Abstract
Introduction
Clinical guidelines have recommended a 1-year trastuzumab regimen as standard care for early human epidermal growth factor receptor 2 (HER2)-positive breast cancer; however, this recommendation can have a dramatic impact on total drug expenditures in middle-income countries (MICs). We performed a cost-effectiveness analysis from the Iranian healthcare perspective to find an optimum duration of trastuzumab use in Iran.
Method
We compared four treatment strategies comprising chemotherapy and varying durations of trastuzumab use (no trastuzumab, 6, 9 months, and 1 year), and a Markov model and probabilistic sensitivity analysis were used to estimate the costs and effects of the strategies. We then examined the cost effectiveness of the strategies at different willingness-to-pay (WTP) thresholds and ages at onset of treatment.
Results
Incremental costs (versus no trastuzumab) were €8826 (6 months), €13,808 (9 months) and €18,588 (12 months), while incremental quality-adjusted life-years (QALYs) were 0.65 (6 months), 0.87 (9 months) and 1.14 (12 months). At a threshold of 3 × gross domestic product (GDP)/capita (€21,000/QALY) and for patients younger than 59 years, the 6-month protocol was most likely to be cost effective (probability of 42%). At a threshold of 4 × GDP/capita (€28,000/QALY), the 6-month and 1-year regimens were essentially equal in cost effectiveness (37 and 35%, respectively). At this WTP threshold, the 6-month and 1-year regimens were optimal strategies only for patients up to 66 and 44 years of age, respectively.
Conclusion
In contrast to clinical guidelines, 6 months of trastuzumab may be the most cost-effective option for Iran. The lower absolute WTP threshold and lower life expectancy compared with high-income countries are two crucial parameters in the cost effectiveness of interventions in MICs. It is therefore necessary to strike a balance between maximum population health and maintaining affordability in these countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lower absolute willingness-to-pay and life expectancy are two important issues that can affect conclusions regarding the cost effectiveness of treatment in middle-income countries |
Although most clinical guidelines worldwide recommend 1 year of trastuzumab for early human epidermal growth factor receptor 2 (HER2)-positive breast cancer, it is not the most cost-effective strategy in Iran. |
In Iran, the most cost-effective strategy is 6 months of trastuzumab, but only for patients younger than 59 years of age. |
A significant price reduction is necessary to make trastuzumab cost effective for all patients. |
1 Introduction
Trastuzumab is a monoclonal antibody that is used in the management of breast cancer (BC). It has mostly been used as adjuvant treatment for patients in the early stage of BC who overexpress human epidermal growth factor receptor 2 (HER2). Trastuzumab’s huge share of total drug expenditure, particularly in middle-income countries (MICs), has raised concerns among policymakers regarding efficient resource allocation in their countries [1]. To date, 1 year of trastuzumab use is considered as the optimum duration of therapy for the adjuvant treatment of early-stage, HER2-positive BC [2,3,4], based on the results of various randomized controlled trials (RCTs).
As an MIC, Iran provided a national guideline that recommends a 9-week period of trastuzumab use [5] due to the unaffordability of 1 year of trastuzumab therapy [1]; however, clinical evidence suggests that very short durations of trastuzumab therapy cannot provide significant efficacy [2]. Therefore, the main question in this regard is “What is the maximum obtainable level of health in an MIC when we are dealing with an expensive intervention?”
The aim of this study was to provide a model-based cost-effectiveness analysis (CEA) of adjuvant trastuzumab for patients with early HER2-positive BC from an Iranian healthcare perspective. Subsequently, we undertake a scenario analysis to determine the optimum duration of trastuzumab use in Iran.
2 Methods
In our study, we compared two treatment approaches (chemotherapy with and without trastuzumab) for managing early HER2-positive BC. A Markov model was used to estimate the marginal differences in clinical outcomes and healthcare costs. We designed a cohort for patients with HER2-positive BC and included the necessary information in this model to estimate the incremental cost-effectiveness ratio (ICER) of using trastuzumab for these patients with a lifetime horizon in Iran. Afterward, a scenario analysis was conducted to compare the cost effectiveness of different strategies of trastuzumab therapy in an MIC.
2.1 Model Structure
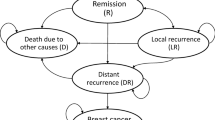
We designed a model structure based on three sources of information (Fig. 1). First, the routine practice of treating HER2-positive BC in Iran was understood based on a previous study of clinical practice using a claims database and clinician survey [1]. We then discussed our interpretations of the results through a number of interviews with two Iranian academic oncologists, and based on our final decision on data availability. The model was designed to be able to compare two main arms (trastuzumab versus no trastuzumab), and included six main health states: (1) early BC; (2) loco-recurrence BC; (3) advanced BC; (4, 5) two progression-free health states after treatment in early BC and loco-recurrence BC; and (6) death. We also included other health-state subgroups for loco-recurrence (loco-regional and second primary BC), and advanced patients (central nervous system [CNS], visceral, bone, and soft tissue metastasis). Moreover, we incorporated long-term cardiotoxicity in all states in which patients use trastuzumab. A 3-month cycle length was used in the model [1].
