 25] without becoming too far removed from the initial infection. We calculated the mean and standard deviation for the attributable RSV case costs stratified by RSV season, age, prematurity, CCI score, and sex and tested for significant differences in case and control costs using the Wilcoxon signed rank test. We selected a matched samples nonparametric test to account for non-normal distribution in costing data. We applied the Bonferroni adjustment as a multiple testing correction to account for the multiple subgroup analyses. Moreover, we calculated the mean and standard deviation of RSV-attributable case costs associated with hospitalization, ED visits and physician billing. All costs were inflated to Canadian dollars ($CAD), year 2020 values, using the healthcare consumer price index as reported by Statistics Canada [26].
2.3.2 Objective 2: RSV Burden
The age-specific weekly rate of RSV was calculated as the number of incident ARIs per week and age group, divided by the total age-specific population in that week in Alberta, multiplied by the percent of total RSV laboratory tests positive for RSV by week (Fig. 1).
RSV incidence rates are presented as RSV cases per 100,000 people per week and per year by age group. As our ARI incidence rates were derived from the total Alberta population, we did not weigh the outcomes by age and sex distributions.
3 Results
3.1 Demographics
There were 12,478 positive tests for RSV across four RSV seasons in the approximately 4.26 million individuals enrolled per year in Alberta’s publicly funded health insurance plan. Following exclusions for loss to follow-up and duplication, 8484 laboratory-confirmed cases of RSV were included in our analysis, with 42,083 matched controls (Table 1). The majority of laboratory-confirmed RSV cases were male, lived in an urban environment and had a CCI score of 0 or 1. Over 59% of the cases occurred in children aged < 2 years. The number of laboratory-confirmed RSV cases varied widely by season, ranging from 1144 cases in 2015–2016 to 2765 in 2016–2017.
We also identified 3,456,562 unique cases of medically attended ARI between November and April across eight RSV seasons. Following random selection and exclusions for cases without a full year of follow-up or without a control match, we included a sample of 345,284 ARI attributed to RSV cases in our analysis (Table 1). Most of these cases were identified in physician billing (86.5%), with 13.5 and 0.08% identified in ambulatory/ED and inpatient care, respectively. The highest number of cases occurred in December (65,789) and January (65,025). Cases were matched to 345,284 controls. Cases and controls had a mean age of 32 years, with cases spread relatively evenly across the eight RSV seasons. The majority of cases lived in an urban environment, had a CCI of 0 and were female (Table 1).
For the burden of disease analysis, we identified 10,706,843 cases that met our definition of ARI from 1 September 2010 through 31 August 2019. The average RSV test positivity rate from September 2010 to September 2018 was 4% in Canada, whereas the in-season positivity rate (November–April) for those eight RSV seasons was 7% [13] (Appendix B in the ESM).
3.2 Objective 1: RSV Case Cost
RSV-attributable costs for laboratory-confirmed cases was $CAD12,713 at 30 days and $CAD40,028 at 365 days following diagnosis (Fig. 2a). For laboratory-confirmed RSV cases, inpatient costs accounted for 70 and 64% of total costs at 30 and 365 days, respectively. The proportion of case costs by care type stayed consistent across age groups for laboratory-confirmed RSV (Fig. 3a). For ARI attributed to RSV, the average attributable cost per case was much lower at $CAD315 at 30 days and $CAD898 at 365 days (Fig. 2b). Inpatient costs accounted for a smaller percentage of total ARI attributed to RSV case costs (compared with laboratory-confirmed RSV cases), but it remained the biggest cost driver, at 41% of costs over both time periods. For ARI attributed to RSV, physician costs accounted for 36% (30 days) and 38% (365 days) of total costs, and ambulatory/ED care represented 23% (30 days) and 21% (365 days) of overall costs. The proportion of case costs by care type varied by age group for ARI attributed to RSV (Fig. 3b), with younger and older age groups having higher inpatient costs and individuals aged 2–49 years having higher physician costs.
