Abstract
Background and Objectives
The MONALEESA-7 trial demonstrated the efficacy and safety of ribociclib plus a nonsteroidal aromatase inhibitor (NSAI) [with goserelin] for pre-/perimenopausal women with hormone receptor-positive and human epidermal growth factor receptor 2-negative advanced breast cancer. This analysis evaluated the cost effectiveness of ribociclib plus NSAI vs NSAI monotherapy and tamoxifen monotherapy from the perspective of the Canadian healthcare system.
Methods
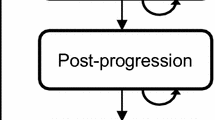
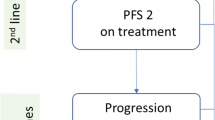
The incremental cost-effectiveness ratio expressed as incremental costs per quality-adjusted life-year (QALY) gained for ribociclib plus an NSAI vs an NSAI and vs tamoxifen was estimated using a semi-Markov cohort model developed in Microsoft Excel with a 15-year time horizon and states for progression-free survival, post-progression survival, and dead. Survival distributions for progression-free survival, post-progression survival, and time to discontinuation as well as health-state utilities were estimated using data from MONALEESA-7. Direct costs of advanced breast cancer treatment were based on Canadian-specific values from published sources. Costs ($CAN 2019) and QALYs were discounted at 1.5% annually.
Results
Ribociclib plus an NSAI was estimated to yield gains of 1.42 life-years and 1.17 QALYs vs an NSAI, and 2.61 life-years and 2.12 QALYs vs tamoxifen, at incremental costs of $209,701 and $220,836, respectively. In probabilistic analyses, the incremental cost-effectiveness ratio for ribociclib plus an NSAI was estimated to be $178,872 per QALY gained vs an NSAI and $104,400 per QALY gained vs tamoxifen. Results of deterministic analyses were similar (incremental cost-effectiveness ratios of $177,245 and $103,316 vs NSAI and tamoxifen, respectively). Results were sensitive to parametric distributions used for projecting progression-free survival and the time horizon.
Conclusions
At its current list price, ribociclib used in combination with NSAI is likely to be co-effective relative to an NSAI alone or tamoxifen alone if the willingness-to-pay threshold is less than approximately $178,000 per QALY. These results have informed deliberations regarding reimbursement and access to this treatment in Canada and may be useful for decision makers in other settings.




Similar content being viewed by others
References
Canadian Cancer Society. Breast cancer statistics. 2020. Available from: https://www.cancer.ca/en/cancer-information/cancer-type/breast/statistics/?region=on. Accessed 21 May 2020.
Surveillance Epidemiology and End Results Program. Cancer stat facts: female breast cancer. National Cancer Institute. 2018. Available from: https://seer.cancer.gov/statfacts/html/breast.html. Accessed 11 May 2018.
Canadian Cancer Society. Breast cancer statistics. 2019. Available from: http://www.cancer.ca/en/cancer-information/cancer-type/breast/statistics/?region=on. Accessed 10 Feb 2019.
Canadian Cancer Society Advisory Committee. Canadian cancer statistics 2015. Toronto (ON): Canadian Cancer Society; 2015. Available from: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2015-EN.pdf?la=en. Accessed 12 Dec 2018.
Cardoso F, et al. 3rd ESO-ESMO International Consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol. 2017;28(12):3111.
Canadian Cancer Statistics Advisory Committee. Canadian cancer statistics 2018. Toronto (ON): Canadian Cancer Society; 2018. Available from: http://cancer.ca/Canadian-Cancer-Statistics-2018-EN. Accessed 12 Dec 2018.
Partridge AH, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(29):3307–29.
Cardoso F, et al. 4th ESO-ESMO International Consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol. 2018;29(8):1634–57.
Surveillance Epidemiology and End Results Program. Cancer Stat Facts: female breast cancer. 2020. Available from: https://seer.cancer.gov/statfacts/html/breast.html. Accessed 21 May 2020.