In the model, patients with early HER2-positive BC received chemotherapy with or without trastuzumab. After treatment, patients moved to a follow-up state and could progress to local or advanced BC. Patients stayed in the follow-up state until the occurrence of progression (local or distance recurrence) or second primary BC. We assumed that patients in both arms were equally likely to receive all other medical services. Patients who had progressed to loco-recurrence after receiving the treatment could move to the disease-free state after the local-recurrence state, or progress to advanced BC; patients who progressed to advanced BC stayed there until they died from BC or other causes. Some of the patients who received trastuzumab developed reversible cardiac toxicity. Patients in all health states could die due to background mortality.
2.2 Patient Characteristics
We set the age at onset of treatment at 45 years, in accordance with the results of a variety of current claims database [6] and epidemiological [7,8,9] studies in Iran.
2.3 Transition Probabilities
The following items describe the input parameters used in the model as well as their sources. Various transition probabilities used in the model are shown in Table 1.
2.3.1 Treatment Effect
The effectiveness of trastuzumab was derived from a Cochrane review [2] that was a comprehensive systematic review and meta-analysis of eight RCTs regarding disease-free survival (DFS) of trastuzumab. Due to our study design, we relied on a DFS hazard ratio (HR) based on the results of the meta-analysis of those trials (five of eight) that investigated the effects of more than 6 months of trastuzumab therapy. To include the treatment effect duration in the model, we used an assumption that was used in a CEA of trastuzumab in the UK [10], based on discussions with experts.
2.3.2 Cancer Progression
For the first 4 years, the recurrence rate in the ‘no trastuzumab’ arm was obtained from the latest HERceptin Adjuvant (HERA) trial [11] report after a 4-year patient follow-up [12]. For years 5–10, the recurrence rates were taken from the anthracycline-treated HER2-positive subgroup of the National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol B-15 trial [13]. Since that trial only focused on lymph node-positive patients, we applied a relative risk of 0.815 based on a study reporting that 63% of Iranian patients have node-positive BC at the time of diagnosis [14], and the assumption that node-positive patients have a recurrence rate that is two times greater than node-negative patients [15]. We also assumed no chance of recurrence after 10 years based on expert opinion. The ratio of loco-recurrences to distant recurrences was obtained from the results of the HERA trial [16]. Two HRs were applied to represent the increased risk of distant metastases after local recurrence. These HRs were 3.22 (95% confidence interval [CI] 2.02–6.55) and 6.14 (95% CI 2.02–6.55) for years 1–4 and 5–10, respectively [17].
2.3.3 Cardiac Toxicity
The results of a Cochrane systematic review and meta-analysis [2] were used to estimate the probabilities of decline in left ventricular ejection fraction (LVEF) and congestive heart failure (CHF) occurring during 1 year of trastuzumab therapy and other chemotherapy drugs. The time of onset and toxicity duration for patients who had reversible cardiac toxicity were taken from the HERA trial [18]. Moreover, patients suffer from CHF based on an age-specific background, and these probabilities were obtained from a cohort study of 6504 individuals in one province of Iran [19].
2.3.4 Mortality
Age-specific background mortality rates were obtained from online data of the National Organization for Civil Registration (NOCR) [20], which included data on all causes of death in Iran. We used another study of BC mortality rates in three different age ranges [7] to subtract the BC mortality rate from the NOCR data. Data on the age-specific CHF mortality rate in Iran was obtained from an Iranian cohort study [19]. Other parameters regarding BC mortality rates in various metastatic states were extracted from international studies.
2.4 Outcomes
Life-years (LYs) and quality-adjusted life-years (QALYs) were estimated for both arms. Table 2 shows the utility values used in this study. We used the results of a study that provided the utility weights for various health states, based on the EQ-5D questionnaire in Sweden [21]. Another study that used the EQ-5D was selected to find the utility weight for patients who suffer from symptomatic heart failure in the UK [22]. Finally, the utility value (EQ-5D) for patients with brain metastases was obtained from a CEA study in the UK [10].
2.5 Costs
Direct medical costs were calculated from an Iranian healthcare perspective. We assumed 100% coverage for all healthcare services by payers, which helps policymakers to examine the cost effectiveness of complete coverage of all services. We used a recent study that investigated healthcare costs and resource use in both the public and private sectors in Iran, which covers approximately 50% of all Iranians (approximately 40 million) [6]. Due to the variety of health insurance coverage in Iran [23], out-of-pocket payments had already been included in cost calculations [6]; therefore, the costs shown in Table 2 comprise the total costs of medical services. The average patient weight must be known to calculate the cost of trastuzumab; therefore, we used data from a 3-year observational period [1] to estimate the average weight based on the average dose of trastuzumab per patient using trastuzumab. The average dose per patient per prescription was 420 mg (95% CI 415–424; n = 1295), which corresponds with an average weight of 70 kg (69–71). While this may not represent the true average weight of the patients, in reality these real-world data represent the total amounts of trastuzumab, both used and wasted. Patients received trastuzumab in a 3-week cycle until the end of therapy.