Attributable costs over 30 days presented by age group and care type for laboratory-confirmed RSV and ARI attributed to RSV care types are divided into MD (physician billing claims for both outpatient and inpatient), Amb (ambulatory care, such as emergency department visits) and Inp (inpatient costs excluding physician costs). ARI acute respiratory infection, RSV respiratory syncytial virus
For both case definitions, costs varied with age, sex, comorbid conditions, prematurity (for children aged < 1 year), RSV season and location of residence (Tables 2 and 3). Individuals with a CCI score of 5 had an RSV-attributable cost between 3 and 13 times that of individuals with a CCI score of 0. Being born premature was also associated with high RSV treatment costs. For ARI attributed to RSV cases, attributable costs were higher in the youngest and oldest age groups and lowest in those aged 6–17 years. In comparison, costs for the laboratory-confirmed RSV cases were high in adult populations. In conjunction with increases in the overall costs of RSV over time, the percent of cases with inpatient and ambulatory costs within 30 days increased, with RSV laboratory-confirmed cases with inpatient costs within 30 days increasing from 11% in 2014–2015 to 31% in 2017–2018. In every category, for both laboratory-confirmed RSV and ARI attributed to RSV and at both time points, the cases had significantly higher costs than their matched controls, even following the Bonferroni adjustment (p < 0.0001 < α = 0.00044) (Tables 2 and 3).
3.3 Objective 2: RSV Burden
Based on the ARI incident cases and the percent positive of RSV test estimates, the incidence rate of medically attended RSV in Alberta from 2010 through 2019 was estimated at 61 cases per 100,000 people per week in RSV season or an average of 1743 cases per 100,000 people per year. RSV incidence rate varied dramatically by season and month, with the highest rate occurring annually in January and February (Fig. 4; Appendix B in the ESM). The 2017–2018 season had the lowest yearly rate (1022 per 100,000 per year), and the 2011–2012 season had the highest rate (2390 per 100,000 per year). RSV rates varied by age group, with the highest incidence rate in children aged 6 months to < 1 year (6461 per 100,000 per year) and the lowest rates in adults aged 50–64 years (1330 per 100,000 per year) (Appendix B in the ESM).
4 Discussion
This is one of the first studies to analyze RSV case costs across multiple years and age groups. For laboratory-confirmed RSV, older adults (aged >65 years) had one of the highest RSV-attributable costs, particularly at 365 days following infection. One potential explanation for the high costs in older adults is the potential for RSV to trigger other costly healthcare visits, which is supported by the observation that the 365-day cost estimates were so much higher than the 30-day estimates, although more research in this area is needed. Of note, the costs in younger adults (aged 50–64 years) with laboratory-confirmed RSV were also high. The breakdown of the costs demonstrated that this group had high physician costs and, perhaps surprisingly, the second highest inpatient costs. Because RSV infection is not well recognized in this age group, only the most severe cases (e.g., inpatient) may be tested compared with cases in young children and older adults, who are tested on a more regular basis, leading to a high average cost of disease for those aged 50–64 years. Capturing costs across age groups is essential to the evaluation of a range of potential RSV immunization programs.
In one of the only other studies to look at total healthcare case costs of RSV across age groups, Amand et al. [29] estimated substantially higher RSV costs in the USA than our ARI attributed to RSV costs but lower costs than our RSV laboratory-confirmed costs. One key difference between the current study and Amand et al. [29] is that they included only individuals with RSV-specific ICD codes, which is neither as specific as our laboratory-confirmed case definition nor as sensitive as our ARI case definition, thereby giving an intermediate estimate of RSV costs. Although studies have validated RSV-specific ICD codes for children aged < 3 years [30], they are generally considered to be less accurate for adults. This is likely because adults are less likely to be hospitalized and therefore tested for RSV. Having more specific and more sensitive RSV case definitions allowed us to capture a range of RSV costs. Moreover, Amand et al. [29] estimated costs in the USA, which typically has higher healthcare costs than other countries, as illustrated by the costs of controls in their study. Finally, Amand et al. [29] only studied one RSV season. It is recognized that the burden of RSV varies markedly by year, as demonstrated by the current study.
Our analysis was also unique in that it looked at how RSV costs change based on a variety of factors, including comorbidities and location of residence. Specifically, our study highlighted the overall impact of comorbidities on case costs across all age groups, with CCI score having a substantial impact on RSV-attributed costs. McLaurin et al. [9] found that RSV hospitalization costs for children born premature were at least double the costs for children born at term. This evidence is consistent with our findings for both ARI attributed to RSV and laboratory-confirmed RSV healthcare costs for children born prematurely.
We observed large differences in the incremental healthcare RSV cost estimates for the two case definitions, which is unsurprising considering the testing criteria for RSV in Alberta, which is generally focused on inpatients and severe cases. These differences in costs are particularly important to consider when estimating the cost effectiveness of RSV interventions, as costing studies that use solely laboratory-confirmed cases or even RSV-specific ICD codes may overestimate the costs of the majority of medically attended RSV infections. Capturing the costs of ARI attributed to RSV is important, as these individuals may use healthcare resources, but these costs often go unreported as testing is generally not recommended for uncomplicated cases.