American Cancer Society. Breast cancer facts & figures 2015–2016. Atlanta (GA): American Cancer Society, Inc.; 2015. Available from: https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html. Accessed 14 Apr 2018.
Rugo HS, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol. 2016;34(25):3069–103.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines). Breast cancer. 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 18 July 2019.
Matutino A, et al. Hormone receptor-positive, HER2-negative metastatic breast cancer: redrawing the lines. Curr Oncol. 2018;25(Suppl 1):S131–41.
Tripathy D, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–15.
Im SA, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–16.
pan-Canadian Oncology Drug Review. Final economic guidance report: ribociclib (Kisqali) for metastatic breast cancer. 2018. Available from: https://cadth.ca/sites/default/files/pcodr/pcodr_ribociclib_kisqali_mbc_fn_egr.pdf. Accessed 11 Apr 2019.
pan-Canadian Oncology Drug Review. Final economic guidance report: palbociclib (Ibrance) with fulvestrant for metastatic breast cancer. 2019. Available from: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10150PalbociclibFulvestrantMBC_fnEGR_NOREDACT-ABBREV_Post_03May2019_final.pdf. Accessed 29 May 2019.
pan-Canadian Oncology Drug Review. Initial economic guidance report: abemaciclib (Verzenio) for metastatic breast cancer. 2019. Available from: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10161AbemaciclibMBC_inEGR_NOREDACT-ABBREV_Post_03May2019_final.pdf. Accessed 11 June 2019.
Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 2019. Available from: https://www.cadth.ca/about-cadth/how-we-do-it/methods-and-guidelines/guidelines-for-the-economic-evaluation-of-health-technologies-canada. Accessed 10 Feb 2019.
Jackson C. Package ‘flexsurv’: flexible parametric survival and multi-state models. 2016. Available from: https://cran.r-project.org/web/packages/flexsurv/flexsurv.pdf. Accessed 26 Sep 2016.
Statistics Canada. Life tables, Canada provinces and territories, 1980/1982 to 2014/2016. 2018. Available from: https://www150.statcan.gc.ca/n1/pub/84-537-x/2018002/xls/2014-2016_Tbl-eng.xlsx. Accessed 28 Sep 2018.
Xie F, et al. A time trade-off-derived value set of the EQ-5D-5L for Canada. Med Care. 2016;54(1):98–105.
EuroQol. EQ-5D-5L: valuation: crosswalk index value calculator. 2018. Available from: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/valuation-standard-value-sets/crosswalk-index-value-calculator/. Accessed 6 Sep 2018.
Lloyd A, et al. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–90.
Guertin JR, Feeny D, Tarride JE. Age- and sex-specific Canadian utility norms, based on the 2013–2014 Canadian Community Health Survey. CMAJ. 2018;190(6):E155–61.
Statistics Canada. Consumer Price Index by product group, monthly, percentage change, not seasonally adjusted, Canada, provinces, Whitehorse, Yellowknife and Iqaluit (Table: 18-10-0004-13). 2019. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000413. Accessed 30 Oct 2019.
DeltaPA. Ottawa (ON): IQVIA; 2019.
Statistics Canada. Table 14-10-0307-01: employee wages by occupation, annual, inactive. 2019. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410030701. Accessed 16 June 2019.
Ministry of Health, Ontario Health Insurance Plan Laboratories and Genetics Branch. Schedule of Benefits for Laboratory Servicse (effective July 1, 2019). 2019.
Ministry of Health and Long Term Care. Ontario Schedule of Benefits Physician Services under the Health Insurance Act. 2015. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/. Accessed 12 Dec 2018.
Government of Ontario. Ministry of Health and Long-Term Care. Ontario case costing initiative. Health Data Branch Web Portal. Available from: https://hsim.health.gov.on.ca/hdbportal/. Accessed 10 May 2017.
Dawson H, Zinck G. CIHI survey: ED spending in Canada: a focus on the cost of patients waiting for access to an in-patient bed in Ontario. Healthc Q. 2009;12(1):25–8.