The annual national healthcare tariff [24] was used to inflate or adjust the costs attained from years other than the 2014–2017 values.
2.6 Analysis
In the absence of a national guideline for economic evaluations in Iran, both costs and effects were discounted by 3.5% per annum, as suggested by the World Health Organization’s Choosing Interventions that are cost-effective (WHO-CHOICE) project [25]. A half-cycle correction was also applied.
The influence of specific input parameters on the ICER of CEA was examined using deterministic sensitivity analysis (DSA). The ranges in values were determined based on either the literature or on expert opinion.
A probabilistic sensitivity analysis (PSA) was performed in order to quantify the overall uncertainty in the expected output measures [26, 27]. The log-normal distribution was applied for relative risks, HRs, and costs, while the Dirichlet distribution was used for multinomial proportions and the beta distribution was used for binomial proportions and utility weights. The analysis used 10,000 iterations obtained via Markov Chain Monte Carlo simulation.
Based on the WHO-CHOICE, the willingness-to-pay (WTP) threshold in Iran would be 3 × gross domestic product (GDP)/capita, which is equal to €21,000 per QALY (€1 = 34,000 rials) [28]. Because widespread international economic sanctions on Iran began in 2012, we used GDP per capita in 2011, as reported by the World Bank [29], to avoid the effects of these sanctions on Iran’s GDP.
The building of the model, calculations, and statistical analysis were performed using R software for Microsoft Windows, version 3.2.2 (The R Project, Vienna, Austria) [30].
2.7 Estimation of the Optimum Duration and Maximum Age Threshold for Trastuzumab Use
Three scenarios of 6, 9 months, and 1 year of trastuzumab use were designed to compare their cost effectiveness. We used the results of the randomized, non-inferiority PHARE trial [3], which compared 6 months versus 1 year of adjuvant trastuzumab therapy, and the results of a Cochrane meta-analysis [2], which compared 1 year of trastuzumab versus no trastuzumab therapy. For 9 months of trastuzumab use, we assumed that the effectiveness HR was half of the non-inferiority HR in the PHARE trial (Table 1). The ICERs of the three strategies were calculated and, eventually, their probability of being cost effective was compared with that of the no trastuzumab arm. Furthermore, due to the importance of patient age, we investigated how the age threshold for treatment can affect the optimal duration of trastuzumab use. Multiple PSAs were performed, with various ages (40–70 years) as the age at onset of treatment, to determine the ‘maximum age threshold’ for the best-case scenario. The ‘maximum age threshold’ represents the maximum age at the onset of treatment that still results in the strategy being optimal, versus other strategies included in the comparison at a particular WTP threshold.
2.8 Model Validation
Internal validation was performed to assess how well the model’s results for DFS and overall survival (OS) corresponded with the no trastuzumab and 1-year trastuzumab arms from the HERA trial. Similarly, the results of the PHARE trial were used to validate the model’s results for the 1-year and 6-month trastuzumab strategies.
3 Results
Over a lifetime horizon, all trastuzumab strategies were cost effective versus no trastuzumab at a WTP of 3 × GDP (Fig. 2). The results of CEA, reported in Table 3, show that at the WTP threshold of 3 × GDP, and a 42% probability, the 6-month protocol was the most cost-effective strategy, while other strategies showed lower rates of being cost effective. The acceptability curves representing the probabilities that various strategies are cost effective across various WTP thresholds in the base-case scenario can be found in Fig. 3. The 1-year trastuzumab strategy was cost effective in only 21% of simulations, and the 9-month and no trastuzumab strategies were cost effective in 21 and 17% of cases, respectively. When the WTP threshold was increased to 4 × GDP, the 6-month and 1-year regimens were essentially equal in cost effectiveness (37 and 35%, respectively). The 9-month regimen had a 20% probability of cost effectiveness. However, the chance of the no trastuzumab strategy being cost effective fell to 8% when a WTP threshold of 4 × GDP was applied.