Furthermore, this study measured the overall incidence of medically attended RSV over multiple years and age groups. Many of the previous studies of the burden of RSV illness in Canada and the USA have focused on children and hospitalized cases [18, 30]. Our RSV incidence estimates established a baseline of disease incidence. Our incidence estimates for RSV in children aged < 5 years are generally consistent with those of Hall et al. [4], although ours were slightly lower in the youngest age groups. Moreover, our findings were in line with previous studies that demonstrated that the highest rates of medically attended RSV were in young children, with cases decreasing in adults before rising again in the oldest age groups [6, 31].
The rate of medically attended RSV incidence changed dramatically from year to year, as is consistent with previous findings. For example, Pitzer et al. [32] highlighted a biennial pattern of RSV rates, alternating between small and large epidemics. Although our RSV incidence rates changed from season to season, the last two RSV seasons had notably lower incidence rates, which was largely due to a decrease in the percentage of RSV-positive rates in those years. We hypothesize that this is related to changes in laboratory testing criteria, which Canadian research has shown are inconsistent, even for hospitalized children [33]. Although evidence indicated that costs for both laboratory-confirmed RSV and ARI attributable to RSV were increasing over time, we did not observe a substantial increase or decrease in costs in the 2017–2018 season, when the incidence of RSV decreased. However, more research is needed on how RSV testing criteria in Canada have changed over time to accurately depict how these changes could affect RSV positivity rates and healthcare costs associated with RSV, particularly laboratory-confirmed RSV.
Although the RSV healthcare costs we present in this analysis were estimated using Alberta data, they were derived using Canadian Institute of Health Information methods, which are applied across Canada and therefore may be applicable outside of Alberta. Moreover, the relationships and factors associated with higher costs were consistent with other findings in the literature and are unlikely to be province specific. We also used national positivity estimates to estimate RSV incidence, which makes our findings more nationally relevant, especially as positivity rates are more likely to change across provinces because of diverse testing strategies.
Our study has some limitations. First, we chose to use a broader range of ARI ICD codes, instead of using RSV-specific ICD codes, so some of the ARI cases were likely not caused by RSV. We did this to keep the ARI definition as sensitive as possible to capture all levels of RSV severity; we accounted for the potential misclassification by using a very specific laboratory-confirmed RSV definition. Second, we did not include pharmaceutical costs or laboratory costs, as they are not captured in the MACARS database, and we therefore underestimated case costs of RSV. Previous research has demonstrated that, although prescriptions are not a large cost driver, they still contribute to 5–16% of total costs, depending on age group [29]. Third, we did not have Alberta-specific laboratory data for all respiratory tests for 2010 through 2019; as such, we used national estimates to calculate the RSV test positivity rate. Moreover, as noted, changes and inconsistencies in laboratory testing strategies can have a substantial impact on our estimates of RSV incidence rates, and more research is needed in this area. Finally, national RSV test rates were not age specific and so we could not capture differences in positivity rate by age group.
5 Conclusions
While RSV costing studies have largely focused on infants and hospitalized cases, this study demonstrates that older adults have some of the highest costs associated with RSV disease, both for laboratory-confirmed cases and ARI associated with RSV. Therefore, immunization programs targeting infants and/or older populations may be important for the reduction of the overall health and economic impact of RSV infection. Our findings further suggest that, if vaccine allocation decisions are needed, a viable strategy would be to focus on older adults with multiple comorbidities, as they have some of the highest RSV-attributable costs per case. In children, since the youngest age groups have some of the highest RSV-attributable costs, a maternal vaccine may be a worthwhile strategy to prevent these cases and their associated costs. However, given the timing of maternal vaccination, it may not offer full protection against RSV in very premature infants, in which case there may be a role for monoclonal antibody products. In addition, although RSV infection rates are undeniably high in infants and young children, we found that RSV remains a relatively common infection in all age groups and is associated with high healthcare costs. These findings together suggest we need to look at the burden of disease beyond the youngest age groups to appropriately target future immunization programs.
References
Henderson FW, Collier AM, Clyde WAJ, Denny FW. Respiratory synctial virus infections, reinfections and immunity. N Engl J Med. 1979;300:530–4.
