Abstract
Objective
Given the high economic burden of disease among adult patients with chronic heart failure with reduced ejection fraction (HFrEF) following a worsening heart failure event in the US, this study aimed to estimate the cost effectiveness of vericiguat plus prior standard-of-care therapies (PSoCT) versus PSoCT alone from a US Medicare perspective.
Methods
A four-state Markov model (alive prior to heart failure hospitalization, alive during heart failure hospitalization, alive post-heart failure hospitalization, and death) was developed to predict clinical and economic outcomes, based on the results of the VICTORIA trial, in which patients with chronic HFrEF following a worsening heart failure were randomized to placebo or vericiguat, in addition to PSoCT, which consisted of β-blockers, renin-angiotensin-aldosterone inhibitors, mineralocorticoid receptor antagonists, and the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan. Risks of heart failure hospitalization and cardiovascular mortality were based on multivariable regression models derived from VICTORIA data. Utilities were derived from VICTORIA EQ-5D data and the literature. Costs included drug acquisition, heart failure hospitalization, routine care, and terminal care. Primary outcomes included heart failure hospitalization, cardiovascular mortality, life-years, quality-adjusted life-years (QALYs), and incremental costs per QALY gained over a 30-year lifetime horizon, discounted at 3.0% annually.
Results
For the VICTORIA overall intent-to-treat population, compared with PSoCT, vericiguat plus PSoCT resulted in 19 fewer heart failure hospitalizations and 13 fewer cardiovascular deaths per 1000 patients, as well as 0.28 QALY gained per patient at an incremental cost of $23,322, leading to $82,448 per QALY gained.
Conclusions
Based on the results of VICTORIA, patients treated with vericiguat had lower rates of heart failure hospitalization and cardiovascular death. The addition of vericiguat to PSoCT was estimated to increase QALYs and to be cost effective at a willingness-to-pay threshold of $100,000 per QALY gained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Given the high economic burden in patients with heart failure with reduced ejection fraction (HFrEF) who have recently experienced worsening HF event(s) in the US, this study intends to assess whether vericiguat is cost effective as an add-on therapy to prior standard-of-care therapies (PSoCT) versus PSoCT alone in the VICTORIA trial population from a US Medicare perspective. |
Vericiguat plus PSoCT compared with PSoCT alone resulted in an incremental cost of $82,448 per QALY gained. |
Vericiguat plus PSoCT was estimated to result in longer life expectancy and to be cost effective at a willingness-to-pay threshold of $100,000 per QALY gained in the overall VICTORIA intent-to-treat population. |
1 Introduction
Heart failure (HF) is an increasingly common condition in the US, with a projected prevalence of 8 million adults by the year 2030 [1]. Approximately 31–56% of patients with HF have reduced ejection fraction (rEF), defined as a left ventricular ejection fraction (LVEF) of 40% or less [2,3,4,5,6,7,8]. Despite the use of prior standard-of-care therapies (PSoCT)—which may consist of β-blockers (BBs), renin-angiotensin-aldosterone (RAAS) inhibitors, mineralocorticoid receptor antagonists (MRAs), and angiotensin receptor–neprilysin inhibitors (ARNIs)—patients with HF may experience multiple worsening HF events (WHFEs), such as unscheduled HF hospitalization (HFH) or initiation of intravenous diuretic therapy [9, 10]. Approximately 30% of patients with chronic HF with rEF (HFrEF) experience at least one WHFE within the first year of diagnosis [11, 12], and each event places patients at increased risk of additional events [13, 14].
WHFEs negatively impact prognosis and are associated with considerable clinical and economic burden in the US. The 2-year mortality rate in patients with HFrEF who experienced a WHFE was estimated at 23% [14, 15]. Patients with HFrEF who experience a WHFE are at high risk for recurrent HFH [14], and after each subsequent HFH, the mortality rates significantly increase [16, 17]. Inpatient hospitalization costs were the primary driver of total costs in HF [11, 18,19,20], and direct medical costs of HF in the US were projected to increase from $20.9 billion in 2012 to $53.1 billion in 2030 [20]. Medical resource use and costs are also significantly higher for patients with chronic HFrEF following a WHFE compared with patients with stable chronic HFrEF [11, 12], therefore further treatment options are needed in this population.
Vericiguat, a soluble guanylate cyclase (sGC) stimulator, was approved by the US FDA in January 2021 and is indicated to reduce the risk of cardiovascular (CV) death and HFH following a hospitalization for HF or need for outpatient intravenous diuretics in adults with symptomatic chronic HF and ejection fraction <45%. The approval was based on the results of the pivotal VICTORIA trial (NCT02861534).