The base-case and DSA of the model yields ICERs for 6, 9 months and 1 year of €15,108, €16,800, and €17,086 per QALY versus no trastuzumab, respectively. Subsequently, deterministic one-way sensitivity analysis (summarized in Table 4) revealed that the key drivers were the acquisition cost and clinical effectiveness of 1 year of trastuzumab use versus no trastuzumab. Additionally, probabilistic one-way sensitivity analysis showed that when the cost of trastuzumab was changed from −30 to +30% in the base-case, the probability of the 1-year regimen being cost effective decreased from 41 to 8% (Online Resource 1). However, 6 months of trastuzumab therapy showed less of a change (9; 34–43%) at a threshold of 3 × GDP. In contrast, changes in the HR of DFS for 6 months of trastuzumab use versus no trastuzumab showed a range of 16% (54–38%) in the lower (0.50) and upper (0.74) limits (Online Resource 2). At a threshold of 3 × GDP, the 6-month regimen was the most cost-effective strategy (54%) for the lower limit of HR, and the no trastuzumab strategy was the cost-effective strategy for the upper limit of HR. Finally, our results (Fig. 4) show that the 6-month strategy is only cost effective when treated patients are not older than 59 years (the maximum age threshold). At this age, and at a WTP threshold of 3 × GDP, the two strategies of 6 months of trastuzumab and no trastuzumab had almost equal chances of being cost effective (39.5 versus 40%, respectively). However, this age threshold can change if the WTP is increased or decreased. At the WTP threshold of 4 × GDP, 1 year of trastuzumab use is only cost effective for patients who are younger than 44 years, and 6 months of trastuzumab use may be the best strategy for patients between 44 and 66 years of age (Online Resource 3). Finally, the impact of various ages (40–70 years) on the cost effectiveness of different strategies are shown in Online Resource 4.
The impact of life expectancy on the probabilities of being cost effective among different treatment strategies. Max age threshold represents the maximum patient age at onset of treatment that, for those patients, strategy remains optimal at a particular WTP threshold, WTP willingness-to-pay, GDP gross domestic product
Figure 5 illustrates the effect of price reductions on the maximum age threshold. Our analyses revealed that trastuzumab is only cost effective if the price was to be reduced by 30%; however, this reduction would make 1 year of trastuzumab use the optimal strategy only if it was given to patients younger than 49.5 years of age. As Fig. 5 shows, a 10% reduction in price increases the maximum age threshold by 3 and 6 years for the 6-month and 1-year treatment regimens, respectively.
The internal validation results for the three strategies, including no trastuzumab and 1 year and 6 months of trastuzumab use revealed no significant differences between the results of the model and the results of the trials used to perform this study (Table 5).
4 Discussion
The use of monoclonal antibodies such as trastuzumab is a hotly debated topic among policymakers, patients, and healthcare professionals in MICs due to their considerable impact on the healthcare budgets of these nations. For example, in Iran, trastuzumab alone accounts for approximately €93–140 million (approximately 4–6%) of total pharmaceutical expenditure [1]. Trastuzumab is an effective but very expensive drug for patients with HER2-positive BC; therefore, policymakers in MICs can neither ignore it because of its effectiveness nor reimburse it with the same duration, as clinical guidelines have recommended in high-income countries (HICs), due to its cost. Specifically, more money spent on trastuzumab means less money for other treatments [31]. The questions that healthcare professionals and policymakers in these countries must answer include: “What is the optimum duration of trastuzumab use to provide an efficient and affordable treatment?” and “How can we balance population health and affordability?” In response to these questions, our study provides valuable information regarding the cost effectiveness of various trastuzumab strategies in Iran, which may also be useful for other MICs. Moreover, to our knowledge this is the first CEA that examines age heterogeneity of patients and explores the impact of life expectancy on the cost effectiveness of various trastuzumab strategies in an MIC.
The effectiveness of shorter durations of trastuzumab therapy has been studied in various RCTs. The FinHER (Finland Herceptin) trial [32] investigated clinical outcomes in 9 weeks of trastuzumab use and reported an HR of 0.42 (95% CI 0.21–0.83, p = 0.01) for the DFS of trastuzumab use versus no trastuzumab. However, this study was faced with some limitations, such as small sample size, and the results of other trials (SOLD [33] and SHORT-HER [34]) that also focused on 9-week trastuzumab use have not yet published. As a result, we therefore excluded the 9-week strategy as a comparator in this study. The clinical outcomes of 6 months of trastuzumab use were the subject of two RCTs (PHARE [3] and PERSEPHONE [35]). Since only the results of PHARE are currently available, we used the PHARE results in this study. Our assumption regarding the DFS HR in the 9-month strategy was not supported by a real RCT. It was therefore necessary to make assumptions to investigate a potentially cost-effective strategy between 6 months and 1 year of trastuzumab use. Finally, the model was internally validated based on the results of the aforementioned RCTs.
A previously published CEA reported that 1 year of trastuzumab use is not cost effective in Iran [36]; however, this study had some methodological inconsistencies when assessed using the Drummond economic evaluation checklist [37] and also used an inappropriate trastuzumab cost. The cost of 1 year of trastuzumab use by a 70-kg woman was estimated at $48,850, which is inconsistent with the public price of trastuzumab dosage forms (€500 and €1294 for 150 and 440 mg vials, respectively [€22,992] [38]). Consequently, it was necessary to conduct a new study using appropriate methods and more reliable input parameters. For this purpose, we attempted to design a standard economic evaluation using country-specific information, such as the results of a real-world cost analysis that exclusively investigated HER2-positive direct medical costs in Iran [6].