Colosia AD, Yang J, Hillson E, Mauskopf J, Copley C, Shinde V, et al. The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: a systematic review. PLoS ONE. 2017;12:1–20.
Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018;18:e295-311. https://doi.org/10.1016/S1473-3099(18)30292-5.
Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. https://doi.org/10.1056/NEJMoa0804877.
Simpson MD, Kieke BA, Sundaram ME, McClure DL, Meece JK, Sifakis F, et al. Incidence of medically attended respiratory syncytial virus and influenza illnesses in children 6–59 months old during four seasons. Open Forum Infect Dis. 2016;3:1–6. https://doi.org/10.1093/ofid/ofw081.
Fowlkes A, Giorgi A, Erdman D, Temte J, Goodin K, Di Lonardo S, et al. Viruses associated with acute respiratory infections and influenza-like illness among outpatients from the influenza incidence surveillance project, 2010–2011. J Infect Dis. 2014;209:1715–25. https://doi.org/10.1093/infdis/jit806.
Chan P-K, Abdel-Latif M-A. Cost of hospitalization for respiratory syncytial virus chest infection and implications for passive immunization strategies in a developing nation. Acta Paediatr. 2007;92:481–5. https://doi.org/10.1111/j.1651-2227.2003.tb00582.x.
Homaira N, Oei J, Mallitt K, Abdel-Latif M, Hilder L, Bajuk B, et al. High burden of RSV hospitalization in very young children: a data linkage study. Epidemiol Infect. 2016;144:1612–21. https://doi.org/10.1017/S0950268815003015.
McLaurin K, Farr A, Wade S, Diakun D, Stewart D. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol. 2016;36:990–6. https://doi.org/10.1038/jp.2016.113.
Ledbetter J, Brannman L, Wade SW, Gonzales T, Kong AM. Healthcare resource utilization and costs in the 12 months following hospitalization for respiratory syncytial virus or unspecified bronchiolitis among infants. J Med Econ. 2020;23:139–47. https://doi.org/10.1080/13696998.2019.1658592.
Langley JM, Wang EEL, Law BJ, Stephens D, Boucher FD, Dobson S, et al. Economic evaluation of respiratory syncytial virus infection in Canadian children: a Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. J Pediatr. 1997;131:113–7. https://doi.org/10.1016/S0022-3476(97)70133-1.
Banerji A, Lanctôt KL, Paes BA, Masoud ST, Tam DY, MacDonald WA, et al. Comparison of the cost of hospitalization for respiratory syncytial virus disease versus palivizumab prophylaxis in Canadian Inuit infants. Pediatr Infect Dis J. 2009;28:702–6. https://doi.org/10.1097/INF.0b013e31819df78e.
Public Health Agency of Canada (PHAC). Respiratory virus detections in Canada 2020. 2020. https://www.canada.ca/en/public-health/services/surveillance/respiratory-virus-detections-canada.html. Accessed 23 Mar 2020.
Canadian Institute of Health Information. CMG + Methodology Overview. 2012.
Canadian Institute of Health Information. Comprehensive Ambulatory Classification System (CACS). 2020. https://www.cihi.ca/en/comprehensive-ambulatory-classification-system-cacs. Accessed 30 Sep 2020.
Jacobs P, Budden A, Lee K. Guidance document for the costing of health care resources in the Canadian Setting. 2nd edition. Ottawa; 2016.
Schanzer DL, Langley JM, Tam TWS. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children. Pediatr Infect Dis J. 2006;25:795–800. https://doi.org/10.1097/01.inf.0000232632.86800.8c.
Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatr Infect Dis J. 2012;31:5–9. https://doi.org/10.1097/INF.0b013e31822e68e6.
Alberta Health Services. Alberta Respiratory Syncytial Virus (RSV) prevention program information for health professionals. 2022. https://www.albertahealthservices.ca/info/rsvprogram.aspx. Accessed 31 Jan 2022.
Ontario Ministry of Health. Respiratory Syncytial Virus prophylaxis for high-risk infants program reference manual. 2019.
Charlson M, Wells MT, Ullman R, King F, Shmukler C. The Charlson comorbidity index can be used prospectively to identify patients who will incur high future costs. PLoS ONE. 2014;9:1–16. https://doi.org/10.1371/journal.pone.0112479.
Charlson M, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Shing E, Wang J, Nelder MP, Parpia C, Gubbay JB, Loeb M, et al. The direct healthcare costs attributable to West Nile virus illness in Ontario, Canada: a population-based cohort study using laboratory and health administrative data. BMC Infect Dis. 2019;19:1059. https://doi.org/10.1186/s12879-019-4596-9.