VICTORIA was a randomized, placebo-controlled, double-blind, event-driven, multicenter, phase III clinical outcomes trial that tested the efficacy and safety of vericiguat versus placebo on top of PSoCT [9]. VICTORIA had a specific population of patients living with HFrEF who had a recent HFH of <6 months or outpatient intravenous diuretic therapy for <3 months. Vericiguat reduced the primary composite outcome of CV death or HFH compared with placebo (897 of 2526 patients in the vericiguat group vs. 972 of 2524 patients in the placebo group; hazard ratio 0.90, 95% confidence interval 0.82–0.98; p = 0.02).
Given the economic burden of HFrEF following a WHFE, it is important for US payers and other healthcare decision makers to understand the clinical and economic benefits of adding vericiguat to the formulary for treatment in this population. Thus, the objective of this study was to estimate the cost effectiveness of vericiguat in US patients with HFrEF following a WHFE.
2 Methods
2.1 Overview
An HF disease simulation model was developed in Microsoft Excel 365 (Microsoft Corporation, Redmond, WA, USA) to assess the cost effectiveness of vericiguat plus PSoCT versus PSoCT alone for the treatment of adult patients with chronic HFrEF (LVEF <45%) following a WHFE, in line with the definitions of patient population and PSoCT in the VICTORIA trial [9]. The analysis was conducted from a US Medicare perspective over a lifetime horizon, which was assumed to be 30 years, based on the baseline mean age of 67.3 years of the VICTORIA intent-to-treat (ITT) population [9]. This assumption was also supported by the simulation result that more than 99.9% of patients would experience mortality within 30 years since model baseline. Cost and clinical outcomes were discounted at 3.0% annually, in line with the Institute for Clinical and Economic Review’s Reference Case for Economic Evaluations [21].
2.2 Model Structure
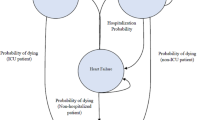
A multistate Markov model with four mutually exclusive states (i.e., alive prior to the first HFH since baseline, alive during the first HFH, alive post the first HFH, and dead) was developed to predict the occurrence of HFH and CV death and to estimate the associated costs (Fig. 1). At model baseline, all patients started in the ‘alive prior to the first HFH’ health state and moved through the model’s health states according to a set of transition probabilities on a cycle basis (see more details of health state transition in the Supplementary Methods section of the electronic supplementary material [ESM]). A cycle length of 1 month was adopted because those in the VICTORIA trial population were considered to be in an advanced disease stage and the duration of the first HFH is explicitly restricted to be, at most, 30 days. Furthermore, this cycle length provides the appropriate level of detail in the model. Half-cycle correction was applied in the model. The proportion of patients occupying the four different health states in each model cycle was applied to estimate the accrued costs and clinical outcomes of the patient cohort over time.
Markov model structure. Note: Transitions to death were based on two components: CV death (based on risk equations from VICTORIA) and non-CV death (based on a US life table adjusted by removing CV mortality). Details of each health state transition are provided in the Supplementary Methods section in the electronic supplementary material. HFH heart failure hospitalization, CV cardiovascular
2.3 Model Inputs
2.3.1 Heart Failure Hospitalization (HFH), Cardiovascular (CV) Death, and Non-CV Death
The clinical outcomes of HFH and CV death in the ITT population of the VICTORIA trial were used to inform the base-case analysis. Multivariable regression models fitted to VICTORIA trial data were applied to predict HFH and CV death over time, with treatment arm (i.e., vericiguat plus PSoCT versus PSoCT alone) and baseline patient characteristics included as covariates (Table 1; Supplementary Table 1 and Supplementary Table 2 in the ESM). Four regression models were applied to inform four health state transitions: (1) first HFH after inclusion into VICTORIA since trial baseline; (2) risk of CV death among patients who had not experienced the first HFH since trial baseline; (3) risk of CV death among patients during the first HFH; and (4) risk of CV death among patients who had experienced the first HFH. These transitions were derived from (1) a parametric regression model with an exponential distribution; (2) a parametric regression model with a generalized gamma distribution; (3) a logistic regression model; and (4) a parametric regression model with a Gompertz distribution, respectively (Supplementary Fig. 1 in the ESM) in the base-case analysis. The selection of parametric distributions was based on clinical assumption as assessed via clinical experts’ opinions. Specifically, two clinical assumptions were made: (1) the baseline risks of CV mortality and HFH would increase over time (age), in line with prior cost-effectiveness models for HFrEF [22,23,24]; and (2) the life expectancy of the modeled population was expected to be relatively short, given that the VICTORIA trial population represented patients who had experienced a WHFE with a poor prognosis.