Our results show that the most cost-effective strategy in the treatment of early HER2-positive BC in Iran is 6 months of trastuzumab as an adjuvant therapy. This strategy remains optimal, even if the price of trastuzumab is reduced by almost 30% (Online Resource 1). The 1-year strategy is only cost effective at higher WTP thresholds. Since, the 9-month strategy was dominated by the 6-month and 1-year strategies, from an efficiency perspective, the 6-month and 1-year strategies are the two best strategies. If the 6-month protocol is considered the standard of care, and doctors continue to adhere to this strategy, Iran can save €40 million/year compared with a 1-year trastuzumab strategy. It is worth noting that all these results can vary if the age of patients at the onset of treatment is changed, and that these various strategies for trastuzumab use would not be cost effective in patients older than 59 years of age (Fig. 4). In other words, in addition to a lower absolute WTP threshold for expensive drugs in MICs, life expectancy in these countries (e.g. 76.6 years for Iranian women) is also generally lower than in HICs. These two issues can affect the results of a CEA in MICs.
We compared our results with the results of model-based CEAs [10, 39,40,41,42] in other countries that used the same study perspective (healthcare), time horizon (lifetime), and trastuzumab use duration (1 year). Two studies (in China [39] and Belgium [42]) calculated ICER values that were lower than those in our study ($8041 and €10,315, respectively), while four CEAs (in the US [40, 41], Australia [43], and the UK [10]) estimated higher ICERs ($39,982, $26,417, $A22,793, and £25,803, respectively). Regardless of this variation, in all studies, including our study, ICERs were primarily affected by the cost of trastuzumab. Despite the methodological similarities between this study and other studies, we cannot easily compare our results with other CEAs because of differences in various parameters, such as costs of trastuzumab, prices for healthcare services, discount rates, background mortality rates, and sources of estimation of the effectiveness of trastuzumab. On the other hand, a CEA in Colombia, using a shorter time horizon (20 years), concluded that trastuzumab is not cost effective, even though trastuzumab was cheaper (US$4,219 versus €22,992) and advanced-stage treatment (downstream costs) was more costly (US$52,093 versus €16,926) compared with costs in Iran [44]. However, comparisons between that study and our study are difficult, not only because of differences in time horizon and cost components but also because of differences in the sources of clinical effectiveness and utility values that were used. This means that comparisons between CEAs of trastuzumab, and between MICs, are also prone to the same problems that arise when comparisons between CEAs in HICs are performed.
Our study faced four notable limitations. First, we had to use the results of studies performed in HICs due to the lack of country-specific health-related quality of life (HRQoL). In fact, there are no good-quality publications on HRQoL in Iran. However, this limitation has no major impact on the overall results since our one-way sensitivity analysis showed ±30% changes in utility values caused less than ±8% changes in the estimated ICER. Second, the HERA trial has recently reported 11 years of median follow-up, and data from this trial could have been used to update the parameter estimates in our model [45]. However, the updated estimate of effectiveness of trastuzumab after 11 years was lower than the effectiveness after 2 years (i.e. an increase in the HR for DFS from 0.62 to 0.73). However, the results of one-way sensitivity analysis (Online Resource 2) that any reduced effectiveness of trastuzumab would not change the conclusion that the price of trastuzumab must be reduced significantly in order for it to have any chance of being cost effective, even for a subgroup of the patient population (i.e. younger patients). Third, the cost effectiveness of the 9-month strategy was estimated based on an assumption of effectiveness due to a lack of data. Despite its low impact on the overall results, as demonstrated by sensitivity analysis (Table 4), our CEA can be updated when the results of the SOLD [33] and/or SHORT-HER [34] trials become available. Finally, while it is known that women with hormone receptor-negative tumors have an increased risk of recurrence and death, we did not examine the cost effectiveness of treatment decisions based on hormone receptor status [46]. This is something that should be examined in the future.
New drugs launched in MICs do not necessarily have the same effectiveness or affordability as in HICs. Policymakers and other primary stakeholders, such as healthcare professionals and patient communities, should focus on a strategy that will help to create a balance between the highest attainable level of health and affordability of the new drug. However, if there is a desire to implement widely accepted clinical guidelines in MICs, concessions are unavoidable. There are four approaches that would lead to recommending 1 year of trastuzumab use in Iran: (1) price reduction; (2) use of a higher WTP threshold; (3) a combination of these two methods; and (4) ‘watchful waiting’ for a generic product. Our results showed that the first two options, either price reduction (by −30%) [Fig. 3] or the use of a higher WTP threshold (4 × GDP), cannot achieve a new balancing point between population health and affordability to switch to a longer course of trastuzumab therapy (Online Resource 3). The third option could be a potential solution; however, due to changes in the price of trastuzumab and the WTP threshold, the maximum age threshold for 1 year of trastuzumab use should be re-estimated. Moreover, the use of a higher WTP would set a precedent for other treatments. The ‘watchful waiting’ approach can indeed enhance affordability in MICs; however, this would mean waiting many years before drugs such as trastuzumab would be reimbursed in MICs. In many cases, ‘watchful waiting’ would mean a large amount of health loss. Therefore, a more equitable solution would involve revamping pharmaceutical pricing systems to make new drugs more affordable for lower income countries. For example, a 75% price reduction would be needed to make 1 year of trastuzumab, the strategy recommended in clinical guidelines in HICs, cost effective for all patients younger than 76.6 years (the average life expectancy) in Iran (Fig. 5).