Government of Canada. Job bank: pharmacist: Ontario: median hourly wage NNOC 3131-A. Date modified: 2017-04-04. Available from: https://www.jobbank.gc.ca/LMI_report_bynoc.do?lang=eng&noc=3131&reportOption=wage. Accessed 10 May 2017.
Beauchemin C, et al. A global economic model to assess the cost-effectiveness of new treatments for advanced breast cancer in Canada. J Med Econ. 2016;19(6):619–29.
. Kisqali® product monograph. Dorval (QC): Novartis Pharmaceuticals Canada Inc.; 2018.
British Columbia Ministry of Health. Schedule of fees for the laboratory services outpatient: payment schedule. 2017. Available from: https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/laboratory-services/laboratory_services_-_schedule_of_fees.pdf. Accessed 15 Feb 2019.
Thein HH, et al. Estimates and predictors of health care costs of esophageal adenocarcinoma: a population-based cohort study. BMC Cancer. 2018;18(1):694.
pan-Canadian Oncology Drug Review. Final recommendation for palbociclib (Ibrance) plus fulvestrant (Faslodex) for advanced breast cancer. 2019. Available from: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10150%20PalbociclibFulvestrantMBC_fnRec_2019-05-03_Approved_Post_03May2019_final.pdf. Accessed 27 May 2019.
Raphael J, et al. Palbociclib in hormone receptor positive advanced breast cancer: a cost-utility analysis. Eur J Cancer. 2017;85:146–54.
Forsythe A, et al. Progression-free survival/time to progression as a potential surrogate for overall survival in HR+, HER2- metastatic breast cancer. Breast Cancer. 2018;10:69–78.
Cristofanilli M. Overall survival with palbociclib + fulvestrant in women with hormone recptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: analyses from PALOMA-3. ESMO Congress, 20 October 2018; 2018; Munich.
Slamon D. Overall survival results from the phase III MONALEESA-3 study of fulvestrant ± ribociclib in postmenopausal patients with HR+/HER2− advanced breast cancer. ESMO Congress, 29 September 2019; 2019; Barcelona.
Sledge Jr GW, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–24. https://doi.org/10.1001/jamaoncol.2019.4782.
Sledge Jr G. MONARCH 2: overall survival of abemaciclib plus fulvestrant in patients with HR+, HER2 advanced breast cancer. ESMO Congress, 29 September 2019; 2019; Barcelona.
Turner NC, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this evaluation was provided by Novartis Pharmaceuticals Corp., East Hanover, NJ, USA.
Conflict of interest
Thomas E. Delea is a partner at Policy Analysis Inc., a privately held, healthcare research consultancy that has received research funding and/or consulting fees from AbbVie, Alexion, Amgen, Bristol Myers Squibb, EMD Serono, GlaxoSmithKline, Jazz Pharmaceuticals, Lilly, Merck, Merck Group, Novartis, Pfizer, Sanofi, Seattle Genetics, and Takeda. Daniel Stellato is currently an employee of Policy Analysis Inc. David Chandiwana is an employee of Novartis and owns stock and/or stock options in Novartis. Marroon E. Thabane was an employee of Novartis at the time the study was conducted and owns stock and/or stock options in Novartis. Jinhee Park was an employee of Novartis at the time this evaluation was conducted and owns stock and/or options in Novartis.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The information reported in this article and the supplementary material is sufficient to replicate the results of the study.
Code Availability
The information reported in this article is sufficient to replicate the results of the study. The model used in the article includes a proprietary code and is not provided.
Author contributions
All authors were involved with the conception and design of the evaluation, analysis and interpretation of the data, and drafting and critically revising the paper. All authors provided final approval and agreed to be accountable for the work reported herein.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stellato, D., Thabane, M.E., Chandiwana, D. et al. Cost Effectiveness of Ribociclib Plus a Nonsteroidal Aromatase Inhibitor in Pre-/Perimenopausal, HR+ and HER2− Advanced Breast Cancer: A Canadian Healthcare Perspective. PharmacoEconomics 39, 853–867 (2021). https://doi.org/10.1007/s40273-021-01028-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-021-01028-3