For transition to the first HFH after inclusion into VICTORIA, the exponential distribution was applied, as all other distributions were ruled out due to a lack of clinical plausibility. More specifically, all other distributions were associated with decreasing hazards in the long term that were either monotonically decreasing or first increasing for a short period of time (<3 months) and then decreasing for the rest of the lifetime horizon. Therefore, the exponential distribution was considered the most clinically plausible one among all the distributions and was therefore applied in the base-case analysis. The risk of CV death among patients during the first HFH was modeled by using logistic regression because the first HFH was restricted to a very short time (at most 30.4375 days). For the risk of CV death among patients who were not in HFH, similar to the risk of HFH, the distributions with increasing hazards were applied in the base-case and scenario analyses. More details of the regression models are provided in the Supplementary Methods section of the ESM, including, but not limited to, the coefficient estimates and the functions used to derive the health state transition probabilities by parametric distribution.
Non-CV mortality was based on US life tables adjusted by removing CV mortality [25, 26], in line with previous cost-effectiveness analyses in HFrEF [23, 24, 27, 28]. The model did not include adverse events, given that there was no significant difference in safety profiles between the vericiguat plus PSoCT and placebo plus PSoCT arms in VICTORIA [9].
2.3.2 Utilities
To estimate the quality-adjusted life-years (QALYs) for patients over time, we multiplied the time spent in each health state by the utility associated with each health state. A utility of 0 usually represents death and a utility of 1 represents perfect health. The utility for health states not in HFH (i.e., alive pre- or post-HFH) was derived from the baseline EQ-5D-5L in the VICTORIA ITT population using the US value set (Table 2). As patients who were hospitalized due to HF were expected to have poorer health-related quality of life (HRQoL) than patients not in HFH, a utility decrement was applied on top of the utility without HFH to estimate the utility with HFH. Due to a lack of EQ-5D measurement during HFH in the VICTORIA trial to capture the acute effect of HFH on quality of life, the utility decrement of HFH was based on external literature [22] (Table 2).
2.3.3 Healthcare Resource Use and Cost Inputs
The model included costs for drug acquisition, HFH, routine care, and terminal care. Cost inputs were inflated to 2020 US dollars using the medical care component of the Consumer Price Index [29].
Daily drug acquisition costs for vericiguat and each individual PSoCT (including angiotensin-converting enzyme inhibitors [ACEi] or angiotensin receptor blockers [ARBs], BBs, sacubitril/valsartan, and MRAs) (Table 2) were calculated as the product of cost per tablet and number of tablets per day for each drug (Supplementary Table 3 in the ESM). Target doses of PSoCT and vericiguat were obtained from the products’ prescribing information [30,31,32,33,34]. Drug wholesale acquisition costs (WACs) and package information for PSoCT drugs and vericiguat were obtained from the RED BOOK [35]. It was also assumed that patients would initiate treatment upon model entry and continue until death, in line with drug prescribing information where no discontinuation rule was specified [30,31,32,33,34]. A one-time cost per HFH [29, 36] was applied upon HFH admission; a monthly routine care cost [11, 29, 36] was applied for as long as a patient remained alive; and a one-time terminal care cost [29, 37] was applied upon death, regardless of the cause of death (Table 2).
2.4 Outcomes
Clinical and economic outcomes were compared between the vericiguat plus PSoCT arm and the PSoCT-alone arm, including the average number of HFHs and CV deaths per 1000 patients, as well as average life-years, QALYs, and costs per patient. Per patient incremental cost per QALY gained was estimated, defined as the difference in total costs divided by the difference in total QALYs between the two treatment arms. A willingness-to-pay (WTP) amount of $100,000 per QALY gained was used to assess a strategy’s cost effectiveness, an incremental cost per QALY below which is considered cost effective [21].
2.5 Sensitivity Analyses
Scenario analyses were conducted for structural or input assumptions, including time horizon, annual discounting rates for future costs and clinical outcomes, alternative distributions of parametric regression models that were potentially clinically plausible, potential drug cost discount for both vericiguat and sacubitril/valsartan, and medical cost inputs.
Uncertainty of quantitative parameters was assessed via univariate (or one-way) and probabilistic sensitivity analyses (PSA). The uncertainty around the central estimate was set according to variance information provided in the original source. Where variance information was not available (for three parameters: HFH cost, routine care cost, and terminal care cost), the standard error was typically assumed to be 20% of the mean. For probabilities (including the proportion of patients treated with each individual PSoCT component) and utility of being alive but not in HFH, a beta distribution was used. For disutility of HFH, costs, and resource use estimates, a gamma distribution was fitted. For regression models, multivariate normality on the scale of estimation was assumed using the Cholesky decomposition approach employed to capture the correlation structure in the variance–covariance matrix [38]. Detailed standard error and distribution information for the model parameters varied in univariate sensitivity analyses and PSA is provided in Supplementary Table 6 in the ESM.