Effectiveness and affordability are not the only two factors to consider when policymakers make reimbursement decisions. Other factors, such as budget impact and equity analysis, are also important to consider. In fact, using multicriteria decision analysis (MCDA) that includes various factors is likely the best way to help policymakers reach a satisfactory conclusion [47].
5 Conclusions
Most clinical guidelines prepared in HICs have recommended 1 year of trastuzumab use due to the efficacy of the drug; however, this treatment strategy would not be an affordable recommendation in MICs due to the lower absolute value of the WTP threshold and the lower life expectancy compared with HICs. Our study showed that 6 months of trastuzumab use is the most cost effective option for the Iranian healthcare setting at a WTP threshold of 3 × GDP and a maximum age threshold of 59 years. Policymakers and other stakeholders in MICs must find the best way to balance population health and affordability of an expensive intervention.
Change history
23 February 2018
Page 95, Table 2, final row, second column: the mean Advanced treatment annual cost which
References
Ansaripour A, Uyl-de Groot CA, Foroozanfar M, Rahimimoghadam S, Redekop WK. Which is more important for doctors in a middle-income country, a national guideline or the medical literature? An adherence survey of trastuzumab use for breast cancer in Iran. J Cancer Policy. 2016;9:8–13.
Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, D’Amico R. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;(4):CD006243
Pivot X, Romieu G, Debled M, Pierga J, Kerbrat P, Bachelot T, Lortholary A, Espie M, Fumoleau P, Serin D, Jacquin J, Jouannaud C, Rios M, Abadie-Lacourtoisie S, Tubiana-Mathieu N, Cany L, Catala S, Khayat D, Pauporte I, Kramar A. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14:741–8.
Goldhirsch A, Gelber RD, Piccart-Gebhart M, de Azambuja E, Procter M, Suter TM, Jackisch C, Cameron D, Weber HA, Heinzmann D, Lago LD, McFadden E, Dowsett M, Untch M, Gianni L, Bell R, Köhne CH, Vindevoghel A, Andersson M, Brunt AM, Otero-Reyes D, Song S, Smith I, Leyland-Jones B, Baselga J. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382:1021–8.
CRNN. Guideline for trastuzumab prescription and an eligibility priority setting for Iranian breast cancer patients. Part 1 (Ultimate guideline). First ed. Tehran: Cancer Research National Network, Iran Ministry of Health; 2012
Ansaripour A, Zendehdel K, Tadayon N, Sadeghi F, Uyl-de Groot C, Redekop WK. Use of data-mining to perform a real world cost analysis of HER2-positive breast cancer in Iran. Value Health. 2016;19:A355.
Vostakolaei FA, Broeders MJM, Rostami N, van Dijck JAAM, Feuth T, Kiemeney LALM, Verbeek ALM. Age at diagnosis and breast cancer survival in Iran. Int J Breast Cancer. 2012;2012:517976.
Rezaianzadeh A, Heydari ST, Hosseini H, Haghdoost AA, Barooti E, Lankarani KB. Prevalence of breast cancer in a defined population of Iran. Iran Red Crescent Med J. 2011;13:647–50.
Haghighat S, Akbari ME, Ghaffari S, Yavari P. Standardized breast cancer mortality rate compared to the general female population of Iran. Asian Pacific J Cancer Prev. 2012;13:5525–8.
Hall P, Hulme C, McCabe C, Oluboyede Y, Round J, Cameron D. Updated Cost-effectiveness analysis of trastuzumab for early breast cancer. PharmacoEconomics. 2011;29:415–32.
Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sánchez Rovira P, Piccart-Gebhart MJ. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36.
Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch C, Cameron D, Mano M, Pedrini JL, Veronesi A, Mendiola C, Pluzanska A, Semiglazov V, Vrdoljak E, Eckart MJ, Shen Z, Skiadopoulos G, Procter M, Pritchard KI, Piccart-Gebhart MJ, Bell R. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–44.
Paik S, Bryant J, Tan-Chiu E, Yothers G, Park C, Wickerham DL, Wolmark N. HER2 and choice of adjuvant chemotherapy for invasive breast cancer: national surgical adjuvant breast and bowel project protocol B-15. J Natl Cancer Inst. 2000;92:1991–8.