Fauroux B, Simões EAF, Checchia PA, Paes B, Figueras-Aloy J, Manzoni P, et al. The burden and long-term respiratory morbidity associated with Respiratory Syncytial Virus infection in early childhood. Infect Dis Ther. 2017;6:173–97. https://doi.org/10.1007/s40121-017-0151-4.
Simões EAF, Chirikov V, Botteman M, Kwon Y, Kuznik A. Long-term assessment of healthcare utilization 5 years after Respiratory Syncytial Virus infection in US infants. J Infect Dis. 2020;221:1256–70. https://doi.org/10.1093/infdis/jiz278.
Statistics Canada. Consumer Price Index, monthly, percentage change, not seasonally adjusted, Canada, provinces, Whitehorse and Yellowknife—Health and personal care. 2020. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000408&pickMembers%5B0%5D=1.2&cubeTimeFrame.startMonth=01&cubeTimeFrame.startYear=2019&referencePeriods=20190101%2C20190101.
Muenchhoff M, Goulder PJR. Sex differences in pediatric infectious diseases. J Infect Dis. 2014;2014:209. https://doi.org/10.1093/infdis/jiu232.
Groeneveld JM, Ballering AV, van Boven K, Akkermans RP, Olde Hartman TC, Uijen AA. Sex differences in incidence of respiratory symptoms and management by general practitioners. Fam Pract. 2020;37:631–6. https://doi.org/10.1093/FAMPRA/CMAA040.
Amand C, Tong S, Kieffer A, Kyaw MH. Healthcare resource use and economic burden attributable to respiratory syncytial virus in the United States: a claims database analysis. BMC Health Serv Res. 2018;18:294. https://doi.org/10.1186/s12913-018-3066-1.
Pisesky A, Benchimol EI, Wong CA, Hui C, Crowe M, Belair M-A, et al. Incidence of hospitalization for respiratory syncytial virus infection amongst children in Ontario, Canada: a population-based study using validated health administrative data. PLoS ONE. 2016;11: e0150416. https://doi.org/10.1371/journal.pone.0150416.
McClure DL, Kieke BA, Sundaram ME, Simpson MD, Meece JK, Sifakis F, et al. Seasonal incidence of medically attended respiratory syncytial virus infection in a community cohort of adults ≥50 years old. PLoS ONE. 2014;9: e102586. https://doi.org/10.1371/journal.pone.0102586.
Pitzer VE, Viboud C, Alonso WJ, Wilcox T, Metcalf CJ, Steiner CA, et al. Environmental drivers of the spatiotemporal dynamics of respiratory syncytial virus in the United States. PLoS Pathog. 2015;11:1–14. https://doi.org/10.1371/journal.ppat.1004591.
Robinson JL, Nicole LS, Canadian Pediatric Society Infectious Diseases and Immunization Committee. Preventing hospitalizations for respiratory syncytial virus infection. Clin Cancer Res 2015;20:321–33.
Acknowledgements
We extend our sincere thanks to the members of the Epidemiology & Surveillance team in Analytics and Performance Reporting at Alberta Health for their support of this work.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This research was supported by a grant from the Canadian Immunization Research Network, grant number: MD05 AB16.
Conflict of interest
ER, MP, SAB, JR, JAB, MK, LWS, and SEM have no conflicts of interest that are directly relevant to the content of this article.
Availability of data and material
Individual-level data are held by the Government of Alberta Ministry of Health and therefore are not available publicly. Additional aggregate level data are available on author request.
Ethics approval
Ethics approval was obtained from the University of Alberta Human Research Ethics Board, ID: Pro00102401
Consent
Not applicable.
Author contributions
LWS acquired the data. ER & SEM designed the analysis. ER conducted the analysis and completed initial interpretation of the data. ER wrote the first draft of the manuscript. SEM, LWS & MP supervised the work. All authors (ER, MP, SAB, JLR, JAB, MK, LWS, SEM) contributed to conceptualizing the project, interpreting the data, drafting the manuscript and approving of the final submitted version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rafferty, E., Paulden, M., Buchan, S.A. et al. Evaluating the Individual Healthcare Costs and Burden of Disease Associated with RSV Across Age Groups. PharmacoEconomics 40, 633–645 (2022). https://doi.org/10.1007/s40273-022-01142-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-022-01142-w