The results of the univariate sensitivity analyses, in which each parameter was varied within the 95% confidence interval, were presented using tornado plots to show the top parameters that created the widest range in model results. The PSA sampled from the distribution of each model parameter 1000 times. The results of each probabilistic model run were presented via the cost-effectiveness acceptability curve.
3 Results
3.1 Base Case
In the base case over a 30-year lifetime horizon, our model predicted 681 HFHs and 427 CV deaths per 1000 patients treated with vericiguat plus PSoCT, compared with 700 HFHs and 440 CV deaths per 1000 patients treated with PSoCT alone, resulting in 19 fewer HFHs and 13 fewer CV deaths with vericiguat plus PSoCT. A discounted average of 4.18 years of survival and 3.34 QALYs was estimated per patient treated with vericiguat plus PSoCT compared with 3.82 years of survival and 3.05 QALYs per patient treated with PSoCT alone, resulting in an incremental gain of 0.35 years of survival and 0.28 QALYs with vericiguat plus PSoCT. Treatment with PSoCT was associated with lifetime costs of $60,228 per patient. The addition of vericiguat increased the lifetime costs by $23,322, including an additional $22,526 in drug acquisition costs and $1093 in routine care costs, but with savings of $202 and $95 in HFH and terminal care costs, respectively. At the commonly accepted WTP threshold of $100,000 per QALY gained in the US [21], adding vericiguat to PSoCT is considered cost effective ($82,448 per QALY gained) [Table 3].
3.2 Scenario Analyses
The costs per QALY gained from treatment with vericiguat plus PSoCT were estimated to be $90,494 and $82,557 over 10- and 20-year time horizons, respectively. Use of alternative parametric distributions for risks of CV death post-HFH was associated with costs of $76,049–$89,004 per QALY gained. Using alternative Medicare medical costs and commercial payer-specific medical costs led to $80,507 and $80,983 per QALY gained, respectively. In a more conservative scenario where terminal care cost was set as zero, the cost per QALY gained was estimated to be $82,785. Decreasing the WAC of both vericiguat and sacubitril/valsartan by 20, 30, and 35% was associated with costs of $85,329, $74,720, and $69,415 per QALY gained, respectively, in comparison with $82,448 in the base case, where WAC was decreased by 25% (Table 4, Supplementary Table 7).
3.3 Univariate Sensitivity Analyses
The results of the univariate sensitivity analyses showed that the costs per QALY gained were most sensitive to the coefficients for (1) rate of the Gompertz distribution related to the risk of CV death post HFH; (2) baseline predose sodium level related to the risk of CV death post HFH; and (3) interaction between the treatment and baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) level related to the risk of first HFH after inclusion into VICTORIA (Fig. 2).
Univariate sensitivity analyses for parameter impact on incremental costs per QALY gained. Notes: The top 10 most impactful parameters included in univariate sensitivity analyses are shown above. For the list of parameters included in the analyses and the associated deterministic point estimates, standard errors, and distributions, please refer to Supplementary Table 6 in the electronic supplementary material. In these sensitivity analyses, negative costs per QALY gained indicate that the vericiguat plus PSoCT arm is dominated by PSoCT alone. CV cardiovascular, exp. Exponential, Gen. gamma generalized gamma, HFH heart failure hospitalization, NT-proBNP N-terminal pro-B-type natriuretic peptide, PSoCT prior standard-of-care therapies, QALY quality-adjusted life-year
3.4 Probabilistic Sensitivity Analyses
At a WTP threshold of $100,000 per QALY gained, the probability of vericiguat plus PSoCT being cost effective compared with PSoCT alone was estimated to be 70%; however, at a WTP threshold of $150,000, the probability of vericiguat plus PSoCT being cost effective increased to 88% (Supplementary Figs. 2 and 3 in the ESM).
3.5 Validation
Both internal and external validations were conducted. For external validation, as discussed earlier, the selection of parametric regression models was based on clinical experts’ opinions regarding clinical plausibility. In particular, we consulted with cardiologists and clinical experts in the field of HF through virtual meetings, during which we presented all the fitted parametric regression models (i.e., exponential, Weibull, Gompertz, log-normal, log-logistic, gamma, and generalized gamma) for each health state transition to ensure appropriate hazard patterns over time. The projected outcomes were plausible from a clinical standpoint. A further external validation with real-world data was planned but could not yet be carried out due to a paucity of data on survival outcomes in the VICTORIA patient population. Prior studies in HFrEF—both clinical and observational—included relatively heathier HFrEF populations and therefore cannot be used for the validation of our model results. In addition, the predicted life expectancy was validated against previous US cost-effectiveness analyses for patients with HFrEF [22, 28, 39, 40].