Mousavi SM, Montazeri A, Mohagheghi MA, Jarrahi AM, Harirchi I, Najafi M, Ebrahimi M. Breast cancer in iran: an epidemiological review. Breast J. 2007;13:383–91.
Untch M, Gelber RD, Jackisch C, Procter M, Baselga J, Bell R, Cameron D, Bari M, Smith I, Leyland-Jones B, de Azambuja E, Wermuth P, Khasanov R, Feng-yi F, Constantin C, Mayordomo JI, Su C-, Yu S-, Lluch A, Senkus-Konefka E, Price C, Haslbauer F, Sahui TS, Srimuninnimit V, Colleoni M, Coates AS, Piccart-Gebhart MJ, Goldhirsch A, the HERA Study Team. Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann Oncol. 2008;19:1090–6.
Piccart-Gebhart M, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang C, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Sütő T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72.
Tanis E, van de Velde CJH, Bartelink H, van de Vijver MJ, Putter H, van der Hage JA. Locoregional recurrence after breast-conserving therapy remains an independent prognostic factor even after an event free interval of 10 years in early stage breast cancer. Eur J Cancer. 2012;48:1751–6.
Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JGM, Ageev FT, Hitre E, Groetz J, Iwata H, Knap M, Gnant M, Muehlbauer S, Spence A, Gelber RD, Piccart-Gebhart MJ. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859–65.
Talaei M, Sarrafzadegan N, Sadeghi M, Oveisgharan S, Marshall T, Thomas GN, Iranipour R. Incidence of cardiovascular diseases in an Iranian population: the Isfahan Cohort Study. Arch Iran Med J. 2013;16:138–44.
National Organization for Civil Registration. Age-specific mortality rate regarding gender in rural and urban areas. 2015. https://www.sabteahval.ir/Upload/Modules/Contents/asset99/fg93.pdf. Accessed 10 Jul 2015.
Lidgren M, Wilking N, Jonsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16:1073–81.
Calvert MJ, Freemantle N, Cleland JGF. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–51.
Ansaripour A, Uyl-de Groot CA, Steenhoek A, Redekop WK. The drug reimbursement decision-making system in Iran. Value Health Reg Issues. 2014;3:174–81.
The Official Gazette of the Islamic Republic of Iran. Healthcare services tariffs in public and private sectors. 2017. http://www.rrk.ir/Laws/. Accessed 3 July 2017.
WHO. Choosing interventions that are cost-effective. 2015. http://www.who.int/choice/en/. Accessed 20 Oct 2015.
Briggs A, Fenn P. Confidence intervals or surfaces? Uncertainty on the cost-effectiveness plane. Health Econ. 1998;7:723–40.
Halpern EF, Weinstein MC, Hunink MGM, Gazelle GS. Representing both first- and second-order uncertainties by Monte Carlo simulation for groups of patients. Med Decis Making. 2000;20:314–22.
CBI. History of exchange rate. 2016. http://www.cbi.ir/nonRefExRates/nonRefRates_fa.aspx. Accessed 18 Feb 2016.
The World Bank. GDP per capita (current US$). 2015. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 20 Oct 2015.
R Core Team (2015). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Barrett A, Roques T, Small M, Smith RD. How much will Herceptin really cost. BMJ. 2006;333(7578):1118–20.
Joensuu H, Kellokumpu-Lehtinen P, Bono P, Alanko T, Kataja V, Asola R, Utriainen T, Kokko R, Hemminki A, Tarkkanen M, Turpeenniemi-Hujanen T, Jyrkkiö S, Flander M, Helle L, Ingalsuo S, Johansson K, Jääskeläinen A, Pajunen M, Rauhala M, Kaleva-Kerola J, Salminen T, Leinonen M, Elomaa I, Isola J. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–20.
US National Institutes of Health. The synergism or long duration (SOLD) Study. A randomized phase III study comparing trastuzumab plus docetaxel (HT) followed by 5-FU, epirubicin, and cyclophosphamide (FEC) to the same regimen followed by single-agent trastuzumab as adjuvant treatments for early breast cancer. 2015. https://clinicaltrials.gov/ct2/show/NCT00593697. Accessed 20 June 2016.
Guarneri V, Frassoldati A, Bruzzi P, D’Amico R, Belfiglio M, Molino A, Bertetto O, Cascinu S, Cognetti F, Di Leo A, Pronzato P, Crinó L, Agostara B, Conte P. Multicentric, randomized phase III trial of two different adjuvant chemotherapy regimens plus three versus twelve months of trastuzumab in patients with HER2-positive breast cancer (short-HER trial; NCT00629278). Clin Breast Cancer. 2008;8:453–6.
Cancer Research UK. A trial comparing 6 months and 12 months of trastuzumab (Herceptin) for early breast cancer (PERSEPHONE). 2015. http://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/a-trial-comparing-6-months-and-12-months-of-trastuzumab-for-early-breast-cancer. Accessed 20 June 2016.
