Abstract
Background
Trial-based cost-utility analyses require health-related quality of life data that generate utility values in order to express health outcomes in terms of quality-adjusted life years (QALYs). Assessments of baseline health-related quality of life are problematic where trial participants are incapacitated or critically ill at the time of randomisation. This review aims to identify and critique methods for handling non-availability of baseline health-related quality of life data in trial-based cost-utility analyses within emergency and critical illness settings.
Methods
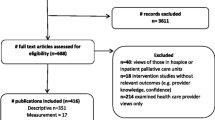
A systematic literature review was conducted, following PRISMA guidelines, to identify trial-based cost-utility analyses of interventions within emergency and critical care settings. Databases searched included the National Institute for Health Research (NIHR) Journals Library (1991–July 2016), Cochrane Library (all years); National Health Service (NHS) Economic Evaluation Database (all years) and Ovid MEDLINE/Embase (without time restriction). Strategies employed to handle non-availability of baseline health-related quality of life data in final QALY estimations were identified and critiqued.
Results
A total of 4224 published reports were screened, 19 of which met the study inclusion criteria (mean trial size 1670): 14 (74 %) from the UK, four (21%) from other European countries and one (5%) from India. Twelve studies (63%) were based in emergency departments and seven (37%) in intensive care units. Only one study was able to elicit patient-reported health-related quality of life at baseline. To overcome the lack of baseline data when estimating QALYs, eight studies (42%) assigned a fixed utility weight corresponding to either death, an unconscious health state or a country-specific norm to patients at baseline, four (21%) ignored baseline utilities, three (16%) applied values from another study, one (5%) generated utility values via retrospective recall and one (5%) elicited utilities from experts. A preliminary exploration of these methods shows that incremental QALY estimation is unlikely to be biased if balanced trial allocation is achieved and subsequent collection of health-related quality of life data occurs at the earliest possible opportunity following commencement of treatment, followed by an adequate number of follow-up assessments.
Conclusion
Trial-based cost-utility analyses within emergency and critical illness settings have applied different methods for QALY estimation, employing disparate assumptions about the health-related quality of life of patients at baseline. Where baseline measurement is not practical, measurement at the earliest opportunity following commencement of treatment should minimise bias in QALY estimation.


Similar content being viewed by others
References
Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic evaluation in clinical trials. Oxford: Oxford University Press; 2007.
Petrou S, Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ. 2011;342:d1548.
CDR Directorate at Canadian Coordinating Office for Health Technology Assessment. Canada common drug review submission guidelines for manufacturers. CCOHTA, 2013.
Guidelines for the Pharmaceutical Industry on Preparation of Submissions to the Pharmaceutical Benefits Advisory Committee. Canberra: PBAC; 2002.
Dolan P, Roberts J. Modelling valuations for Eq-5d health states: an alternative model using differences in valuations. Med Care. 2002;40(5):442–6.
Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–92.
Furlong WJ, et al. The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med. 2001;33(5):375–84.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–108.
Devlin N, Shah K, Feng Y, Mulhern B, Van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Research Paper 16/01, Office of Health Economics; 2016.
Brazier J, Roberts JR. The estimation of a preference-based index from the SF-12. Med Care. 2004;42(9):851–9.
Shah HA, Dritsaki M, Pink J, Petrou S. Psychometric properties of Patient Reported Outcome Measures (PROMs) in patients diagnosed with Acute Respiratory Distress Syndrome (ARDS). Health Qual Life Outcomes. 2016;14:15.
Smith K, Andrew E, Lijovic M, Nehme Z, Bernard S. Quality of life and functional outcomes 12 months after out-of-hospital cardiac arrest. Circulation. 2015;131:174–181.
Gates S, et al. Beta-Agonist Lung injury TrIal-2 (BALTI-2): a multicentre, randomised, double-blind, placebo-controlled trial and economic evaluation of intravenous infusion of salbutamol versus placebo in patients with acute respiratory distress syndrome. Health Technol Assess. 2013;17(38): v–vi, 1–87.
Young D, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368(9):806–13.
Mouncey PR, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–11.
Goodacre S, et al. The RATPAC (Randomised Assessment of Treatment using Panel Assay of Cardiac markers) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Health Technol Assess. 2011;15(23):iii–xi, 1–102.
Goodacre S, et al. Health utility and survival after hospital admission with acute cardiogenic pulmonary oedema. Emerg Med J. 2011;28(6):477–82.
Bohmer E, et al. Health and cost consequences of early versus late invasive strategy after thrombolysis for acute myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2011;18(5):717–23.
Powell CV, et al. MAGNEsium Trial In Children (MAGNETIC): a randomised, placebo-controlled trial and economic evaluation of nebulised magnesium sulphate in acute severe asthma in children. Health Technol Assess. 2013;17(45):v–vi, 1–216.
Pines JM, Fager SS, Milzman DP. A review of costing methodologies in critical care studies. J Crit Care. 2002;17(3):181–6.
Lopez AD, Mathers CD, Ezzati M, Jamison D, Murray CJL. The global and refgional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–57.
Moher D, LiberatiA, Tetzlaff J, Altman DG, The PRISMA Group. The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine (OPEN ACCESS). 2009.
Kendrick D, Young B, Mason-Jones AJ, Ilyas N, Achana FA, Cooper NJ, Hubbard SJ, Sutton AJ, Smith S, Wynn P, Mulvaney C, Watson MC, Coupland C. Home safety education and provision of safety equipment for injury prevention (Review). Evid Based Child Health. 2013;8(3):761–939.
Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–50.
Gyrd-Hansen D, et al. Cost-effectiveness estimate of prehospital thrombolysis: results of the PHANTOM-S study. Neurology. 2015;84(11):1090–7.
Lall R, et al. A randomised controlled trial and cost-effectiveness analysis of high-frequency oscillatory ventilation against conventional artificial ventilation for adults with acute respiratory distress syndrome. The OSCAR (OSCillation in ARDS) study. Health Technol Assess. 2015;19(23):1–177, vii.
Rosenthal VD, Udwadia FE, Kumar S, Poojary A, Sankar R, Orellano PW, Durgad S, Thulasiraman M, Bahirune S, Kumbhar S, Patil P. Clinical impact and cost-effectiveness of split-septum and single-use prefilled flushing device vs 3-way stopcock on central line-associated bloodstream infection rates in India: a randomized clinical trial conducted by the International Nosocomial Infection Control Consortium (INICC). Am J Infect Control. 2015;43:1040–1045.
Macrae D, Grieve R, Allen E, Sadique Z, Betts H, Morris K, et al. A clinical and economic evaluation of Control of Hyperglycaemia in Paediatric intensive care (CHiP): a randomised controlled trial. Health Technol Assess. 2014;18(26):1–210. doi:10.3310/hta18260
Harvey S, et al. An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial. Health Technol Assess. 2006;10(29):iii–iv, ix–xi, 1–133.
National Institute for Health and Clinical Excellence. NICE guide to the methods of technology appraisal. NICE, 2013.
Schuster A, Faulkner M, Zeymer U, Ouarrak T, Eitel I, Desch S, Hasenfuß G, Thiele H. Economic implications of intra-aortic balloon support for myocardial infarction with cardiogenic shock: an analysis from the IABP-SHOCK II-trial. Clin Res Cardiol. 2015;104:566–73.
Sierink JC, Treskes K, Edwards MJR, Beuker BJA, den Hartog D, Hohmann J, Dijkgraaf MGW, Luitse JSK, Beenen LFM, Hollmann MW, Goslings JC, For the REACT-2 study group*. Immediate total-body CT scanning versus conventional imaging and selective CT scanning in patients with severe trauma (REACT-2): a randomised controlled trial. Lancet. 2016;388:673–83.
Peek GJ, et al. Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR). Health Technol Assess. 2010;14(35):1–46.
Sintonen H. Comparing properties of the 15D and the EQ-5D in measuring health-related quality of life. Arch Hellen Med. 2001;18(2):156–160.
Perkins GD, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet. 2015;385(9972):947–55.
Varni JW, Limbers CA. The PedsQL 4.0 Generic Core Scales Young Adult Version: feasibility, reliability and validity in a university student population. J Health Psychol. 2009;14(4):611–22.
Luengo-Fernandez R, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. 2013;81(18):1588–95.
Sznajder M, Aegerter P, Launois R, Merliere Y, Guidet B, CubRea V. A cost-effectiveness analysis of stays in intensive care units. Intensive Care Med. 2001;27:146–53.
Dick AW, Perencevich EN, Pogorzelska-Maziarz M, Zwanziger J, Larson EL, Stone PW. A decade of investment in infection prevention: a cost-effectiveness analysis. Am J Infect Control. 2015;43:4–9.
Goodacre S, Cohen J, Bradburn M, Stevens J, Gray A, Benger J, et al. 3Mg trial: a randomised controlled trial of intravenous or nebulised magnesium sulphate versus placebo in adults with acute severe asthma. Health Technol Assess. 2014;18(22):1–168.
Goodacre S, et al. Randomised controlled trial and economic evaluation of a chest pain observation unit compared with routine care. BMJ. 2004;328(7434):254.
Lamb SE, et al. Managing Injuries of the Neck Trial (MINT): a randomised controlled trial of treatments for whiplash injuries. Health Technol Assess. 2012;16(49):iii–iv, 1–141.
Dixon S, et al. Is it cost effective to introduce paramedic practitioners for older people to the ambulance service? Results of a cluster randomised controlled trial. Emerg Med J. 2009;26(6):446–51.
Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ. 2005;14(5):487–96.
Schmier JK, Halpern MT. Patient recall and recall bias of health state and health status. Expert Rev Pharmacoecon Outcomes Res. 2004;4(2):159–63.
Wilson R, et al. Retrospective evaluation versus population norms for the measurement of baseline health status. Health Qual Life Outcomes. 2012;10:68.
Author contributions
The concept of this manuscript was jointly conceived by Melina Dritsaki, Felix Achana, and Stavros Petrou. Melina Dritsaki and Felix Achana gathered and reviewed the literature and data and drafted the initial manuscript. James Mason drafted the initial work presented in Sect. 4. All authors participated in the interpretation of data and preparation of the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Melina Dritsaki, Felix Achana, James Mason, and Stavros Petrou have no conflicts of interest to disclose. There was no research funding support for this study.
Data availability statement
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dritsaki, M., Achana, F., Mason, J. et al. Methodological Issues Surrounding the Use of Baseline Health-Related Quality of Life Data to Inform Trial-Based Economic Evaluations of Interventions Within Emergency and Critical Care Settings: A Systematic Literature Review. PharmacoEconomics 35, 501–515 (2017). https://doi.org/10.1007/s40273-016-0485-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-016-0485-x