For internal validation, a comparison of the predicted model results and the observed trial results per the study by Armstrong et al. [9] is provided (Supplementary Table 5) and shows an alignment in the results, especially for the incremental outcomes between the two treatment arms at 12 months. In addition to clinical inputs, the medical cost and HRQoL inputs applied in the base-case and scenario analyses were validated through a review of two recently published systematic literature reviews for costs and utilities in patients with HF [41, 42], which showed that the input values in our model were plausible. Lastly, a quality check of the model was conducted using the TECHnical VERifcation (TECH-VER) checklist [43].
4 Discussion
Following FDA approval based on the results of the VICTORIA trial, vericiguat represents an innovative treatment that fills the medical needs for a distinct HFrEF population with recent worsening HF and higher natriuretic peptide levels, i.e., a sicker population associated with a higher economic burden compared with the HFrEF populations in other recent trials (e.g., DAPA-HF and PARADIGM-HF). Our analysis suggests that vericiguat plus PSoCT could be a cost-effective treatment option for patients with chronic HFrEF following a WHFE, compared with PSoCT alone. Using VICTORIA trial data in the model, we estimated, over a 30-year lifetime horizon, vericiguat plus PSoCT would result in 19 fewer HFHs and 13 fewer CV deaths per 1000 patients, and an additional 0.28 QALYs gained per patient at an incremental cost of $82,448 per QALY gained.
Sensitivity analyses showed that the results of the base-case analysis were robust. In the scenario analyses in which time horizon, annual discounting, drug acquisition costs of both vericiguat and sacubitril/valsartan, parametric distribution for risk of CV death, and medical cost inputs were varied, vericiguat plus PSoCT was consistently associated with fewer HFHs and CV deaths compared with PSoCT alone, with incremental costs per QALY gained lower than $100,000. The PSA results showed that the probability of vericiguat plus PSoCT being cost effective was 70% at a WTP threshold of $100,000 per QALY gained. Univariate sensitivity analyses showed that the costs per QALY gained were most sensitive to the variation in the coefficients of risk equations, e.g., the coefficient of baseline predose sodium level related to the risk of CV death post HFH, and the coefficient of interaction between vericiguat treatment effect and baseline NT-proBNP level related to the risk of first HFH. These univariate sensitivity analyses are important given the consistency with the subgroup analysis of VICTORIA. Vericiguat was associated with a more favorable treatment effect in patients with a lower baseline NT-proBNP compared with patients who had a higher baseline NT-proBNP level [9].
The lifetime estimates of life expectancy, QALYs, and costs for PSoCT alone from our analysis were slightly lower than those reported in previous US cost-effectiveness analyses for patients with HFrEF [22, 28, 39, 40] (Supplementary Table 3 in the ESM). For example, our estimate of discounted life expectancy of 3.82 years for PSoCT was slightly lower than previous estimates (6.04–8.40 years over a lifetime horizon) [22, 28, 39, 44], while our estimate of 3.05 discounted QALYs for PSoCT was also lower than that in the literature (4.53–6.02 QALYs over a lifetime horizon) [22, 28, 39, 40, 44]. Such differences were likely due to the fact that the VICTORIA population had recently experienced a WHFE [9] and therefore had worse prognoses and clinical outcomes. Furthermore, the event rate in the control arm of VICTORIA [9] was much higher than that of the other trials in HFrEF, such as PARADIGM-HF [45] and DAPA-HF [46]. In addition, unlike the previous analyses that examined ACEi specifically [22, 28, 39, 40], the PSoCT-alone arm of our analyses included a mix of ACEi, ARBs, BBs, sacubitril/valsartan, and MRAs. In addition, consistent with the comparisons above of life-years and QALYs, our estimate of the discounted cost of $21,685 for PSoCT was lower than that in most of the prior models ($83,303–$145,371 over a lifetime horizon) [22, 39, 40] One exception is one model that reported a cost of $21,758 for the standard-of-care arm [28], likely driven by the fact that the longer patients lived, the more costs would be incurred over time, and vice versa. Another reason might be that our study adopted a Medicare perspective in which lower medical cost inputs were applied, whereas the prior studies adopted a general US payer perspective without being restricted to public or private payers.