Aboutorabi A, Hadian M, Ghaderi H, Salehi M, Ghiasipour M. Cost-effectiveness analysis of trastuzumab in the adjuvant treatment for early breast cancer. Glob J Health Sci. 2015;7:98–106.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4th ed. New York: Oxford University Press; 2015.
SSO. Price of trastuzumab 150 mg and 440 mg vials in Iran. 2016. http://darman.tamin.ir/Forms/DrugStore/DrugLstLocal.aspx?pagename=hdpDrugLstLocal. Accessed 1 Dec 2016.
Chen W, Jiang Z, Shao Z, Sun Q, Shen K. An economic evaluation of adjuvant trastuzumab therapy in HER2-positive early breast cancer. Value Health. 2009;12:S82–4.
Kurian AW, Thompson RN, Gaw AF, Arai S, Ortiz R, Garber AM. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu—positive breast cancer. J Clin Oncol. 2007;25:634–41.
Garrison LP, Lubeck D, Lalla D, Paton V, Dueck A, Perez EA. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer. 2007;110:489–98.
Van Vlaenderen I, Canon JL, Cocquyt V, Jerusalem G, Machiels JP, Neven P, Nechelput M, Delabaye I, Gyldmark M, Annemans L. Trastuzumab treatment of early stage breast cancer is cost-effective from the perspective of the Belgian health care authorities. Acta Clin Belg. 2009;64:100–12.
Millar JA, Millward MJ. Cost effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a lifetime model. Pharmacoeconomics. 2007;25:429.
Buendía JA, Vallejos C, Pichón-Rivière A. An economic evaluation of trastuzumab as adjuvant treatment of early HER2-positive breast cancer patients in Colombia. Biomédica. 2013;33:411–7.
Cameron D, Piccart-Gebhart M, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G Jr, Untch M, Smith I, Gianni L, Baselga J, Al-Sakaff N, Lauer S, McFadden E, Leyland-Jones B, Bell R, Dowsett M, Jackisch C. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–205.
Leung W, Kvizhinadze G, Nair N, Blakely T. Adjuvant trastuzumab in HER2-positive early breast cancer by age and hormone receptor status: a cost-utility analysis. PLOS Med. 2016;13:e1002067.
Marsh K, IJzerman M, Thokala P, Baltussen R, Boysen M, Kaló Z, Lönngren T, Mussen F, Peacock S, Watkins J, Devlin N. Multiple criteria decision analysis for health care decision making—emerging good practices: report 2 of the ISPOR MCDA emerging good practices task force. Value Health. 2016;19:125–37.
Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates AS, Goldhirsch A, Cardoso F. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from International Breast Cancer Study Group Trials VIII and IX. J Clin Oncol. 2013;31:3083–90.
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2009;28:92–8.
Niwinska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol. 2010;21:942–8.
SSO. Drug list. 2017. http://www.darman.tamin.ir/Forms/Public/Druglist.aspx?pagename=hdpDrugList. Accessed 15 July 2017.
Data Availability Statement
The model developed within this study is available from the corresponding author upon request. All model inputs are described in the paper.
Author information
Authors and Affiliations
Contributions
AA, CAU, and WKR designed the study, AA programmed the model and performed the statistical analyses, and AA and WKR interpreted the results. AA provided a draft of the manuscript, and WKR and CAU critically reviewed the manuscript.
Corresponding author
Ethics declarations
Funding
All authors declare that they received no financial support for this study.
Conflict of interest
Amir Ansaripour, Carin A. Uyl-de Groot, and W. Ken Redekop declare that they have no competing interests.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s40273-018-0634-5.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40273_2017_557_MOESM1_ESM.tif
Online Resource 1 Acceptability curves at the lower and upper limits of cost of trastuzumab as the results of one-way sensitivity analysis. (TIFF 280 kb)
40273_2017_557_MOESM2_ESM.tif
Online Resource 2 Acceptability curves at the lower and upper limits of effectiveness of tra**stuzumab as the results of one-way sensitivity analysis.DFS HR: Disease-free survival hazard ratio. (TIFF 292 kb)
40273_2017_557_MOESM3_ESM.tif
Online Resource 3 The impact of WTP thresholds and life expectancy on the probabilities being cost effective among different treatment strategies at two WTP thresholds of 2×GDP and 4×GDP. (TIFF 224 kb)
40273_2017_557_MOESM4_ESM.tif
Online Resource 4 Acceptability curves of various patient age at the onset of treatment as the results of one-way sensitivity analysis (TIFF 2813 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ansaripour, A., Uyl-de Groot, C.A. & Redekop, W.K. Adjuvant Trastuzumab Therapy for Early HER2-Positive Breast Cancer in Iran: A Cost-Effectiveness and Scenario Analysis for an Optimal Treatment Strategy. PharmacoEconomics 36, 91–103 (2018). https://doi.org/10.1007/s40273-017-0557-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-017-0557-6