Our analysis has several strengths. First, in contrast to many previous cost-effectiveness analyses in HFrEF that adopted a Markov model based on either the occurrence of mortality (with two health states: alive, death) [23, 24, 40], the progression per New York Heart Association (NYHA) class [28, 47] and/or treatment intolerance (Supplementary Table 5 in the ESM) [22, 47], this analysis adopted a four-state Markov model based on the occurrence of HFH and CV death (alive prior to the first HFH, alive during the first HFH, alive post the first HFH, and death), which better reflected the clinical pathway of patients with chronic HFrEF, especially for patients who had experienced a WHFE (e.g., the VICTORIA trial population) who were at higher risk of CV mortality pre and post the next WHFE. More specifically, as shown in Supplementary Fig. 1 in the ESM, patients who had not experienced HFH since the model entry were associated with a lower risk of CV death (indicated by the slower drop of survival curves) compared with the patients in or post-HFH (with a faster drop of survival curves). By modeling separately patients who had or had not experienced HFH since the model baseline, this model allows for the differentiation of these patients regarding CV mortality. This is particularly important for the VICTORIA trial population, who were relatively sicker (driven by the recent WHFE experience right before the trial randomization) and therefore at higher risk of CV death post any WHFE compared with the HFrEF population included in other trials (e.g., PARADIGM-HF, SHIFT, or DAPA-HF) and modeled for cost effectiveness [22, 28, 39, 40, 44]. In contrast, the previously commonly adopted Markov model structure used to model based on the PARADIGM-HF, SHIFT, or DAPA-HF trial populations (relying on the occurrence of mortality, NYHA class progression, transplantation, or treatment intolerance) [22,23,24, 28, 40, 44, 47] would not be able to capture the differences in baseline risk and treatment effect pre versus post the first HFH, and was therefore less appropriate for this model specific to the VICTORIA trial population.
Second, the analysis relied on clinical experts’ opinions as to which parametric distributions (e.g., Gompertz) were associated with clinically plausible outcomes of HFH and CV death over time. This led to more clinically relevant choices of the analysis results. Third, by including baseline patient characteristics that were key prognostic factors as covariates in the parametric models for HFH and CV death, this analysis captured the effect of these factors on long-term outcomes, which may be more clinically meaningful.
Despite the strengths and robustness of the base-case results, as demonstrated by sensitivity analyses above, some limitations should be considered when interpreting the results. First, this analysis relied on VICTORIA trial data with a median follow-up of 10.8 months [9] to predict the risks of HFH and CV death over a lifetime horizon, which could lead to uncertainty of long-term extrapolation. In particular, at the end of the trial follow-up, 27.4–29.6% of VICTORIA patients had the first HFH since trial baseline, while CV death occurred in 16.4–17.5% of patients [9]. Still, this is a common limitation of most disease simulation models, and the uncertainty of extrapolation has been examined through both clinical validation by clinicians and statistical assessment via the PSA.
Second, similar to the first limitation above, due to the limited number of events observed within the VICTORIA trial period, this analysis did not capture the subsequent HFH, despite the expectation that patients with HF would experience multiple WHFEs (i.e., HFH or outpatient initiation of intravenous diuretic therapy) [9, 10] and that each WHFE could increase the risk of additional WHFEs [13, 14].
Third, no real-world data are currently available on vericiguat adherence to understand the potentially waning differences in treatment effect for those who discontinued over time. Therefore, adherence was not considered in the analysis. When real-world data become available, a further external validation for the current model is advisable on survival and HFH outcomes for the VICTORIA patient population.
Fourth, this study did not model any numeric differences in adverse events, given that the VICTORIA trial did not show statistically significant differences in the safety profiles of the vericiguat plus PSoCT and placebo plus PSoCT arms (32.8% of patients in the vericiguat arm experienced at least one serious adverse event vs. 34.8% in the placebo arm) [9].
Fifth, the results of this model based on VICTORIA trial data may not always be generalizable to the real-world HFrEF population in the case of evolving treatment paradigms. For example, emerging treatments such as sodium-glucose cotransporter-2 (SGLT2) inhibitors were not generally used in VICTORIA because SGLT2 inhibitors were not part of the guideline-directed medical therapies at the time of the trial. However, a small fraction of patients did use SGLT2 inhibitors in VICTORIA, with a balance between both groups. Despite the recent FDA approval of the SGLT2 inhibitors dapagliflozin and empagliflozin, it might take time for SGLT2 inhibitors to be widely used in real-world practice. There are a lack of clinical or real-world data regarding whether vericiguat would be prescribed on top of newer treatments or in lieu of them.
Furthermore, while we adopted a US Medicare perspective to be aligned with the baseline mean age of the VICTORIA population and applied Medicare-specific medical cost inputs, the costs could vary by health plan and site of care. Therefore, we conducted scenario analyses to examine the impact of using alternative Medicare costs and commercial payer-specific costs separately, which showed that the medical costs had limited impact on the model results. In addition, we expected that the cost of hospital-based terminal care could differ from the cost in the community setting. Therefore, we also conducted a conservative scenario analysis by zeroing out terminal care cost from the model, which had only a limited effect on the model results. In addition, for the drug costs, we relied on WAC and assumed a discount of 25% to approximate the net price for both vericiguat and sacubitril/valsartan, as the net price of the recently launched vericiguat was not available at the time of this analysis. To mitigate this limitation, scenario analyses were conducted to examine the impact of varying the drug discount on the model results.
Finally, non-drug costs can be determined based on different sources, as recently consolidated [42]. We chose to base our model on the most recently published HCRU cost data in the US that further allow to distinguish Medicare patients who had, versus those who did not have, a recent worsening HF event based on the unique population described [11, 36].
5 Conclusions
VICTORIA enrolled a population with recent worsening HF and higher natriuretic peptides and consequently addresses a previous research gap. Our cost-effectiveness model leverages risk equations to allow representative disease progression modeling for a multifactorial condition such as HFrEF.
Over a 30-year lifetime horizon, vericiguat plus PSoCT was associated with gains in life expectancy and QALYs and an incremental cost of $82,488 per QALY gained compared with PSoCT alone in the VICTORIA ITT population in the base case. Sensitivity analyses showed that the base-case analysis results were robust and suggest that vericiguat on top of PSoCT is cost effective at a WTP threshold of $100,000 in the US. It will be important to understand the real-world effectiveness of vericiguat in the upcoming years and to update our cost-effectiveness analysis with recent real-world data once they become available.
References
Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596.
Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996–1004.
Gurwitz JH, Magid DJ, Smith DH, et al. Contemporary prevalence and correlates of incident heart failure with preserved ejection fraction. Am J Med. 2013;126(5):393–400.
Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure. Circ Heart Fail. 2013;6(2):279–86.
Ibrahim NE, Song Y, Cannon CP, et al. Heart failure with mid-range ejection fraction: characterization of patients from the PINNACLE Registry®. ESC Heart Fail. 2019;6(4):784–92.
Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476–86.
Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction. Circulation. 2012;126(1):65–75.
Vasan RS, Xanthakis V, Lyass A, et al. Epidemiology of left ventricular systolic dysfunction and heart failure in the Framingham study: an echocardiographic study over 3 decades. JACC Cardiovasc Imaging. 2018;11(1):1–11.
Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883–93.
Butler J, Braunwald E, Gheorghiade M. Recognizing worsening chronic heart failure as an entity and an end point in clinical trials. JAMA. 2014;312(8):789–90.
Butler J, Djatche LM, Sawhney B, et al. Clinical and economic burden of chronic heart failure and reduced ejection fraction following a worsening heart failure event. Adv Ther. 2020;37(9):4015–32.
Mentz RJ, Djatche L, Pulungan Z, et al. Characteristics and outcomes of heart failure with reduced ejection fraction patients enrolled in Medicare Advantage with and without recent worsening heart failure. J Am Coll Cardiol. 2020;75(11 Supplement 1):809.
DeVore AD, Hammill BG, Sharma PP, et al. In-hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. J Am Heart Assoc. 2014;3(4):e001088.
Butler J, Yang M, Manzi MA, et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(8):935–44.
Bress AP, King JB, Brixner D, et al. Pharmacotherapy treatment patterns, outcomes, and health resource utilization among patients with heart failure with reduced ejection fraction at a US academic medical center. Pharmacotherapy. 2016;36(2):174–86.
Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–6.
Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116(13):1482–7.
Olchanski N, Vest AR, Cohen JT, et al. Comparing inpatient costs of heart failure admissions for patients with reduced and preserved ejection fraction with or without type 2 diabetes. Cardiovasc Endocrinol Metab. 2020;9(1):17.
Echouffo-Tcheugui JB, Bishu KG, Fonarow GC, et al. Trends in health care expenditure among US adults with heart failure: the Medical Expenditure Panel Survey 2002–2011. Am Heart J. 2017;186:63–72.
Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–19.
Institute for Clinical and Economic Review. ICER’s reference case for economic evaluations: principles and rationale. 2020. https://icer.org/wp-content/uploads/2020/10/ICER_Reference_Case_013120.pdf. Accessed 4 Oct 2021.
Sandhu AT, Ollendorf DA, Chapman RH, et al. Cost-effectiveness of sacubitril-valsartan in patients with heart failure with reduced ejection fraction. Ann Intern Med. 2016;165(10):681–9.
McMurray JJ, Trueman D, Hancock E, et al. Cost-effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart. 2018;104(12):1006–13.
UK National Institute of Health and Care Excellence. Single technology appraisal. Sacubitril valsartan for treating heart failure with systolic dysfunction. Committee Papers. 2015.
US Centers for Disease Control and Prevention. National vital statistics reports. United States Life Tables, 2017. 2019.
US Centers for Disease Control and Prevention. National vital statistics reports. Deaths: Final Data for 2017. 2019.
McEwan P, Darlington O, McMurray JJ, et al. Cost‐effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health‐economic analysis of DAPA‐HF. Eur J Heart Fail. 2020;22(11):2147–56.
King JB, Shah RU, Bress AP, et al. Cost-effectiveness of sacubitril-valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2016;4(5):392–402.
Bureau of Labor Statistics. Consumer Price Index. Medical care component 2021 [cited 2021 January 11]. Available from: https://data.bls.gov/timeseries/CUUR0000SAM?output_view=data
PRINIVIL [prescribing information]. Whitehouse Station, NJ, USA: Merck Sharp & Dohme Corp. 2019.
COREG [prescribing information]. Research Triangle Park: GlaxoSmithKline. 2017.
ENTRESTO [prescribing information]. East Hanover: Novartis. 2019.
ALDACTONE [prescribing information]. New York: Pfizer Inc. 2008.
VERQUVO [prescribing information]. Whitehouse Station, NJ, USA: Merck Sharp & Dohme Corp. 2021.
IBM Micromedex. RED BOOK® 2021 [cited 2021 January 11]. Available from: http://micromedex.com
Mentz RJ, Pulungan Z, Kim S, et al. Quality outcomes, healthcare resource utilization and costs in Medicare patients with chronic heart failure with reduced ejection fraction with and without a worsening event. J Med Econ. 2021;24(1):698–705.
Obi EN, Swindle JP, Turner SJ, et al. Health care costs for patients with heart failure escalate nearly 3-fold in final months of life. J Manag Care Spec Pharm. 2016;22(12):1446–56.
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. OUP Oxford; 2006.
Gaziano TA, Fonarow GC, Velazquez EJ, et al. Cost-effectiveness of sacubitril-valsartan in hospitalized patients who have heart failure with reduced ejection fraction. JAMA Cardiol. 2020;5(11):1236–44.
Gaziano TA, Fonarow GC, Claggett B, et al. Cost-effectiveness analysis of sacubitril/valsartan vs enalapril in patients with heart failure and reduced ejection fraction. JAMA Cardiol. 2016;1(6):666–72.
Di Tanna GL, Urbich M, Wirtz HS, et al. Health state utilities of patients with heart failure: a systematic literature review. Pharmacoeconomics. 2021;39(2):211–29.
Urbich M, Globe G, Pantiri K, et al. A systematic review of medical costs associated with heart failure in the USA (2014–2020). PharmacoEconomics. 2020;38(11):1219–36.
Büyükkaramikli NC, Rutten-van Mölken MP, Severens JL, et al. TECH-VER: a verification checklist to reduce errors in models and improve their credibility. Pharmacoeconomics. 2019;37(11):1391–408.
Parizo JT, Goldhaber-Fiebert JD, Salomon JA, et al. Cost-effectiveness of dapagliflozin for treatment of patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6(8):926–35.
McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Zueger PM, Kumar VM, Harrington RL, et al. Cost-effectiveness analysis of sacubitril/valsartan for the treatment of heart failure with reduced ejection fraction in the United States. Pharmacotherapy. 2018;38(5):520–30.
Acknowledgments
The authors thank the patients, along with their families and caregivers, who participated in the VICTORIA trial, from which the clinical inputs of this study were obtained. The authors thank Nicholas Durno, an employee of OPEN Health, York, UK, for development of an early version of the cost-effectiveness model. They also thank Catherine Mirvis and Christina DuVernay, employees of OPEN Health, Bethesda, MD, USA, for editorial assistance with the manuscript, and Svetlana Bizjajeva, PhD, an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, for statistical consultation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The design and conduct of this study and manuscript development were funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Conflicts of Interest
Rongzhe Liu, Ibrahim Diakite, and Dipen Patel are employees of OPEN Health, which received funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, to conduct the study. Adnan Alsumali, Laurence M. Djatche, Dominik Lautsch, and Yufei Wang were employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may hold stock in Merck & Co., Inc., Kenilworth, NJ, USA. Andrew Briggs received compensation from Merck & Co., Inc., as a consultant for this work. He has also been contracted by Bayer, Eisai, Janssen, Novartis, Sword Health, Amgen, and Daichii Sankyo and received compensation outside of the submitted work.
Availability of Data and Material
The corresponding author has full access to all the data in the study. Data sharing is available upon request.
Code Availability
The cost-effectiveness model was developed in Microsoft Excel 365 (Microsoft Corporation). Any additional information about model programming is available from the corresponding author upon request.
Ethics Approval
This study relied on VICTORIA estimates and published literature as analysis inputs, therefore Ethics Committee approval was not required.
Consent to Participate
Patients included in VICTORIA provided informed consent. This model did not involve human subjects.
Consent for Publication
Patients included in VICTORIA provided informed consent. This model did not involve human subjects
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution- Non-commercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alsumali, A., Djatche, L.M., Briggs, A. et al. Cost Effectiveness of Vericiguat for the Treatment of Chronic Heart Failure with Reduced Ejection Fraction Following a Worsening Heart Failure Event from a US Medicare Perspective. PharmacoEconomics 39, 1343–1354 (2021). https://doi.org/10.1007/s40273-021-01091-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-021-01091-w